Abstract
The primary objective of this study was to describe the cytotoxicity on HEPG-2 cells and to study the COVID‑19 activities of the novel H2L ligand and its Cr and Cu nano-complexes. As well as exploring the chemistry of the prepared nano-complexes. In this paper novel Schiff base, N-(4, 6-dimethyl pyrimidin-2-yl)-4-(((2-hydroxyl naphthalene-1-y l) methylene) amino) benzene—sulfonamidesulfonyl) amide has been synthesized. The novel Schiff base H2L is used to synthesize novel nano and micro-complexes with CrCl2.6H2O and CuCl2.2H2O. The prepared ligand and micro complexes were interpreted by different spectroscopic techniques. The nano-sized Cr and Cu complexes were synthesized in an environmentally friendly manner using Coriandrum sativum (CS) media extract in ethanol. The structure, morphologies and particle size of the nano-sized complexes were determined using FT-IR, TEM, and PXRD. The results showed that the nano-domain complexes are on the Sub-nano scale. Furthermore, using TGA, we studied the effect of heat on the size of newly prepared nano-complexes. Experimental data were supported by DFT calculations. The findings revealed that the metal complexes investigated are more stable than the free ligand H2L. The antitumor activity was examined before and after heating the nano-complexes at 200 °C. The results reveal the Cr nano complex, after heating, exhibited strong antitumor activity with IC50 value (3.349 μg/ml). The tested Cu nano-complex shows good DNA cleavage. The liver cancer and COVID19 proteins were examined using molecular docking to identify the potential binding energy of inhibitors.
Similar content being viewed by others
Introduction
Sulfadimidine is a sulfa-based drug that is used to prevent and treat bacterial infections such as influenza, eye infections, actinomices, meningitis infections, and urinary tract infections1,2 .They can also be utilized as model molecules to study drug action mechanisms3. In addition to other sulfonamides, it has been altered by Schiff-base formation4 and the formed complexes with transition elements5,6. Because they have multiple potential donor sites, such as amino, pyrimido, and sulfonamidic nitrogen, as well as sulfonyl oxygen atoms, they are good complexing agents. Several Schiff bases generated from sulfa drugs have been produced and employed as ligands in the production of powerful metal complexes5,7,8. Schiff base compounds have received a lot of attention from researchers. Schiff bases are useful in many different areas such as polymer stabilizers, catalysts, intermediates in organic synthesis, dyes and pigments9. Furthermore, Schiff bases are an essential class of ligands in coordination chemistry. Schiff bases have also been demonstrated to have antibacterial, antifungal, antimalarial, antiviral, anti-inflammatory, and antipyretic effects, among other biological actions10,11,12,13,14,15. The creation of novel compounds with unique physical, chemical, and biological properties results from the synthesis of nano-sized complexes16. Plant extracts are used to prepare green nano-domain complexes, which is an environmentally friendly method17,18. There is no published literature for Schiff base generated from sulfadimidine sodium with 2-hydroxy-1-naphthaldehyde, its metals, or nano-metal complexes.
Quantum chemical calculations are being used to elucidate molecular interaction mechanisms. Recently, DFT (density functional theory) calculations were performed to comprehend the molecular geometry and molecular electrostatic potential of the investigated compounds19,20,21,22,23. Moreover, molecular docking studies were carried out in order to further evaluate and identify the actual molecular recognition interaction between the prepared compounds under study and the predicted target24,25,26,27,28,29,30.
Therefore, in this study, we focus on the synthesis of a novel Schiff base ligand generated from sulfadimidine sodium and 2-hydroxy-1-naphthaldehyde in addition to the preparation of novel micro and nano-sized complexes. Different physicochemical and spectral analysis techniques were used to characterize the H2L ligand and its micro and nano-sized complexes. In addition, molecular modeling was studied to acquire more information about the properties of the prepared compounds under study. Furthermore, the antitumor activities and DNA cleavage of the nano-complexes were evaluated to examine the bio-efficiency. Also, molecular docking studies were used to predicate possible biological applications, using computer software.
Experimental
Materials
All chemicals used were obtained from Aldrich or BDH. They included 2-Hydroxy-1-naphthaldehyde, sulfadimidine sodium, CrCl2.6H2O or CuCl2.2H2O and LiOH. The solvents, such as absolute ethyl alcohol and Dimethylformamide (DMF) were used as received without pretreatment.
Instrumentation and spectral measurements
The CHNS contents were evaluated using a Perkin-Elmer elemental analysis (240c). Melting points of all synthesized compounds were measured on Gallenkamp apparatus. 1HNMR spectra in DMSO-d6 were obtained on a BRUKER 400 MHz. With an (MS-5988 GC–MS) at 70 eV, mass spectra were recorded. The molar conductance was measured on a Jenway conductometer. A Jasco P-530 spectrophotometer was used to acquire electronic spectra. FTIR spectra were recorded on Shimadzu spectrophotometer applying KBr pellet in the range of (4000–400 cm−1). With an (MS-5988 GC–MS) at 70 eV, mass spectra were recorded. PXRD patterns were created on a Bruker AXS D8 advanced diffractometer. A Jeol Jem-1200 EX II electron microscope was used to produce the SEM images, with an acceleration voltage of 25 kV. The TEM micrographs were executed using JEOL JEM-1200 EX II, Japan at 60–70 kV. Thermogravimetric analyses of the micro and nanocomplexes were performed in an inert atmosphere on a Shimadzu DTG-60H at 10 °C/min. A Jasco P-530 spectrophotometer was used to acquire electronic spectra. Using Varian E-109 X-band or Q-band spectrophotometers, ESR measurements were done at 77 and 300 K. DPPH was employed as a standard material.
