Abstract
The identification of BRAF V600 mutation in multiple cancers beyond melanoma and the development of combined BRAF and MEK targeting agents have altered the landscape of tissue-agnostic precision oncology therapies with an impact on survival outcomes. Despite initial efficacy, resistance emerges, and it is pertinent to identify putative resistance mechanisms. We report a case of recurrent glioblastoma (GBM) harboring BRAF V600E alteration who initially responded to combined BRAF + MEK inhibition and subsequently developed treatment resistance by histological transformation to gliosarcoma and acquisition of oncogenic KRAS G12D and an NF1 L1083R mutation. This documented case represents an initial evidence of a developing phenomenon in cancer research as it provides the first evidence of an emergent KRAS G12D/NF1 L1083R aberration with histological transformation occurring concurrently with primary BRAF V600E-altered glioblastoma as a previously unrecognized acquired mechanism of resistance in the setting of combined BRAF and MEK inhibition. This novel finding not only sheds new light on the RAS/MAPK pathway but also highlights the potential for morphological transformation to gliosarcoma, underscoring the critical need for further investigation in this area.
Similar content being viewed by others
Introduction
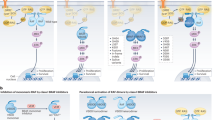
v-raf murine sarcoma viral oncogene homolog B1 (BRAF) is a Rapidly accelerated fibrosarcoma (RAF) kinase that is activated by Rat sarcoma virus (RAS) and activates the mitogen-activated protein kinase kinase (MEK) pathway. It has emerged as a major oncogenic driver and agnostic target in a wide variety of solid tumors and hematological malignancies1. BRAF signaling is instrumental for cell growth and activating BRAF mutations stimulate pro-oncogenic processes. Over 90% of activating BRAF mutations in cancer cells occur within the kinase domain at amino acid V600, most commonly resulting in V600E mutation2,3. Combined BRAF and MEK inhibition is now the standard of care in melanoma, non-small cell lung cancer, and anaplastic thyroid cancer4,5,6. Recently the combination of Dabrafenib + Trametinib received the Food and Drug Administration (FDA) approval in adult and pediatric solid tumors (except colorectal cancer) based on a demonstration of anti-tumor activity in more than 20 different cancer histologies including BRAF V600 mutant low-grade glioma (LGG) and high-grade glioma (HGG)6,7,8. However, a greater impetus to identify mechanisms of BRAF inhibitor resistance is emerging to guide treatment strategies. Resistance mechanisms to BRAF and MEK inhibition beyond melanoma have been reported in lung and thyroid cancer however, this has been infrequently described in gliomas9,10. Lehmann et al reported on pediatric HGG where increased activation of the RAS/ Extracellular signal-regulated kinase (ERK) caused alterations in RAS signaling through Neurofibromatosis type 1 (NF1) missense mutations. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3K)/Protein kinase B (AKT) signaling is also indicted in BRAF targeted resistance secondary to aberrations in PIK3C2G11,12.
In this paper, we report a case of recurrent glioblastoma (GBM) harboring BRAF V600E alteration who initially responded to BRAF and MEK inhibition and subsequently developed treatment resistance by histological transformation to gliosarcoma and acquired an emerging KRAS G12D and NF1 L1083R mutations. To our knowledge, this is the first documented case of emergent KRAS G12D and NF1 L1083R aberration concurrently occurring with primary BRAF V600E altered glioblastoma as an acquired mechanism of resistance in the setting of BRAF and MEK inhibition leading to morphological transformation to gliosarcoma.
Results
Case report
A female patient in her 30 s presented with a five-day history of headaches, slurred speech, and dyscoordination in fine motor skills. Baseline imaging of the brain via MRI with contrast demonstrated a 3.3 cm ring-enhancing lesion in the right frontoparietal subcortical white matter, suspicious for a glioma. A timeline of the patient’s clinical course and management is depicted in Fig. 1.
