Abstract
Anti-tuberculosis treatment can cause significant drug-drug interaction and interfere with effective anticoagulation. However, there is a lack of evidence and conflicting data on the optimal oral anticoagulation in patients treated for tuberculosis. We investigated the safety and effectiveness of anticoagulation with non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in patients on anti-tuberculosis treatment. Patients on concomitant oral anticoagulation and anti-tuberculosis treatment including rifampin were identified from the Korean nationwide healthcare database. Subjects were censored at discontinuation of either anticoagulation or rifampin. The outcomes of interest were major bleeding, death, and ischemic stroke. A total 2090 patients (1153 on warfarin, 937 on NOAC) were included. NOAC users, compared to warfarin users, were older, had a lower prevalence of hypertension, heart failure, ischemic stroke, and aspirin use and a higher prevalence of cancer, with no significant differences in CHA2DS2-VASc or HAS-BLED scores. There were 18 major bleeding events, 106 deaths, and 50 stroke events during a mean follow-up of 2.9 months. After multivariable adjustment, the use of NOAC was associated with a lower risk of incident ischemic stroke (HR 0.51, 95% CI 0.27–0.94), while there was no significant difference in risk for major bleeding or death compared with warfarin. These results suggest that NOACs have better effectiveness for stroke prevention and similar safety compared with warfarin in patients on concomitant anti-tuberculosis treatment. This is the first study assessing the safety and effectiveness of NOACs compared to warfarin in this clinical scenario.
Similar content being viewed by others
Introduction
Tuberculosis (TB), caused by the bacillus Mycobacterium tuberculosis through air-borne transmission, remains a major cause of morbidity and mortality globally with approximately a quarter of the worldwide population affected1. Individuals with TB are categorized into active TB disease and latent TB infection. The standard treatment for people with drug-susceptible active TB disease is a 6-month regimen including four first-line drugs: isoniazid, rifampin, ethambutol and pyrazinamide2. Of the anti-TB medication, rifampin plays an essential role but is also the main cause of drug-drug interactions.
Patients with tuberculosis have increased risk of venous thromboembolism3,4, and can have comorbidities including atrial fibrillation and stroke, which require administration of anticoagulation. However, there is a lack of evidence and conflicting data on the optimal oral anticoagulation strategy in patients being treated for tuberculosis. Rifampin is a potent inducer of multiple pathways affecting drug metabolism including the hepatic cytochrome P450 (CYP) and P-glycoprotein (P-gp) efflux transporter system5. Warfarin is mainly metabolized in the liver via the CYP 2C9, 1A2, and 3A46, and induction of the CYP enzymes leads to a significant decrease (about 15–74%) in warfarin area under the concentration–time curve (AUC)7. A three- to five-fold increase in warfarin dosage is usually required to attain sufficient warfarin levels measured by prothrombin time international normalized ratio (INR) testing7,8. The non-vitamin K antagonist oral anticoagulants (NOACs) are substrates of the either or both the CYP3A4 and P-gp system9, and induction of these systems by rifampin can also lead to reduced exposure to NOACs. Previous pharmacokinetic studies on co-administration of NOACs with rifampin reported that the AUC of dabigatran, apixaban, and rivaroxaban decreased 50–67%, while that of edoxaban decreased 35% but with compensatory increase of active metabolites7,10,11,12.
Thus, recent guidelines state that dabigatran, apixaban, and rivaroxaban are contraindicated while edoxaban may be used with caution when co-administered with rifampin, due to reduced NOAC plasma levels13. On the other hand, the risk of major bleeding was reported to be increased with the concurrent use of rifampin and NOACs in a retrospective cohort14, contrary to what may be expected from reduced NOAC levels. However, up to now, there have been no clinical studies focusing on the safety and effectiveness of NOACs compared to warfarin when used concomitantly with anti-TB medication15. In this study, we analyzed a nationwide cohort of patients on oral anticoagulation to compare the safety and effectiveness of NOACs and warfarin when co-administered with rifampin.
