Abstract
In this study, we sought to establish the prevalence of leptospirosis among renal patients and general outpatients attending Mulago National Referral Hospital, Uganda. A total of 254 patients were recruited, their blood samples collected and interviewer-administered semi-structured questionnaires provided between July and October 2018. These questionnaires captured data on sociodemographic characteristics and symptoms of leptospirosis disease. An individual with an average body temperature of 37.3 ± 1.1 °C was considered to be having fever. The blood samples were analyzed using the standard Microscopic Agglutination Test (MAT) with a panel of 14 Leptospira-serovars belonging to 11 serogroups. Prevalence was reported with confidence intervals while questionnaire data was analyzed using logistic regression analysis. We present an overall prevalence of leptospirosis at 4.70% (95% CI = 2.60–8.30) after analysis of samples from recruited patients. This seropositivity (12/254) was classified into 7 serovars, among which, Canicola and Djasiman presented with titers between ≥ 200 and ≥ 400 in samples of both renal patients and outpatients, indicative of the active disease. Djasiman was the highest contributor to the reported prevalence. Overall, most examined participants presented with common symptoms of abdominal pain (AOR = 24.4, 95% CI (2.42–267.89), p = 0.02) and dehydration (AOR = 0.1, 95% CI (0.01–0.69), p = 0.05). Our study suggests that these symptoms and previous history of abdominal pain may be caused by Leptospira infections among the studied participants. We therefore recommend inclusion of leptospirosis in the differential diagnosis for renal and febrile illnesses. Indeed, abdominal pain and dehydration should be further studied with a bigger sample size and for other related febrile illnesses.
Similar content being viewed by others
Introduction
Leptospirosis is a zoonotic infection caused by bacteria of genus Leptospira1. It is considered one of the world’s public health challenges due to its greatest spread at global level2. Studies have indicated that domestic animals (dogs) as well as wild animals (rodents) are great reservoirs for this disease causing bacteria3,4. Infected animals are able to shed the Leptospira-spirochetes (being distinguished by the helical shape of the bacteria) through their urine into the environment where other hosts easily pick them1. These Leptospira-spirochetes in addition have the ability to withstand harsh environmental conditions such as pH and temperature5. This ultimately accounts for their wide geographical distribution and increased chances for new host infections worldwide. Indeed, estimates at global scale indicate 1.03 million cases and 58,900 deaths occur annually as revealed by Leptospirosis Epidemiology Reference Group (LERG)6. Additionally, various studies across Sub-Saharan Africa (SSA) reveal a (2.3–19.8)% prevalence of acute leptospirosis among febrile patients who attend healthcare facilities7,8,9.
In Uganda, studies that have been conducted among dogs and humans reveal a leptospirosis prevalence of 27% and 35% respectively, using Microscopic Agglutination (MAT) test10,11. Like it is across Africa, the human patients diagnosed with leptospirosis normally present with fever during their visits to the health facilities12. Despite this documented burden of leptospirosis, its febrile nature makes the disease to be misdiagnosed, under or un-diagnosed especially in resource constrained healthcare settings13. Indeed, late detection of this bacteria may lead to complications associated with progression of the infection into complicated stages of multi-organ involvement such as kidney and lung dysfunction14,15,16. It is important to note that health care facilities and hospitals tend to diagnose causes of febrile illness using blood culture techniques with media that does not support growth of Leptospira thus ending up missing leptospirosis diagnosis. For instance, majority of the samples from febrile patients submitted for bacterial blood culture usually turn out to be negative for any infectious organism according to the laboratory records and other published literature about Mulago Hospital17.
Adequate data on the prevalence, signs and symptoms associated with leptospirosis would facilitate the design of diagnostic algorithms for use in resource limited settings. This would be important in SSA countries like Uganda where healthcare systems are fragile to allow for early detection of such diseases. Therefore, our study sought to establish the prevalence of leptospirosis as well as ascertaining the signs and symptoms related to the infection among patients attending renal and general outpatient units in Mulago National Referral Hospital, Uganda.