Synthesis of H2L Schiff base ligand
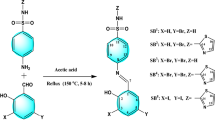
In a round-bottomed flask, a mixture of sulfadimidine sodium (2.7833 g; 10 mmol) and 2-hydroxy-1-naphthaldehyde (1.7218 g, 10 mmol) in 20 mL EtOH was refluxed for 4 h. The reaction mixture was then acidified with 5% HCl to form a neutral (pH=7) solution, and a solid of H2L Schiff-base was isolated and recrystallized from ethanol.
Synthesis of Schiff base complexes
The H2L solution in ethanol was mixed with a solution of CrCl2.6H2O or CuCl2.2H2O in ethanol in a 1:1 ratio. The resulting solution was refluxed for 4–6 h at 70 °C. The precipitate was separated by filtration and washed with ethanol, bi-distilled water followed by diethyl ether and lastly dried at room temperature in a desiccator under vacuum (Fig. 1).
Synthesis of Schiff base nano-complexes
Nano-sized complexes of Cr(II) and Cu(II) were produced in a 1:1 ratio, in ethanol by adding Coriandrum sativum (CS)/EtOH media, as previously reported in our publications31.
Computational calculations
The H2L and its complexes were optimized using the Gaussian 09 program32, using the DFT method comprising the B3LYP/6-311G + (dp) level of theory for all atoms except metal ions and at the B3LYP/LANL2DZ level of theory for metal ions33.
Antitumor activity
The antitumor activity of the ligand and its nanocomplexes was tested against HepG2 cells using cis-platin (cis-diamminedichloroplatinium). The cell lines were obtained from American Type Culture Collection (ATCC; Rockville, MD, USA). The experiments were carried out in the tissue culture unit at Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. Graphed prism version (San Diego, CA.USA) was used to calculate the IC50 values.
DNA cleavage studies
The DNA cleavage experiment was was carried out using CT-DNA (Calf Thymus DNA) by gel electrophoresis with the corresponding nanocomplex. Test samples were dissolved in DMSO and added separately to the CT-DNA. The sample mixtures were incubated at 37ºC for 2 h. The samples were electrophoresed for 2 h at 100 V on 0.25 g agarose gel using 25 ml of TAE buffer (pH8.0). After electrophoresis, the gel was stained using 10 μg/ml ETBR solution for 10–15 min, then the bands were observed and photographed under UV transilluminator.
Docking simulation
Molecular docking simulation was executed to anticipate the binding sites and affinity score, using MOE2019 version software34, and the crystal structures of different targets were reclaimed from the protein data bank (https://www.rcsb.org) in the pdb format. The target proteins include human methionine adenosyl-transferases (PDB ID: 5A19) which is tightly linked to liver cancer35, and the crystal structure of COVID-19 main protease viral protein (PDB ID: 6lu7)36.
Results and discussion
Analytical data
The CHNS contents, chemical formula, melting points, Color and yields of the synthesized compounds are postulated in Table 1. This illustrates the purity of the ligand and its complexes.
1H-NMR interpretations
The 1H NMR spectra of the synthesized H2L ligand were detected in DMSO and D2O (Figs. S1 and S2). For H2L, The CH3 and OH protons signals were detected as a singlet at δ2.21 and 9.64 ppm, respectively. The imine (–CH=N) signal was observed at δ 8.58 ppm37. The NH proton signal was observed at δ 10.61 ppm38. The aromatic proton signals of the H2L ligand were detected between δ 7.24 and 7.91 ppm39. The signal at δ 2.51 ppm for DMSO-d6 37. The hydroxyl group signal has completely disappeared upon the addition of D2O, indicating the purity of the prepared ligand.
Molar conductivity analysis
The molar conductance (Ωm) of the prepared ligand H2L and its micro-complexes in 10–3 M DMSO solution lie in the 0.7 to 5.35 Ohm−1 cm2 mol−1 range (Table 1), revealing the non-electrolytic nature of these compounds40.
IR spectra
Infrared spectral studies of micro-complexes
The infrared spectra of the investigated ligand and its metal(II) Chelates are depicted in (Fig. 2A). The H2L ligand spectral exhibited absorption bands at 3200, 1384, and 1156 cm−1 that is, respectively, assigned to υ(OH), υas(SO2) and υsy(SO2) groups. The synthesis of the H2L ligand is revealed by the imine signal of the ligand (C=N) at 1633 cm−1; however, in the Cr(II) and Cu(II) complexes, this signal shifted to 1607 and 1613 cm−1, suggesting that imine nitrogen is involved in coordination41. The phenolic (O–H) of the H2L disappeared in the Cr(II) and Cu(II) complexes, showing proton replacement during complex formation. The three characteristic peaks of υ(NH) at 3457, υas(SO2) at 1384 cm−1 and υsy(SO2) at 1156 cm−1 in the spectra of the Cr(II) and Cu(II) micro-complexes and their free ligand all appear at the same position, excludes the coordination through the NH and SO2 groups42. The new bands in the IR spectra of the prepared compounds at 520–528 and 570–573 cm−1 are ascribed to (M–N) and (M–O), respectively. Furthermore, in the spectra of the Cr(II) complex, the broadband near 3477 could be attributed to the υ(OH2) (Table 2).