Results
Baseline characteristics of the study population
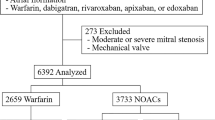
A total of 2090 subjects on concomitant oral anticoagulation and rifampin were included in the study, including 1153 patients on warfarin, and 937 patients on NOACs (Fig. 1). Indication for anticoagulation was atrial fibrillation in 1290 (61.7%), and venous thromboembolism in 800 (38.3%). The proportion of patients on NOAC versus warfarin increased over the years (Table 1). The proportion of atrial fibrillation as the indication for anticoagulation was higher in patients on warfarin. Patients on NOACs were on average 3 years older, had a lower prevalence of hypertension, heart failure, ischemic stroke, and aspirin use, higher prevalence of cancer, and less concomitant use of aspirin, compared to patients on warfarin. Meanwhile, while there were no significant differences in CHA2DS2-VASc or HAS-BLED scores, and history of intracranial hemorrhage or gastrointestinal bleeding between NOAC and warfarin users.
Risk of outcomes associated with use of warfarin or NOACs
The mean follow-up duration was 2.9 ± 3.0 months. Overall, the incidence of major bleeding events during concomitant anticoagulation and anti-TB medication was low. Major bleeding events occurred in 6 of warfarin users (0.52%) and 12 of NOAC users (1.28%). Death occurred in 48 of warfarin users (4.2%) and 58 of NOAC users (6.2%). Ischemic stroke occurred in 35 patients on warfarin (3.0%) and 15 patients on NOAC (1.6%); when confined to the subgroup of patients on anticoagulation for atrial fibrillation (n = 1290), ischemic stroke occurred in 34 of 805 warfarin users (4.2%) and 12 of NOAC users (2.5%).
There was a tendency for higher incidence of major bleeding and death and lower incidence of stroke in NOAC users compared to warfarin users by unadjusted Kaplan–Meier survival analysis (Fig. 2). The use of NOAC was associated with higher risk of death and lower risk of stroke compared to use of warfarin in unadjusted univariable Cox regression analysis (Table 2). After multivariable adjustment for possible confounding factors, the use of NOAC was associated with a lower risk of incident ischemic stroke (HR 0.51, 95% confidence interval [CI] 0.27–0.94, p = 0.030); meanwhile, the risk for major bleeding or death was no longer significantly different compared to use of warfarin (Table 2). The tendency for lower risk of stroke with use of warfarin compared to use of NOAC was consistent when confined to the patients being anticoagulated for atrial fibrillation, though not achieving significance (p = 0.086).
Reduced dose analysis
Among NOAC users, 52.0% (n = 487) were prescribed reduced dose of NOACs. Supplemental Table 1 shows the hazard ratios for the study outcomes for reduced and standard dose of NOACs compared to warfarin. The trend of lower risk for stroke and higher risk for death and bleeding associated with NOACs were consistent regardless of dose regimen, and after multivariable adjustment, only the association between reduced dose of NOACs and increased risk of death was significant. Supplemental Table 2 shows the hazard ratios for the study outcomes for reduced dose of NOACs compared to standard dose of NOACs. After multivariable adjustment, no significant difference was observed between reduced dose of NOACs and standard dose of NOACs for all study outcomes.
Subgroup analysis
Subgroup analyses stratified by age, sex, indication for OAC use, history of cancer or chronic kidney disease, stroke risk according to the CHA2DS2-VASc score, and bleeding risk according to the HAS-BLED score and number of concomitant antiplatelet agents were performed, and no significant subgroup difference detected in the treatment effect of NOAC versus warfarin (all p-for-interaction > 0.100) (Fig. 3). However, this may be due to the lack of power. To note, the incidence of major bleeding events was similar in patients with HAS-BLED score 0–2 and ≥ 3, and there was a tendency for higher bleeding risk associated with NOACs compared to warfarin in patients with low HAS-BLED scores. Most of the stroke events occurred in patients with CHA2DS2-VASc scores ≥ 2, and NOAC was associated with significantly lower risk of stroke in patients with high CHA2DS2-VASc scores compared to warfarin.