Materials and methods
Study design
This cross-sectional study was conducted between July and October 2018 among renal and general outpatients within Mulago National Referral Hospital (MNRH). The study utilized a quantitative approach which involved blood sample collection from patients and administration of a semi-structured questionnaire to collect data on the patient factors, signs and symptoms.
Study site
Uganda’s only national referral hospital, MNRH has approximately 2072 health care professionals as of the (Human Resource for Health Audit Report 2017). The hospital receives referred patients from all districts of the country with official capacity of 1790 beds, although it often houses over 3000 patients.
The hospital has an established renal clinic running every Tuesday of the week. Patients with renal problems are required to present a proof of diagnosis from either the general outpatient of MNRH, or any other private or public healthcare facility. Patients in the general outpatient unit on the other hand normally walk-in with their referral letters from lower-level government or private healthcare facilities even to some extent patients arrive without any medical records (self-referral).
Study population
The study population comprised of both female and male participants with the age range of (5–87) years attending renal and general outpatient units in Mulago hospital Kampala (Uganda) during the study period. The patients in this study presented with febrile illness or renal dysfunction as sampled from the general outpatient and renal units respectively.
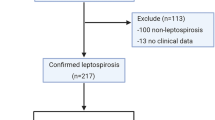
During the study period, the renal unit received a total of 180 patients and of these, only 119 agreed to participate in the study. Following the inclusion criteria, only 135 patients from the general outpatient unit qualified and consented to voluntarily participate in the study. Overall, sample size of 254 (119 and 135) patients were included in the study.
Data and sample collection procedures
Data collection
A structured and pre-tested questionnaire was administered to all the 254 participants recruited to the study. This data collection tool was used to obtain information about the patient, signs and symptoms among others (Details of the data collection tool refer to Supplementary I).
Our study also collected other patient’s information such as body temperature, physical examination and history of previous treatment as assessed by the clinician and filled special laboratory request form (Details of the laboratory request form refer to Supplementary II). Additionally, the urine color (dark-dehydration) and (light for hydration) suggested the hydration status of the patient. An individual with an average body temperature of 37.3 ± 1.1 °C was considered to be having fever.
Blood sample collection
Blood samples were collected from participants and allowed to stand for between 30 and 60 min to enable clotting. This was then centrifuged for 5 min at a speed of 3000 rpm centrifugal force to separate the serum. Serum was then aliquoted in to cryovials, packed in the cool box and transported to Central Diagnostic Laboratory (CDL) at the College of Veterinary Medicine Animal Resources and Biosecurity, Makerere University for analysis.
Laboratory procedures
During testing at CDL, the standard microscopic agglutination test (MAT) with a panel of 14 Leptospira serovars belonging to 11 serogroups was used. All serovars used were maintained in Ellinghausen–McCullough–Johnson–Harris (EMJH) liquid media and sub-cultured weekly to maintain the live serovars panel. Cultures which were 5 days old and showed some growth of Leptospira under dark-field microscopy were selected for use. These serogroups with their respective serovars included were: Autumnalis (L. interrogans serovar Autumnalis), Ballum (L. borgpetersenii serovar Kenya), Canicola (L. interrogans serovar Canicola), Tarassovi (L. borgapetersenii serovar Tarassovi), Hebdomadis (L. borgapetersenii serovar Nona), Pomona (L. interrogansserovar Pomona), Shermani (L. santarosai serovar Shermani), Djasiman (L. interrogans serovar Djasiman), Pyrogenes (L. borgapetersenii serovar Nigeria), Sejroe (L. borgapetersenii serovar Sejroe), Icterohaemorrhagiae (L. kirschneri serovar Sokoline; L. interrogans serovar Icterohaemorrhagiae; L. interrogans serovar Copenhageni), Grippotyphosa (L. kirschneri serovar Grippotyphosa).