Infrared spectral studies of nano-complexes
The FTIR spectra of the Cr and Cu nano-domain complexes generated in CS/EtOH media are given in (Fig. 2B), which reveals the binding modes of the Cr and Cu nano-domain complexes, which are corroborated by a change in band position of the nano-domain complexes when compared to the free ligand.
Mass spectral data
MS of the synthesized H2L and its Cr and Cu complexes are shown in (Fig. S3). The MS of H2L exhibited a molecular ion peak (m/z)=432.45, which is in good agreement with the proposed molecular weight (Fig. S3A). The proposed fragmentation pattern of the H2L ligand was given in (Fig. 3). The mass spectrum of the Cr(II) and Cu(II) complexes displayed molecular ion peaks at m/z 968.27 and 518.92, respectively, approving the molecular weight and the existence of Cr and Cu isotope peaks at m/z 54 and 65, respectively (Fig. S3B, C).
X‑ray study
The X-ray diffraction pattern is an analytical technique that was used to learn more about the chemistry and crystallographic structure of the obtained Cr and Cu nano-complexes (Fig. 4). The crystalline phase of the prepared Cr and Cu nano-complexes in CS/EtOH media was revealed by their sharp and high-intensity diffraction peaks. The nano crystallite sizes of the Cr and Cu nano-complexes were calculated using the Scherrer formula17,37,43, which are 42.5 and 39.2 nm, respectively, which lay inside the range of the nano-scale.
SEM analysis of micro-complexes
The morphologies and particle size of the Cr(II) and Cu(II) micro-complexes have been demonstrated by the scanning electron microscope (SEM). Figure 5 depicts the SEM photographs of the synthesized Cr(II) and Cu(II) Schiff base complexes. In the pictograph, we observed that the produced complexes are arranged in a regular matrix. This suggests that Cr(II) and Cu(II) complexes have homogeneous phase material. The Cr(II) complex has a seashell-like shape with an average particle size of 8 µm. The images of the Cu (II) complex display a spherical shape with an average particle size of 0.33 µm. This indicates that Cr(II) and Cu(II) complexes are found in the microdomain.
TEM measurement of nano-complexes
The transmission electron microscopy (TEM) technique was used to examine the morphologies and particle size of the Cr and Cu nano-sized complexes produced in Coriandrum sativum (CS) media before and after heating the nano-sized chelates at 200 °C for 2 h. The images of the Cr and Cu nano-complexes show that these complexes have a spherical shape. Moreover, the calculated histogram for the average particle size of the prepared nano-sized chelates before heating was 3.85 and 16.42 nm (Sub-nano) for Cr and Cu nano-sized chelates, respectively. While heating Cr and Cu nano-complexes to 200 °C resulted in particle sizes of 2.05 nm and 2.72 nm (Sub-nano), respectively, it also indicates that the particle size of Cr and Cu nano-sized chelates decreases with increasing temperature (Fig. 6).
Thermal analysis
Thermal analysis of micro-complexes
The results of thermogravimetric analysis of mononuclear Cr(II) and Cu(II) complexes are collected in (Table 3). The thermogram of Cr(II) complex comprises four decomposition steps. The weight losses in the temperature range of 45–317 °C are attributed to the removal of one lattice H2O molecule, two coordinated H2O molecules and 2C6H8N2. In the range of 317–395 °C, the loss in weight corresponds to the loss of 2SO2 and 2C6H6. Another loss of 2C10H8 molecules occurs at temperatures ranging from 395 to 578 °C. At higher temperatures (578–736 °C), the found weight loss associated with this step may be attributed to the loss of the 2HCN molecules. The thermal decomposition of the Cu(II) complex has three main degradation steps. The Cu(II) complex exhibits thermal stability up to 400 °C, indicating that crystalline water molecules and coordinated water molecules do not exist in this complex. The first stage of decomposition occurs at the temperature range of 41–451 °C, which corresponds to the elimination of 2C6H8N2. The removal of 2C6H6, SO2 and HCN take place within the temperature range 451–702 °C followed by the loss of 2C11H7NO within the temperature range 702–860 °C. In Cr(II) and Cu(II) complexes, the remaining residues are metal oxides. These results are following the composition of the complexes. A representative TG/DTA diagram is given in (Fig. S4A, B).