Subgroup analyses of risk for outcomes in warfarin and NOAC users. Adjusted for age, sex, CHA2DS2-VASc score, history of intracranial hemorrhage, history of gastrointestinal bleeding, chronic kidney disease, cancer, and concurrent antiplatelet usage. HR hazard ratio, NOAC non-vitamin K antagonist oral anticoagulant, AF atrial fibrillation, VTE venous thromboembolism, CKD chronic kidney disease.
Discussion
To the best of our knowledge, this is the first and largest study to comprehensively compare the safety and effectiveness of NOACs with warfarin in patients during concomitant TB medication. Our key findings are as following: (1) major bleeding events are rare and stroke events mostly occur in patients with high CHA2DS2-VASc scores; (2) NOACs were associated with better effectiveness for stroke prevention and similar safety regarding major bleeding and death on multivariate analysis; (3) stratified analyses according to NOAC dose and subgroups showed results overall consistent with the main analysis.
TB is a global health concern, being the 13th leading cause of death worldwide and the leading cause of death from infection in 20191. TB is prevalent in low and middle income countries with widespread poverty, undernutrition, human immunodeficiency virus infection, smoking and diabetes. Korea still has a high burden of TB, with the highest incidence of TB (49 cases per 100,000 population) and the third highest mortality from TB (3.8 cases per 100,000 population) of the 38 member countries of the Organization for Economic Cooperation and Development1. On the other hand, the prevalence of atrial fibrillation continues to increase rapidly with population aging; the prevalence of atrial fibrillation in Korea was 1.53% in 2015 and continues to increase over time16. The incidence of venous thromboembolism is also on the rise17. Meanwhile, TB infection is associated with increased thrombogenicity3,4. Thus, not infrequently, clinicians encounter cases in which patients on anticoagulation start anti-TB medication or vice-versa; however, there is a lack of evidence on the best oral anticoagulation strategy during concomitant anti-TB medication. Some clinicians make the choice of switching to warfarin and monitoring INR levels in these patients, instead of maintaining NOACs.
The use of rifampin causes induction of the hepatic CYP and P-gp enzymes related to drug metabolism and subsequently leads to a reduction in drug exposure for both warfarin and NOACs according to pharmacokinetic studies7,10,11,12. The dose of warfarin is usually increased during concomitant anti-TB medication with INR monitoring to achieve target levels7,8, while there is no method to monitor NOAC activity and no consensus on increasing NOAC dose during concomitant anti-TB medication. The latest guideline states that edoxaban may be used while the other NOACs are contraindicated due to reduced plasma levels13. One can presume that lower plasma levels of NOACs would result in more stroke events but less bleeding; however, a retrospective cohort study including 1151 patients on concomitant rifampin and NOACs from the Taiwan National Health Insurance database reported that in patients taking NOACs for atrial fibrillation, the incidence of major bleeding increased with the concurrent use of rifampin14.
In the current retrospective cohort, we found an insignificant tendency for increased major bleeding and death, and a significant decrease in stroke events associated with NOACs compared to warfarin. Presuming that the dose of warfarin was increased while that of NOAC was not during concomitant anti-TB medication, we expected that the incidence of bleeding events would be higher in warfarin users. However, results were contrary to our expectation, suggesting that anticoagulation effects of NOAC remain stronger than that of warfarin. This suggests that there may be other drug–drug interactions in addition to those currently established while on concurrent anticoagulation and anti-TB medication, translating into unpredictable drug exposure levels and outcomes. This warrants further research for underlying in vivo mechanisms.
Ischemic stroke events mostly occurred in patients with high CHA2DS2-VASc scores (≥ 2), and NOACs were associated with significantly lower risk of stroke compared to warfarin. While there was a tendency for increased bleeding with NOACs compared to warfarin, the actual incidence of major bleeding was low and the difference was insignificant after multivariable adjustment. Also, the bleeding risk related to NOACs was not especially higher in those with higher bleeding risk (HAS-BLED scores ≥ 3). These results suggest that clinicians should continue anticoagulation in patients at high risk of stroke, preferably with NOACs than warfarin, during treatment for TB.