Briefly, samples were retrieved from the refrigerator and they were run in two steps (screening and titration), first diluted in the ratio of 1:50 by adding 100 μl of test serum in 4.9 mls of s phosphate buffered saline (PBS) for screening. This preparation was screened in 96 well flat bottom microtitre plates with the panel of 14 live serovars. The first row of each plate was filled with controls serovars loaded in 50 μl of PBS and 50 μl of a live serovar making a final volume of 100 μl. The remaining rows were left for a single serum sample and each column for a single serovar. After loading of samples and test serovars, the plates were put into orbital plate shaker for 5 min for optimal mixing of sera and live serovars and then incubated for 2.5 h at 29 °C. The plates were examined by stereo microscope at high light intensity for agglutination18. Results were interpreted as positive and negative. Positive samples referred to those with a titre of ≥ 100.
Data analysis
Data from the questionnaire was first entered in the research laboratory registers and later transferred into EPI DATA software package 2.1. Final analysis was done using R version 3.5.2 (2018-12-20). Prevalence was presented as percentages with respective confidence intervals. Prevalence was presented for the different patient characteristics (Table 1) including the species, serogroups and serovars (Table 2). A bivariable logistic regression was run where one independent variable was compared with the leptospirosis status (positive or negative) (Table 3). p values less than 0.05 were considered significant at 95% confidence interval. In addition, variables that had p value < 0.2 and those with biological and scientific significance were included in our multivariate logistic regression model. Adjusted odds ratio (AOR) at 95% Confidence Interval (CI) were incorporated in Table 4.
Ethics consideration
This study was approved by Mulago National Referral Hospital Research and Ethics Committee (approval number-MHREC 1347). In addition, written informed consent was sought from all the study participants before being recruited into the study. In circumstances were the participants involved minors (below 18 years old), assent was obtained from them while written informed consent was sought through the parent or guardian that escorted them to the health facility. We also followed the Helsinki declaration guidance on ethical principles of involving human subjects in this study19.
Results
Prevalence of leptospirosis
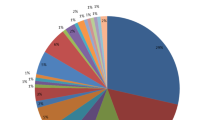
The overall prevalence of leptospirosis was 4.70% (CI = 2.60–8.30) among all the patients recruited in this study. The renal and general outpatient presented a prevalence of 2.70% and 2.00% respectively. In terms of gender, leptospirosis in males was (5.60%) and (3.90%) females. Leptospirosis was highly prevalent among patients whose occupations were professional (5.40%) and non-professional (4.30%). Also, leptospirosis was more prevalent (9.10%) among children below 10 years (Fig. 1). There was no detectable leptospirosis among patients who had been previously on antibiotics treatment (Table 1).
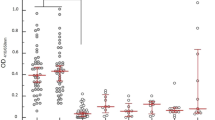
In terms of seropositivity, a total of twelve (12) cases from both renal and general outpatient units were positive for Leptospira with titres ≥ 100. Djasiman and Canicola were more prevalent in both patient categories with titers of ≥ 200 and ≥ 400 respectively (Fig. 2). In the renal unit, one (1) individual was positive for both Djasiman and Hebdomadis serovars. Similarly, in the general outpatient unit, another individual was positive for Icterhaemorhagic strain RGA and Canicola Leptospira serovars (Table 2).
Signs and symptoms associated with leptospirosis disease
Regarding signs and symptoms of patients, individuals who were dehydrated were less likely to be diagnosed with leptospirosis (AOR = 0.10, 95% CI (0.01–0.69), p = 0.05) (Table 4). Patients who reported with signs of abdominal pain were 24.4 times more likely to be diagnosed with leptospirosis (AOR = 24.40, 95% CI (2.42–267.89), p = 0.02) (Table 4).
Discussion
This study investigated prevalence and associated signs and symptoms among patients who attended renal and general outpatient units of Mulago National Referral Hospital. Our study is the first to document leptospirosis status among the renal patients in Uganda. The findings from this study, revealed an overall prevalence of 4.7%. This prevalence was similar to that of 3.8% registered in northern Tanzania among hospitalized febrile patients20. A study conducted in south western Uganda among non-pregnant adult females attending lower health facilities however reported contrary results with leptospirosis prevalence at 35%21. Lower health facilities tend to offer antibiotics to non-malarial febrile patients and any other category that might be un-diagnosed. Since the Leptospira bacteria has been shown to be highly susceptible to prevailing antibiotics22,23, the prevalence goes low at each level of treatment even though the effects of the disease can be long term and often culminate into referrals. Therefore, previous exposure to antibiotics by patients attending referral hospitals such as MNRH could probably explain the observed low prevalence in this study. Lower health facilities should therefore enhance leptospirosis diagnosis in order to prevent progression of the disease to complicated phases of organ involvement such as kidney dysfunction. In addition, non-malarial febrile patients should be referred to health facilities with capacity to detect Leptospira in order to minimize symptomatic treatment which often leads to poor treatment outcomes.