She underwent craniotomy with gross total resection and histology was confirmed as glioblastoma. Immunohistochemistry testing was negative for Isocitrate Dehydrogenase-1 (IDH1) mutant protein although BRAF testing was not performed at that time. The Ki-67 index was 32%. Post-operatively she received concurrent chemoradiation with a total dose of 60 Gy and temozolomide at 75 mg/m2 daily during the course of therapy. Soon after, she received adjuvant therapy as part of a clinical trial with temozolomide at 150 mg/m2 days 1-5, memantine 10 mg twice a day, metformin 500 mg twice a day, and mefloquine 250 mg twice a week (NCT01430351). Due to significant toxicity with severe nausea and fatigue, metformin and, subsequently, mefloquine was discontinued.
Two months after starting adjuvant chemotherapy, restaging scans revealed Progressive Disease (PD) and she was switched to bevacizumab 10 mg/kg every 2 weeks. After one month, capecitabine was added to the regimen at 1250 mg/m2 every 2 weeks. She stopped capecitabine due to hand/foot syndrome-related toxicity after 5 months of therapy. She was subsequently started on lomustine at 75 mg/m2 on day 3 every 6 weeks, which she received for the next four months. Unfortunately, she eventually developed persistent G2 thrombocytopenia and lomustine was discontinued. She was restarted on bevacizumab monotherapy which she tolerated well for the next 18 months. A re-staging MRI brain at this juncture demonstrated two new dural-based lesions in the right frontal lobe and right occipital lobe. She received radiation therapy to a total dose of 40 Gy in 15 fractions for the recurrent dural lesions in September 2016. Thereafter, she continued maintenance therapy with bevacizumab for one more year.
In September 2017, a restaging MRI Brain demonstrated progression in the afore two dural lesions, one in the right inferior frontal lobe and the other in the right occipital lobe. She underwent a repeat craniotomy with gross total resection and pathology confirmed the diagnosis of recurrent glioblastoma. Next-generation sequencing (NGS) of the tumor showed an actionable BRAF V600E mutation and MET N375S mutation of germline origin with unknown actionability while immunohistochemistry testing revealed IDH 1/2 were wild-type.
Based on this BRAF V600E mutation, she was genomically matched to enroll on the ROAR basket trial with dabrafenib at 150 mg twice a day plus trametinib at 2 mg daily (NCT02034110). Initially, her best response was stable disease, but after 10 cycles of therapy, the MRI of the brain confirmed a Partial Response (PR). Her best response was a −55% tumor reduction according to the Response Assessment in Neuro-Oncology (RANO) criteria13. She tolerated the regimen reasonably well with no dose reductions or treatment interruptions.
After 18 cycles of dabrafenib and trametinib therapy, a re-staging MRI of the brain revealed a new lesion in the right inferior frontal lobe, while the other lesions remained stable. Her case was discussed in a multidisciplinary tumor board setting, and the patient underwent craniotomy with the gross total resection of the new lesion. The histology of this specimen showed focal transformation to gliosarcoma in the background of glioblastoma. Figure 2 highlights the radiological metamorphosis of patient’s primary glioblastoma to gliosarcoma through the course of therapy. Figure 3 highlights the histopathological differences in the primary glioblastoma and secondary gliosarcoma lesions. Repeat NGS testing on this specimen revealed the emergence of new activating and actionable oncogenic KRAS G12D and NF1 L1083R mutations co-existing with the primary BRAF V600E mutation.
a Axial contrast-enhanced MRI while the patient was on single-agent bevacizumab shows a dural-based frontal lobe metastasis (arrow). b Axial contrast-enhanced image 9 months after radiation therapy shows significant improvement in the irradiated lesion (arrow). c Axial contrast-enhanced MRI 5 months later shows recurrence of the metastasis (arrow). The lesion demonstrates heterogeneous enhancement. d Axial post-contrast MRI 2 months later shows the rapid growth of the cystic lesion (white arrow) and extensive perilesional enhancement (black arrow). e Axial post-contrast image at treatment nadir 2 months after dabrafenib shows a significant decrease in the size of the lesion. f Axial post-contrast image 3 months later shows recurrence of the lesion, this time with solid enhancement (white arrow). The lesion was subsequently resected, showing gliosarcoma.