Strengths and limitations
A main strength of this study is that it included all Korean patients on a nationwide basis prescribed both oral anticoagulants and anti-TB medication and is thus free from referral bias; also, complete outcomes without loss of follow-up could be obtained from the database. Nevertheless, there are some limitations that are worthy of being mentioned. First, as we limited the study period to the duration of concomitant oral anticoagulation and anti-TB medication, the mean follow-up was around 3 months and the number of outcome events was not high, resulting in decreased statistical power. This was inevitable considering that the standard anti-TB regimen usually lasts only 6 months. Second, we also planned to compare outcomes between the individual NOACs and verify whether edoxaban was associated with better outcome as presumed in the latest guideline13, but the study lacked statistical power to undergo these analyses. Third, due to the small number of major bleeding events, there may be a small positive association between NOAC use and increased bleeding risk which was missed in multivariate analysis related to the lack of power. However, this also suggests that the risk of major bleeding is practically low during the short period of concomitant anticoagulation and anti-TB treatment, and the difference in risk between use of NOAC and warfarin negligible. Fourth, as the INR levels were not available in the database, we could not assess the time in therapeutic range for warfarin users. Last, due to the retrospective observational nature of the study, our study cannot provide causal relationships and there should be caution in interpreting the results. Despite these limitations, we believe that this study is of practical value, considering that randomized clinical trials regarding this issue are unlikely to be performed in this special population.
Conclusions
In a nationwide cohort of patients on concomitant anti-TB medication and oral anticoagulation, NOACs showed better effectiveness for stroke prevention and no significant difference in safety regarding major bleeding and death compared with warfarin. These results suggest that clinicians should continue anticoagulation preferably with NOACs than warfarin during treatment for TB in patients at high risk of stroke, rather than switching to warfarin and monitoring INR levels.
Methods
Study population
The study cohort was derived from the Korean National Health Insurance Service claims database covering the entire Korean population, the details of which have been described previously18,19. De-identified datasets are provided to approved researchers at the National Health Insurance Sharing Service (nhiss.nhis.or.kr). The current study complied with the Declaration of Helsinki, and was approved by the Institutional Review Board of Seoul National University Hospital (E-2208-109-1351). Informed consent was waived by the Institutional Review Board of Seoul National University Hospital due to the retrospective nature of the study and anonymized database.
Adults (age ≥ 20 years) who were prescribed oral anticoagulants for the clinical indications of non-valvular atrial fibrillation or venous thromboembolism between January 2013 and December 2017 were identified. This population was provided after 50% random sampling from the database, according to regulations of the Korean National Health Insurance Service. Oral anticoagulants included warfarin and the NOACs, i.e. rivaroxaban, dabigatran, apixaban, and edoxaban. The starting year was set as 2013, the year NOACs were approved for use in Korea. From this population, we included patients with simultaneous prescription of rifampin (Fig. 1). Rifampin was selected to identify patients on anti-TB treatment, as it is an integral part throughout the standard 6-month anti-TB regimen and is the main cause of drug-drug interaction. We excluded patients with prosthetic heart valves, end-stage renal disease, or missing data. The index date was the first date of concomitant prescription of oral anticoagulants and rifampin. Subjects were censored at the discontinuation of either anticoagulation or rifampin, or at 1 year to reduce confounding effects related to outliers. Follow-up was until the occurrence of the outcomes, death, censoring, or the end of the study (December 31, 2018).
Covariates and study outcomes
Age, sex, and income level were obtained from the database. Comorbidities were assessed for 1 year before the index date. Diseases were defined using diagnostic codes, inpatient and outpatient hospital visits, and prescription codes (Supplemental Table 3)19,20. Concomitant antiplatelet or nonsteroidal anti-inflammatory drug (NSAID) use of at least 1 month or more during the study period was identified. The HAS-BLED score was calculated as a measure of bleeding risk, by assessing the presence of hypertension (1 point), abnormal renal function (1 point; end-stage renal disease, chronic kidney disease, kidney transplantation), abnormal liver function (1 point; liver cirrhosis, liver disease), stroke (1 point; ischemic stroke), bleeding (1 point; previous hospitalization for gastrointestinal bleeding, peptic ulcer), old age (1 point; age > 65 years), heavy alcohol drinking (1 point; > 8 times/week), and antiplatelet or NSAID use (1 point)21. The CHA2DS2-VASc score was calculated as a measure of stroke risk, by assessing the presence of congestive heart failure (1 point), hypertension (1 point), old age (2 points if ≥ 75 years; 1 point if ≥ 65 years), diabetes mellitus (1 point), prior stroke or transient ischemic attack or systemic embolism (2 points), vascular disease (1 point; myocardial infarction or peripheral artery disease), and female sex (1 point)22.