Thermal analysis of nano-complexes
The current thermal analysis techniques aim to acquire more about the chemistry of the Cr and Cu nano-complexes generated in CS/EtOH media by measuring the size of the nano-complexes at each step of thermal heating to determine how the temperature influences the size of the nano-complexes. The particle sizes of Cr and Cu nano-complexes were calculated before and after heating at 200 °C (Tables 4 and 5). After heating at 200 °C, the thermograms of Cr and Cu nano-complexes revealed a reduction in the particle size at each of the thermal heating steps. The heat treatment reduces the accumulation of nano-complexes and thus reduces particles size. Cr and Cu nano-complexes are present in sub-nano structures (Fig. S4C, F).
Electronic spectroscopy
The H2L Schiff base ligand shows four absorption bands in DMF44. H2L Schiff base ligand displayed absorption bands at 386, 445, 468 and 539 nm, which may be assigned to π→π* transition of the benzene rings, n→π* of imine (C=N), n→π* of the phenolic group and charge-transfer (CT), respectively. The Cr(II) complex revealed absorption bands at 334, 404, 434, 485 and 540 nm that can be attributed to π→π* transition of the benzene rings, n→π* of imine (C=N), n→π* of the phenolic group, charge transfer CT (M→L) and 5Eg→5T2g(D) transition, respectively. These absorption bands resemble an octahedral geometry45. The spectrum of the Cu(II) complex exhibits three bands at 313, 364 and 412 nm, which can be attributed, respectively, to the π→π* transition of the benzene rings, n→π* of imine and phenolic groups. Moreover, a broad band at 543 nm has been recorded for the 2T2→2E(D) transition. These electronic bands resemble a tetrahedral geometry46,47. The electronic spectrum of green synthesized nano-complexes revealed a brand that is associated with the surface plasmon resonance (SPR) 48, confirming the reduction process and formation of nano-complexes. The Cr nano-complex exhibits bands at 329, 381, 439 and 523 nm transitions, which are connected to π→π* transitions of the benzene rings, n→π* of imine and phenolic groups and 5Eg→5T2g(D) transitions, respectively. This suggests that the Cr nano-complex is an octahedral structure. The three bands in the spectrum of the Cu nano-complex detected at 364, 405 and 536 nm are attributed to n→π* of imine and phenolic groups and 2T2→2E(D) transitions, respectively, and suggest a tetrahedral geometry (Fig. S5) & (Table 6).
EPR spectroscopy
The X-band EPR spectra of Cu(II) complex were recorded at room temperature (Fig. 7). The effective geff-value of the observed EPR spectrum of the prepared Cu(II) complex was determined and listed in (Table 7). The investigated Cu(II) complex is suggested to have tetrahedral geometry based on the shape of the observed EPR signals and geff-value. According to the results obtained, the g║ value for the Cu(II) complex is lower than 2.3, revealing that the metal–ligand bonds are covalent. The geometric parameter G factor defined as G=(g||–2.0023)/(g⊥–2.0023), which is 0.355, suggests that the exchange interaction is detected in the Cu(II) complex and the exchange interaction was operative between copper centers in the present Cu(II) complex49,50.
Molecular modeling
Geometry optimization of the H 2 L ligand
Figure 8 shows the molecular optimization of the H2L by using the B3LYP method and 6–311 + G(d,p) basis sets. The charges calculated from the natural-bond-orbital method (NBO) are: O1(− 0.693), O2(− 0.953), O3(− 0.924), N1(− 0.531), N2(− 0.528), N3(− 0.891), N4(− 0.427) and S(2.357). Metal ions coordinate to O3 and N4 atoms.
Geometry optimization of the metal complexes
Complexes were optimized using the Gaussian 09 program, using the DFT method comprising the B3LYP/6-311G + (dp) level of theory for all atoms except metal ions and at the B3LYP/LANL2DZ level of theory for metal ions. The computed theoretical infrared frequencies ensure the absence of imaginary frequencies.
Optimization of [CrL2(H2O)2].H2O
Figure 9A displays the molecular optimization of the complex [CrL2(H2O)2].H2O as the lowest-energy configuration. The chromium metal ion is six-coordinated in an octahedral geometry, with atoms O3, N4, O4, and N5 having a dihedral angle of 0.730° (Table 8). The distance between N4 and O1 in the ligand (3.715 Å) is reduced in the [CrL2(H2O)2].H2O complex to 2.786 and 2.779 Å for N4–O3 and N5–O4, respectively, as a result of complex formation. The charges calculated from the natural-bond-orbital method (NBO) on the coordinated atoms are Cr (+ 0.506), O3 (− 0.616), O4 (− 0.619), N4 (− 0.468), N5 (− 0.461), O7 (− 0.873) and O8 (− 0.872).
Optimization of [CuL2]
Figure 9B shows the molecular optimization of the complex [CuL2] as the lowest-energy configuration. The copper metal ion is four-coordinated in a tetrahedral geometry (Table 9). The distance between N4 and O1 in the ligand (3.715 Å) is reduced in the [CuL2] complex to 2.913 and 2.821 Å for N4–O3 and N5–O4, respectively, as a result of complex formation. The charges computed from the natural-bond-orbital method (NBO) on the coordinated atoms are Cu (+ 0.893), O3(− 0.635), N4(− 0.602), O4(− 0.630) and N5(− 0.575).