She continued therapy with dabrafenib and trametinib for four more months as she was deriving clinical benefit. Subsequent MRI of the brain demonstrated further progression and hence she was taken off trial 3 months later she was referred to the hospice due to clinical deterioration.
Discussion
BRAF600 mutations are known to be primary oncogenic drivers in multiple tumors14. In glioblastoma, it occurs in a small portion of IDH-wild type tumors, corresponding to 8% of the cases15. BRAF-targeted therapy has set precedence in demonstrating overall and progression-free survival benefits in multiple tumor types harboring the BRAF V600E mutation. This led to the agnostic approval of dabrafenib and trametinib by the FDA in June 202216,17. Among adult patients with BRAF V600E mutated brain tumors, 15 of the 31 high-grade glioma patients had an objective response rate (ORR) of 33% with 3 Complete Responses (CR) and 12 PRs while the duration of response among all high-grade tumors was 13.6 months. Median progression-free survival was 3·8 months and overall survival was 17·6 months18. Notably, this patient benefitted from Dabrafenib and Trametinib for 21.4 months higher than the median survival outcomes as noted before.
In melanoma, resistance mechanisms emerging after treatment with BRAF-targeted therapy are well known and they correspond mainly to recovery of MEK/ERK signaling or activation of PI3K/AKT signaling, through BRAF amplification and alternative splicing or alterations in RAS, MEK, and ERK10. The mechanism of drug resistance includes alteration of drug targets, expression of drug pumps, expression of detoxification mechanisms, reduced susceptibility to apoptosis through p53, increased ability to repair DNA damage, and altered proliferation19.
Histologic transformation to small cell lung cancer (SCLC) is a widely known resistance mechanism to epidermal growth factor receptor (EGFR) targeted therapy in Non-Small Cell Lung Cancer (NSCLC) occurring in 3-15% of EGFR aberrated NSCLC20. Patients who undergo histologic transformation to small cell lung cancers have dismal outcomes. A systemic review looking at outcomes demonstrated a median survival of 6 months after SCLC transformation21.
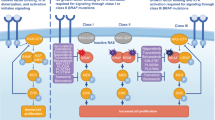
In this patient’s case, acquired mutations in KRAS with oncogenic potential and NF1 with unknown actionability were observed after prolonged exposure to BRAF inhibition along with a morphological transformation to gliosarcoma. Gliosarcoma is a rare histopathological variant of IDH-wildtype GBM and accounts for ~2% of glioblastoma variants. Histopathologically, these tumors demonstrate a combination of glial areas and sarcomatoid and mesenchymal differentiated components. Secondary gliosarcoma usually evolves after treatment of primary glioblastoma. These tumors are distinct from radiation-induced gliosarcoma which occurs after intracranial radiotherapy in patients without any prior presence of glioblastoma22. In our patient, this histological transformation of the right frontal lobe dural lesion to gliosarcoma occurred while on active therapy with dabrafenib and trametinib with initial response and then breakthrough progression with radiological and pathological transformation. It should be noted that on initial diagnosis, the pathology for this tumor raised the possibility of anaplastic pleomorphic xanthoastrocytoma. However, in the 2019 recurrence, this specimen exhibited morphology more consistent with archetypal IDH-wild type glioblastoma. However, the possibility that this tumor may have originated from an anaplastic PXA remains, particularly given the patient’s relatively young age. Furthermore, one must also consider the contiguous situated placement of the lesion’s proximity to the leptomeninges which could be a contributing factor to sarcomatous transformation.