The safety outcomes were hospitalization for major bleeding and all-causes death. Major bleeding was defined as intracranial hemorrhage, gastrointestinal bleeding, respiratory tract bleeding, and internal bleeding such as hemothorax, hemoperitoneum, and hemopericardium. The effectiveness outcome was hospitalization for ischemic stroke. The outcome of ischemic stroke was assessed in the total study population and in the subgroup of patients with atrial fibrillation.
Statistical analysis
Categorical data are presented as numbers (%), and continuous data are presented as mean ± standard deviation. Characteristics were compared between the groups using the t-test or the chi-square test. Incidence rates were calculated by dividing the number of events by the total follow-up period (per 1000 person-years). The risks of the outcomes associated with warfarin or NOAC use were assessed with Cox regression analysis, and the hazard ratios (HR) and corresponding 95% CI were estimated. Cumulative incidence curves for the outcomes were drawn by the Kaplan–Meier method and compared with the log-rank test. Multivariate models were adjusted for age, sex, CHA2DS2-VASc score, history of intracranial hemorrhage, history of gastrointestinal bleeding, chronic kidney disease, cancer, and concurrent antiplatelet usage. We also performed separate analyses for the outcomes stratified by the NOAC dose (i.e., standard and reduced). Reduced doses of NOACs were defined as 15 or 10 mg rivaroxaban once daily, 110 mg dabigatran twice daily, 2.5 mg apixaban twice daily, and 30 mg edoxaban once daily, while regular doses of NOACs were defined as 20 mg rivaroxaban once daily, 150 mg dabigatran twice daily, 5 mg apixaban twice daily, and 60 mg edoxaban once daily. Pre-specified subgroup analyses were performed according to age, sex, indication for anticoagulation, cancer, chronic kidney disease, CHA2DS2-VASc score and HAS-BLED score, and number of antiplatelet agents. P-values < 0.05 were considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Data availability
The dataset generated and analyzed in the current study are available in the Korean National Health Insurance Sharing Service database (https://nhiss.nhis.or.kr) and specifications required for the dataset are available from the corresponding author on reasonable request.
References
World Health Organization. Global Tuberculosis Report 2021 (World Health Organization, 2021).
Nahid, P. et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 63, e147–e195. https://doi.org/10.1093/cid/ciw376 (2016).
Ha, H. et al. Thromboembolism in Mycobacterium tuberculosis infection: Analysis and literature review. Infect. Chemother. 51, 142–149. https://doi.org/10.3947/ic.2019.51.2.142 (2019).
Borjas-Howard, J. F., Bierman, W. F. W., Meijer, K., van der Werf, T. S. & Tichelaar, Y. Venous thrombotic events in patients admitted to a tuberculosis centre. QJM 110, 215–218. https://doi.org/10.1093/qjmed/hcw152 (2017).
Baciewicz, A. M., Chrisman, C. R., Finch, C. K. & Self, T. H. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr. Med. Res. Opin. 29, 1–12. https://doi.org/10.1185/03007995.2012.747952 (2013).
Gelosa, P. et al. Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs). Pharmacol. Res. 135, 60–79. https://doi.org/10.1016/j.phrs.2018.07.016 (2018).
MacDougall, C., Canonica, T., Keh, C. & Louie, J. Systematic review of drug–drug interactions between rifamycins and anticoagulant and antiplatelet agents and considerations for management. Pharmacotherapy 42, 343–361. https://doi.org/10.1002/phar.2672 (2022).