HOMO/LUMO energy evaluation
An important method for analyzing the chemical behavior of ligands and complexes is HOMO/LUMO energy evaluation (Fig. 10). The ability of the compound to acquire electrons is expressed by the LUMO energy, whereas its ability to donate electrons is expressed by the HOMO energy. The total energies of the Cr(II) and Cu(II) complexes are lower than those of the free ligand, indicating that they are more stable. In addition to the frontier molecular orbitals are used in estimation of other chemical parameters such as dipole moment, energy gap (Eg), ionization potential (I), electronegativity (χ), Chemical hardness (η), Chemical softness (S), electrophilicity (ω), electron affinity (A), chemical potential (μ) are tabulated in (Table 10). It was reported that a molecule with a high energy gap (Eg) value resulted in a hard molecule with low reactivity. CuL2 complex has a higher softness (S) value, suggesting a higher chemical reactivity.
Electrostatic potential map (MEP)
The electrostatic potential mapping is based on the total electron density surface, which displays electrostatic potential distribution, size, structural shape and dipole moments. Figure 11 displays the MEP for the investigated ligand and its complexes. Red, blue, yellow, and green color zones on the MEP surface represent, respectively, electron-rich, electron-poor, moderately electron-poor, and neutral zones. Positive potential zones are found over hydrogen atoms in the MEP, while negative potential regions are found over electronegative atoms (oxygen and nitrogen). The green region predominated in the MEP surfaces, corresponding to a potential halfway between the two extremes of red and dark blue color.
Antitumor activity
The ligand H2L and its Cr and Cu nano-complexes prepared in CS/EtOH media before and after heating the nanodomain complexes at 200 °C were measured against a cell line (HepG-2) to establish in vitro antitumor efficiency (Fig. 12A) and compared with the cis-platin anticancer drug (IC50 ~ 1.714 μg/ml). The antitumor and growth-inhibitory effects were investigated by using the IC50 approach, which estimates the concentration of a drug that reduces cell lineout growth by 50%. Compounds with IC50 values less than 5.00 μg/ml, within 5.00–10.00 μg/ml and between 10.00 and 25.00 μg/ml are considered strong, moderate, and mild antitumor activity, respectively51. The nano-sized Cr complex, after heating at 200 °C, exhibited strong antitumor activity with IC50 value (3.349 μg/ml). The nano-sized Cr(II) complex (before heating) and Cu(II) complex (before and after heating) displayed moderate antitumor activity, with IC50 values ranging from 5.73 to 7.49 μg/ml, whereas the H2L ligand showed weak antitumor activity, with IC50 value of 25.6 μg/ml (Fig. 12B). The results reveal that the nanodomain complexes generated in CS/EtOH media after heating at 200 °C have greater efficiency compared to the nano-complexes without heating. This may be due to the heating nano-complexes having a higher ability to bind DNA than others examined due to their small particle size, which can also be used extensively in economic anticancer studies by specialists.
DNA cleavage studies
DNA interacts with nano-domain complexes in many different ways, and these interactions significantly affect the structure and function of DNA52. The Cu nano-domain complex was investigated for its DNA cleavage activity using agarose gel electrophoresis, which was used with different and constant concentrations of the copper nano-domain complex with constant and different concentrations of DNA, respectively, to determine whether the nano-domain Cu complex prepared in CS/EtOH media has any impact on DNA. According to the results, the Cu nano-domain complex could cleave DNA at high concentrations (2 and 1 mg/ml) in the presence of a constant concentration of DNA and variable concentrations of the Cu nano-domain complex (Fig. 13 lane 3,4); Furthermore, when a fixed concentration of Cu nano-domain complex is present in the presence of varying amounts of DNA, the Cu nano-domain complex can cleave DNA at low concentrations of DNA (Fig. 13 lane 8]; under the experimental conditions and full length images (Figs. S6 and S7).
Gel electrophoresis diagram illustrating the cleavage of DNA by Cu nano-complex after incubation at 37 °C for 1 h. Lane L- marker 1 kb DNA Ladder, lane 1 DNA control, lane 2 DNA + DMSO, lane 3: 400 ng DNA + 2 mg/ml of nano-complex, lane 4: 400 ng DNA + 1 mg/ml of nano-complex, lane 5: 400 ng DNA + 0.5 mg/ml of nano-complex, lane 6: DNA control, lane 7: DNA + DMSO, lane 8: 200 ng DNA + 1 mg/ml of nano-complex, lane 9: 400 ng DNA + 1 mg/ml of nano-complex, lane 10: 800 ng DNA + 1 mg/ml of nano-complex.
Molecular docking simulation with liver cancer and COVID-19 protein receptors
The protein-drug affinities could be investigated through molecular docking interaction. The ligand H2L and its nano-scale Cu(II) and Cr(II) complexes were docked with protein targets in liver cancer, specifically the receptors of methionine adenosyl-transferases (PDB ID: 5A19) and COVID-19 main protease viral protein (PDB ID: 6lu7). The binding free energies of prepared compounds in case of (PDB ID: 5A19) are − 26.0, − 28.0, and − 39.0 kcal/mol while in case of (PDB ID: 6lu7) are − 8.0, − 23.3 and − 44.1 kcal/mol for H2L, CuL2 and [CrL2(H2O)2].H2O; respectively, (Tables 11 and 12). The stronger interaction reflects more negative binding energy. Hence, molecular interaction data show the highest binding affinity of the [CrL2(H2O)2].H2O towards both receptors relative to other compounds. The two- and three-dimensional plots of representative docked structures of H2L, [CrL2(H2O)2].H2O and CuL2 with the receptor’s active site of the liver cancer protein (PDB ID: 5A19) and COVID-19 main protease viral protein (PDB ID: 6lu7) were depicted in Figs. 14 and 15, respectively.