Similar to BRAF, the RAS family of genes also works via Mitogen-activated protein kinases (MAPK) signaling pathways and activates RAF and PI3K downstream in independent pathways. Receptor tyrosine kinase signaling via Insulin-like growth factor 1 receptor (IGF1R) promotes activation of PI3K and phosphorylation of AKT23. This does not affect MAPK as is generally thought, however, MAPK and PI3K pathways jointly regulate Mcl-1 which is an anti-apoptotic factor that may promote cancer cell survival and growth. Thus, MAPK and IGF-1R via PI3K and AKT signaling pathways are both implicated in the development of BRAF inhibitor resistance. In one study in BRAF-resistant melanoma, it was found that a combination of MEK inhibitor with PI3K inhibitor led to tumoricidal effects. This study, however, did not observe the development of new mutations after acquired BRAF resistance24.
Additionally, important to note is that KRAS and BRAF do not typically co-occur in gliomas, but a common finding in GBM is aberrant RAS signaling. One paper looking at factors influencing aberrant RAS signaling found that RAS and BRAF mutations contributed to aberrant RAS signaling in a small portion of GBM25. In our case, given that initially neither aberrations were present on pathology and sequentially BRAF and then KRAS and NF1 were noted, the mechanism through which these mutations were acquired seems to be through reduced susceptibility for apoptosis as well as altered molecular signaling pathways as they are all present in common pathways. One study demonstrated that targeting both pathways through co-inhibition was more efficient in inducing apoptosis than inhibition of each pathway23.
To the best of our knowledge and literature review, this is the first case of BRAF V600E mutated GBM with the acquisition of KRAS G12 D and NF1 L1083R mutation both in the RAS/MAPK pathway and histologic transformation to gliosarcoma as a resistance mechanism to BRAF/MEK inhibition. MAPK pathway recovery may act as a secondary mechanism of resistance in glioblastomas harboring BRAF V600E after the treatment with BRAF inhibitors.
Methods
Participant
The patient was treated with dabrafenib and trametinib following enrollment in the phase II study of Efficacy and Safety of the Combination Therapy of Dabrafenib and Trametinib in Subjects With BRAF V600E - Mutated Rare Cancers (NCT02034110) after the collection of the written informed consent.
Materials
Tumor samples were obtained via surgery performed by a neurosurgeon. FFPE specimens derived from fresh tumor biopsies were reviewed by an MD Anderson pathologist to ensure adequate tumor cellularity (≥20%) for analysis. Tumor samples were evaluated using hematoxylin and eosin staining for tumor cellularity. DNA was extracted, purified, and quantified. All procedures were performed in a CLIA-compliant environment. For genomic analysis, the pre-treatment sample was sequenced and subsequently analyzed in the MD Anderson CLIA molecular diagnostic laboratory using the Ion Ampliseq 50-Gene Assay for the detection of mutations in the coding sequence of 50 genes (Thermo Fisher Scientific, MA, USA). DNA was extracted from the recurrent right frontal lobe lesion, in the MD Anderson CLIA molecular diagnostic laboratory utilizing the Oncomine® platform (Thermo Fisher) for the detection of somatic mutations in the coding sequence of 146 cancer-related genes. The radiologic response was assessed according to RANO.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Samples were sequenced and analyzed in a CLIA-compliant MD Anderson laboratory as described above. The raw sequencing data are not publicly available due to data privacy regulations and restrictions for use of such data, as stated in the study protocol and patient consent form.
References
Ross, J. S. et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J. Cancer 138, 881–890 (2016).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Subbiah, V. et al. Pan-cancer efficacy of vemurafenib in BRAFV600-mutant non-melanoma cancers. Cancer Discov. 10, 657–663 (2020).
Subbiah, V., Baik, C. & Kirkwood, J. M. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer 6, 797–810 (2020).
Subbiah, V. et al. Efficacy of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated anaplastic thyroid cancer (ATC). J. Clin. Oncol. 35, 6023–6023 (2017).
Subbiah, V. et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann. Oncol. 33, 406–415 (2022).
Wen, P. Y. et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 23, 53–64 (2022).
Subbiah, V. et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 21, 1234–1243 (2020).
Owen, D. H. et al. KRAS G12V mutation in acquired resistance to combined BRAF and MEK inhibition in papillary thyroid cancer. J. Natl. Compr. Canc Netw. 17, 409–413 (2019).