Lee, C. R. & Thrasher, K. A. Difficulties in anticoagulation management during coadministration of warfarin and rifampin. Pharmacotherapy 21, 1240–1246. https://doi.org/10.1592/phco.21.15.1240.33897 (2001).
Wiggins, B. S., Dixon, D. L., Neyens, R. R., Page, R. L. 2nd. & Gluckman, T. J. Select drug–drug interactions with direct oral anticoagulants: JACC review topic of the week. J. Am. Coll. Cardiol. 75, 1341–1350. https://doi.org/10.1016/j.jacc.2019.12.068 (2020).
Hartter, S. et al. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br. J. Clin. Pharmacol. 74, 490–500. https://doi.org/10.1111/j.1365-2125.2012.04218.x (2012).
Vakkalagadda, B. et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa. Am. J. Cardiovasc. Drugs 16, 119–127. https://doi.org/10.1007/s40256-015-0157-9 (2016).
Mendell, J., Chen, S., He, L., Desai, M. & Parasramupria, D. A. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin. Drug Investig. 35, 447–453. https://doi.org/10.1007/s40261-015-0298-2 (2015).
Steffel, J. et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 23, 1612–1676. https://doi.org/10.1093/europace/euab065 (2021).
Chang, S. H. et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 318, 1250–1259. https://doi.org/10.1001/jama.2017.13883 (2017).
Fiore, M. et al. Is anticoagulation with novel oral anticoagulants an effective treatment for tuberculosis patients not achieving a therapeutic range with vitamin K antagonists? A systematic review. Cardiovasc. Hematol. Disord. Drug Targets 17, 105–110. https://doi.org/10.2174/1871529X17666170703115545 (2017).
Joung, B. et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ. J. 48, 1033–1080. https://doi.org/10.4070/kcj.2018.0339 (2018).
Kim, H. Y. et al. Epidemiology of venous thromboembolism and treatment pattern of oral anticoagulation in Korea, 2009–2016: A nationwide study based on the National Health Insurance Service database. J. Cardiovasc. Imaging 29, 265–278. https://doi.org/10.4250/jcvi.2021.0014 (2021).
Choi, E. K. Cardiovascular research using the Korean National Health Information Database. Korean Circ. J. 50, 754–772. https://doi.org/10.4070/kcj.2020.0171 (2020).
Lee, H. J. et al. Risk of upper gastrointestinal bleeding in patients on oral anticoagulant and proton pump inhibitor co-therapy. PLoS ONE 16, e0253310. https://doi.org/10.1371/journal.pone.0253310 (2021).
Lee, H. J. et al. Novel oral anticoagulants for primary stroke prevention in hypertrophic cardiomyopathy patients with atrial fibrillation. Stroke 50, 2582–2586. https://doi.org/10.1161/STROKEAHA.119.026048 (2019).
Pisters, R. et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 138, 1093–1100. https://doi.org/10.1378/chest.10-0134 (2010).
Lip, G. Y., Nieuwlaat, R., Pisters, R., Lane, D. A. & Crijns, H. J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest 137, 263–272. https://doi.org/10.1378/chest.09-1584 (2010).
Author information
Authors and Affiliations
Contributions
H.J.L. and H.K.K. conceived and designed the study. H.J.L., B.S.K., and T.M.R. acquired the data. B.S.K. and H.D.K. analyzed the data. H.J.L., H.K.K., C.S.P., T.M.R., J.B.P., H.L., and Y.J.K. interpreted the data. H.J.L. wrote the original draft. H.K.K., J.B.P., H.L., Y.J.K. reviewed and edited the manuscript. All authors read and approved the final manuscript. All authors agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
HKK reports research grants from Actelion, Handok, GSK, Dae-Woong, Hanmi, Ildong, JW Pharmaceutical, Samjin, and Norvatis. The remaining authors declare no potential conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, HJ., Kim, HK., Kim, BS. et al. Safety and effectiveness of anticoagulation with non-vitamin K antagonist oral anticoagulants and warfarin in patients on tuberculosis treatment. Sci Rep 13, 2060 (2023). https://doi.org/10.1038/s41598-023-29185-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29185-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.