Conclusion
In this study, a novel ligand was synthesized from sulfadimidine sodium and 2-hydroxy-1-naphthaldehyde and coordinated with Cr(II) and Cu(II) to produce novel micro- and nano-complexes that were characterized by various physicochemical and spectral analyses. XRD and TEM results revealed that the nano-sized Cr and Cu complexes were found to be in the sub-nano scale, and temperature variations have a significant impact on the size of the nano complexes. The obtained results from the theoretical study are in good agreement with the experimental results. The antitumor studies on Cr and Cu nanocomplexes showed an inhibition of HEPG-2 cell line and the antitumor activity of the nanocomplexes was improved after heating them at 200 °C. Additionally, the results obtained indicate the Cu nanocomplex showed good DNA cleavage. Furthermore, a molecular docking study revealed that Cr nanocomplex has the highest activity with the receptor of (PDB ID: 5A19) and (PDB ID: 6lu7) in liver cancer and COVID-19, respectively.
Data availability
The data used to support the findings of this study are included in the article.
References
de Zayas-Blanco, F., Garica-Falcón, M. & Simal-Gándara, J. Determination of sulfamethazine in milk by solid phase extraction and liquid chromatographic separation with ultraviolet detection. Food Control 15, 375–378 (2004).
Yun, M.-K. et al. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science 335, 1110–1114 (2012).
Tawfik, A. M., El-Ghamry, M. A., Abu-El-Wafa, S. M. & Ahmed, N. M. A new bioactive Schiff base ligands derived from propylazo-N-pyrimidin-2-yl-benzenesulfonamides Mn(II) and Cu(II) complexes: Synthesis, thermal and spectroscopic characterization biological studies and 3D modeling structures. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 97, 1172–1180 (2012).
Mansour, A. M. & Ghani, N. T. A. Hydrogen-bond effect, spectroscopic and molecular structure investigation of sulfamethazine Schiff-base: Experimental and quantum chemical calculations. J. Mol. Struct. 1040, 226–237 (2013).
Mansour, A. M. Molecular structure and spectroscopic properties of novel manganese (II) complex with sulfamethazine drug. J. Mol. Struct. 1035, 114–123 (2013).
Mansour, A. Experimental and quantum chemical studies of sulfamethazine complexes with Ni(II) and Cu(II) ions. J. Coord. Chem. 66, 1118–1128 (2013).
Topacli, C. & Topacli, A. PM3 semi-empirical IR spectra simulations for metal complexes of schiff bases of sulfa drugs. J. Mol. Struct. 654, 131–137 (2003).
Mansour, A. M., Abdel-Ghani, N. T. & Ragab, M. S. DNA/bovine serum albumin binding and cytotoxicity of transition metal ternary complexes based on sulfamethazine and bromazepam drugs. Appl. Organomet. Chem. 34, e5995 (2020).
Gümrükçü, G., Karaoğlan, G. K., Erdoğmuş, A., Gül, A. & Avcıata, U. A novel phthalocyanine conjugated with four salicylideneimino complexes: Photophysics and fluorescence quenching studies. Dyes Pigm. 95, 280–289 (2012).
Ceramella, J. et al. A review on the antimicrobial activity of schiff bases: Data collection and recent studies. Antibiotics 11, 191 (2022).
Kuate, M. et al. Synthesis, characterization and antimicrobial studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of (E)-2-(4-dimethylbenzydimino)-glycylglycine,(glygly-DAB) a schiff base derived from 4-dimethylaminobenzaldehyde and glycylglycine. IJOC 8, 298 (2018).
Ragab, M. S., Soliman, M. H., Shehata, M. R., Shoukry, M. M. & Ragheb, M. A. Design, synthesis, spectral characterization, photo-cleavage and in vitro evaluation of anticancer activities of new transition metal complexes of piperazine based Schiff base-oxime ligand. Appl. Organomet. Chem. 36, e6802 (2022).
Elbadawy, H. A., Abd-El-Nabey, B. A., Ali, A. E. D. & Elsayed, E. H. The development of an unexpected Cu(I)-pyrazolo pyrimidine-based complex: Synthesis, spectroscopic characterizations, and biological activity. Appl. Organomet. Chem. 36(10), e6843 (2022).
Patil, S. A., Naik, V. H., Kulkarni, A. D. & Badami, P. S. DNA cleavage, antimicrobial, spectroscopic and fluorescence studies of Co(II), Ni(II) and Cu(II) complexes with SNO donor coumarin Schiff bases. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75, 347–354 (2010).
Abou-Melha, K. S. et al. Synthesis, characterization, DFT calculation, DNA binding and antimicrobial activities of metal complexes of dimedone arylhydrazone. J. Mol. Liq. 334, 116498 (2021).