Niemantsverdriet, M. et al. KRAS mutation as a resistance mechanism to BRAF/MEK inhibition in NSCLC. J. Thorac. Oncol. 13, e249–e251 (2018).
Schreck, K. C. et al. Deconvoluting mechanisms of acquired resistance to RAF inhibitors in BRAF(V600E)-mutant human glioma. Clin. Cancer Res. 27, 6197–6208 (2021).
Lehmann, R., Rayner, B. S. & Ziegler, D. S. Resistance mechanisms in BRAFV600E paediatric high-grade glioma and current therapeutic approaches. Front. Oncol. 12, https://doi.org/10.3389/fonc.2022.1031378 (2022).
Leao, D. J., Craig, P. G., Godoy, L. F., Leite, C. C. & Policeni, B. Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. Am. J. Neuroradiol. 41, 10–20 (2020).
Bouchè, V. et al. BRAF Signaling Inhibition in Glioblastoma: Which Clinical Perspectives? Front. Oncol. 11, https://doi.org/10.3389/fonc.2021.772052 (2021).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med 364, 2507–2516 (2011).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365 (2012).
Wen, P. Y. et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 23, 53–64 (2022).
Cree, I. A. & Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 17, 10 (2017).
Mambetsariev, I. et al. Small cell lung cancer transformation following treatment in EGFR-mutated non-small cell lung cancer. J. Clin. Med. 11, https://doi.org/10.3390/jcm11051429 (2022).
Roca, E. et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat. Rev. 59, 117–122 (2017).
Frandsen, S. et al. Clinical characteristics of gliosarcoma and outcomes from standardized treatment relative to conventional glioblastoma. Front Oncol. 9, https://doi.org/10.3389/fonc.2019.01425 (2019).
Kudchadkar, R., Paraiso, K. H. & Smalley, K. S. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 18, 124–131 (2012).
Villanueva, J. et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 (2010).
Knobbe, C. B., Reifenberger, J. & Reifenberger, G. Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 108, 467–470 (2004).
Acknowledgements
We would like to thank Dr. Ilankumaran Palaniswamy from Novartis for his assistance in the review of the manuscript. Novartis sponsored the ROAR basket clinical trial. V.S. is an Andrew Sabin Family Foundation fellow at the University of Texas MD Anderson Cancer Center. V.S. acknowledges the support of the Jacquelyn A. Brady Fund. V.S. is supported by a US National Institutes of Health (NIH) grant (no. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (no. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (no. P30 CA016672).
Author information
Authors and Affiliations
Contributions
Conception or design of the work: B.E.N. and V.S. Drafting of the article: B.E.N., N.K.R., M.N. Visual Illustrations: J.T.H., B.A., M.G. Critical revision of the article: S.-P.W., V.S. All authors provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
V.S. reports receiving Research funding/Grant support for clinical trials from Roche/ Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint medicines, Loxo oncology, Medimmune, Altum, Dragonfly Therapeutics, Takeda, and National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning point therapeutics, Boston Pharmaceuticals; Travel support from Novartis, Pharmamar, ASCO, ESMO, Helsinn, Incyte and has served on Consultancy/Advisory boards for Helsinn, LOXO Oncology/Eli Lilly, R-Pharma US, INCYTE, QED pharma, MedImmune, Novartis, Relay Therapeutics, Roche; Other: Medscape. The remaining authors declare no competing interests.
Ethical approval
Ethical approval/Institutional review board approval for the above clinical trials was obtained by MD Anderson Cancer Center. Consent to participate was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nelson, B.E., Reddy, N.K., Huse, J.T. et al. Histological transformation to gliosarcoma with combined BRAF/MEK inhibition in BRAF V600E mutated glioblastoma. npj Precis. Onc. 7, 47 (2023). https://doi.org/10.1038/s41698-023-00398-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-023-00398-5
This article is cited by
-
RAF inhibitor re-challenge therapy in BRAF-aberrant pan-cancers: the RE-RAFFLE study
Molecular Cancer (2024)
-
Multiple drugs
Reactions Weekly (2023)