Zare, N. & Zabardasti, A. A new nano-sized mononuclear Cu (II) complex with N, N-donor Schiff base ligands: sonochemical synthesis, characterization, molecular modeling and biological activity. Appl. Organomet. Chem. 33, e4687 (2019).
Al-Hakkani, M. F., Gouda, G. A., Hassan, S. H. & Nagiub, A. M. Echinacea purpurea mediated hematite nanoparticles (α-HNPs) biofabrication, characterization, physicochemical properties, and its in-vitro biocompatibility evaluation. Surf. Interfaces 24, 101113 (2021).
Madani, M. et al. Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes. Nanotechnol. Rev. 11, 731–759 (2022).
Al-Humaidi, J. Y. et al. 1, 2, 3-triazole-benzofused molecular conjugates as potential antiviral agents against SARS-CoV-2 virus variants. Life 12, 1341 (2022).
Albelwi, F. F. et al. Design, synthesis and molecular docking of novel acetophenone-1, 2, 3-triazoles containing compounds as potent enoyl-acyl carrier protein reductase (InhA) inhibitors. Pharmaceuticals 15, 799 (2022).
Hagar, M., Ahmed, H. A., Aljohani, G. & Alhaddad, O. A. Investigation of some antiviral N-heterocycles as COVID 19 drug: molecular docking and DFT calculations. Int. J. Mol. Sci. 21, 3922 (2020).
Alnoman, R. B., Parveen, S., Hagar, M., Ahmed, H. A. & Knight, J. G. A new chiral boron-dipyrromethene (BODIPY)-based fluorescent probe: Molecular docking, DFT, antibacterial and antioxidant approaches. Biomol. Struct. Dyn. 38, 5429–5442 (2020).
Al Sheikh Ali, A. et al. Design, synthesis, molecular modeling, anticancer studies, and density functional theory calculations of 4-(1, 2, 4-triazol-3-ylsulfanylmethyl)-1, 2, 3-triazole derivatives. ACS Omega 6, 301–316 (2020).
Mohammed, F. F. et al. 2-(alkylthio)-3-(naphthalen-1-yl) quinazolin-4 (3 H)-ones: Ultrasonic synthesis, DFT and molecular docking aspects. Polycycl. Aromat. Compd. 42, 4034–4048 (2022).
Alnoman, R. B., Hagar, M., Parveen, S., Ahmed, H. A. & Knight, J. G. Computational and molecular docking approaches of a New axially chiral BODIPY fluorescent dye. J. Photochem. Photobiol. A Chem. 395, 112508 (2020).
Parveen, S., Alnoman, R. B., Hagar, M., Ahmed, H. A. & Knight, J. G. Synthesis, molecular docking, and DFT calculation of a half-strapped BODIPY as potential EGFR inhibitor. ChemistrySelect 5, 13163–13173 (2020).
Alnoman, R. B., Parveen, S., Khan, A., Knight, J. G. & Hagar, M. New quinoline-based BODIPYs as EGFR/VEGFR-2 inhibitors: Molecular docking, DFT and in vitro cytotoxicity on HeLa cells. J. Mol. Struct. 1247, 131312 (2022).
Abdellattif, M. H. et al. Novel 2-hydroselenonicotinonitriles and selenopheno [2, 3-b] pyridines: Efficient synthesis, molecular docking-DFT modeling, and antimicrobial assessment. Front. Chem. 9, 252 (2021).
Hagar, M., Chaieb, K., Parveen, S., Ahmed, H. & Alnoman, R. N-alkyl 2-pyridone versus O-alkyl 2-pyridol: Ultrasonic synthesis, DFT, docking studies and their antimicrobial evaluation. J. Mol. Struct. 1199, 126926 (2020).
Parveen, S. et al. Synthesis, docking and density functional theory approaches on 1, 3-Bis-3-(4-Chlorophenyl)-2, 3-dihydroquinazolin-4 (1H)-on-2-thioxopropane toward the discovery of dual kinase inhibitor. Polycycl. Aromat. Compd. 42, 3736–3747 (2022).
El-ghamry, M. A., Nassir, K. M., Elzawawi, F. M., Aziz, A. A. A. & Abu-El-Wafa, S. M. Novel nanoparticle-size metal complexes derived from acyclovir. Spectroscopic characterization, thermal analysis, antitumor screening, and DNA cleavage, as well as 3D modeling, docking, and electrical conductivity studies. J. Mol. Struct. 1235, 130235 (2021).
Frisch, M. et al. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, 2013. Google Scholar There is no corresponding record for this reference. (b) Laurent, AD; Jacquemin, D. Int. J. Quantum Chem. 113, 2019–2039 (2013).
Ragab, M. S., Shehata, M. R., Shoukry, M. M., Haukka, M. & Ragheb, M. A. Oxidative DNA cleavage mediated by a new unexpected [Pd (BAPP)][PdCl 4] complex (BAPP= 1, 4-bis (3-aminopropyl) piperazine): crystal structure, DNA binding and cytotoxic behavior. RSC Adv. 12, 1871–1884 (2022).
Inc., C. C. G. (Chemical Computing Group Inc. Montreal, QC, Canada, 2016).
Murray, B. et al. Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes. PNAS 113, 2104–2109 (2016).
Jin, Z. et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582, 289–293 (2020).
Hosny, S., Gouda, G. A. & Abu-El-Wafa, S. M. Novel nano copper complexes of a new Schiff base: green synthesis, a new series of solid Cr(II), Co(II), Cu(II), Pd(II) and Cd(II) chelates, characterization, DFT, DNA, antitumor and molecular docking studies. Appl. Organomet. Chem. 36, e6627 (2022).
Tailor, S. M. & Patel, U. H. Synthesis, spectroscopic characterization, antimicrobial activity and crystal structure of silver and copper complexes of sulfamethazine. J. Coord. Chem. 68, 2192–2207 (2015).
Fazli, M., Akbarzadeh-T, N., Beitollahi, H., Dušek, M. & Eigner, V. New Schiff base ligand N-(2-hydroxy-1-naphthylidene)-2-methyl aniline and its nano-sized copper (II) complex: synthesis, characterization, crystal structure and application as an electrochemical sensor of 2-phenylphenol in the presence of 4-chlorophenol. J. Mater. Sci. Mater. Electron. 32, 25118–25136 (2021).
Shah, R. et al. Synthesis and structural elucidation for new Schiff base complexes; conductance, conformational, MOE-docking and biological studies. J. Inorg. Organomet. Polym. Mater. 30, 3595–3607 (2020).
Gaber, G. A., Hosny, S. & Mohamed, L. Z. Experimental and theoretical studies of 2-cyano-N-(4-morpholinobenzyldine) acetohydrazide as corrosion inhibitor for galvanized steel and 304 stainless steel in 1M H2SO4 solution. Int. J. Electrochem. Sci. 16, 2 (2021).
Al-Saidi, H. M. et al. Synthesis and characterization of Ni(II), Cu(II), Zn(II) and Azo dye based on 1, 10-o-phenanthroline binary complexes: Corrosion inhibition properties and computational studies. Int. J. Electrochem. Sci. 17, 2 (2022).
Morsy, M. I. et al. A novel LC-MS/MS method for determination of the potential antiviral candidate favipiravir for the emergency treatment of SARS-CoV-2 virus in human plasma: Application to a bioequivalence study in Egyptian human volunteers. J. Pharm. Biomed. Anal. 199, 114057. https://doi.org/10.1016/j.jpba.2021.114057 (2021).
Mansour, A. M. Selective coordination ability of sulfamethazine Schiff-base ligand towards copper (II): Molecular structures, spectral and SAR study. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 123, 257–266 (2014).
Yousif, M., Cabelof, A. C., Martin, P. D., Lord, R. L. & Groysman, S. Synthesis of a mononuclear, non-square-planar chromium (ii) bis (alkoxide) complex and its reactivity toward organic carbonyls and CO2. Dalton Trans. 45, 9794–9804 (2016).
Rehman, S.-U., Ikram, M., Rehman, S., Islam, N. U. & Jan, N. Synthesis and characterization of Ni(II), Cu(II) and Zn(II) tetrahedral transition metal complexes of modified hydrazine. J. Mex. Chem. Soc. 55, 164–167 (2011).
Shimizu, I. et al. Tetrahedral copper (II) complexes with a labile coordination site supported by a Tris-tetramethylguanidinato ligand. Inorg. Chem. 56, 9634–9645 (2017).
Ghosh, S. K. & Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem. Rev. 107, 4797–4862 (2007).
Onder, A., Turkyilmaz, M. & Baran, Y. Synthesis, spectroscopic, magnetic and thermal properties of copper (II), nickel (II) and iron (II) complexes with some tetradentate ligands: Solvatochromism of iron (II)–L2. Inorg. Chim. Acta 391, 28–35 (2012).
Ali, A. E., Elasala, G. S. & Ibrahim, R. S. Synthesis, characterization, spectral, thermal analysis and biological activity studies of metronidazole complexes. J. Mol. Struct. 1176, 673–684 (2019).
Shier, W. T. Mammalian cell culture on $5 a day: A laboratory manual of low cost methods. Los Banos Univ. Philipp. 64, 9–16 (1991).
Grazul, M. et al. Synthesis, physico-chemical properties and biological analysis of newly obtained copper (II) complexes with pyrazole derivatives. J. Inorg. Biochem. 135, 68–76 (2014).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.H. Conceptualization, Methodology, Sofware, Data curation, Writing – original draft, Visualization, Investigation, Validation, Writing – review & editing. M.S.R and R.F.A discussed part of the results and Writing – original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosny, S., Ragab, M.S. & Abd El-Baki, R.F. Synthesis of a new sulfadimidine Schiff base and their nano complexes as potential anti-COVID-19 and anti-cancer activity. Sci Rep 13, 1502 (2023). https://doi.org/10.1038/s41598-023-28402-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28402-9
This article is cited by
-
Synthesis, characterization, synergistic inhibition, and biological evaluation of novel Schiff base on 304 stainless steel in acid solution
Scientific Reports (2024)
-
Experimental and theoretical insight of Schiff base transition metal complexes: synthesis, characterization, antimicrobial and COVID-19 molecular docking studies
Research on Chemical Intermediates (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.