L. interrogans serovar Djasiman was the main circulating pathogenic Leptospira serovar detected among seropositive individuals in this study. This serovar has not been reported before among humans in any healthcare facility-based study within Uganda. This Leptospira serovar despite having been reported elsewhere24, its high burden reported in this study could probably be due to the non-inclusion of the serovar in the testing panel in earlier studies10,25 or could be due to global travels since zero prevalence has overtime been reported among26,27. The high frequency of serovar Djasiman in this study suggested that a risk of developing severe leptospirosis exists in Uganda24. Other studies have implicated L. interrogans serovar Djasiman for causing massive alveolar hemorrhage28. The low prevalence of Icterohemorrhagiae in our study is in agreement with previous studies conducted on cattle26,27 and humans10 in Uganda. We report a moderate prevalence for L. borgapetersenii serova Sejroe. However, studies in Uganda and elsewhere have reported a high burden and severity of this serovar12,29. L. borgapetersenii serova Sejroe is leptospira associated bovine infection and its circulation in the human population indicates its zoonotic nature. Therefore, there is dire need to pay keen attention to the servars circulating within the country with particular emphasis on the ones with zoonotic potential.
Disease signs and symptoms such as fever, abdominal pain, dehydration, headache among others presented by patients at point of care are relevant in supporting clinical diagnosis of that particular health condition. Our study reveals that patients who reported with signs of abdominal pain were 24.4 times more likely to be diagnosed with leptospirosis. Abdomen pain among individuals diagnosed with leptospirosis was reported in a hospital based case study in Hawaii USA30. Patients attending the renal and general outpatient units with signs of dehydration were less likely to be diagnosed with leptospirosis. However other studies report contrary results due to the fact that leptospirosis infection is associated with fever and vomiting which are known to cause dehydration. Therefore, the observed dehydration among our study participants could probably be due to other disease conditions such as malaria, diarrhea among others.
Conclusion
The prevalence of leptospirosis with previously unknown serovars among patients in Mulago National Referral Hospital of Uganda, indicates the burden and dynamics of the disease remains unknown in the country. This study suggests the need for a large-scale prospective cohort study in hospitals but more so the lower health facilities. The policy makers should advocate for inclusion of leptospirosis in the deferential diagnosis of febrile illnesses especially at lower level and private health facilities.
References
Adler, B. & de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. https://doi.org/10.1016/j.vetmic.2009.03.012 (2010).
Ricardo, T., Bergero, L. C., Bulgarella, E. P. & Previtali, M. A. Knowledge, attitudes and practices (KAP) regarding leptospirosis among residents of riverside settlements of Santa Fe, Argentina. PLoS Negl. Trop. Dis. https://doi.org/10.1371/journal.pntd.0006470 (2018).
Fornazari, F., Langoni, H., Marson, P. M., Nóbrega, D. B. & Teixeira, C. R. Leptospira reservoirs among wildlife in Brazil: Beyond rodents. Acta Trop. 178, 205–212 (2018).
Samir, A., Soliman, R., El-Hariri, M., Abdel-Moein, K. & Hatem, M. E. Leptospirosis in animals and human contacts in Egypt: Broad range surveillance. Rev. Soc. Bras. Med. Trop. 48, 272–277 (2015).
Vijayachari, P., Sugunan, A. P. & Shriram, A. N. Leptospirosis: An emerging global public health problem. J. Biosci. https://doi.org/10.1007/s12038-008-0074-z (2008).
Costa, F. et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. https://doi.org/10.1371/journal.pntd.0003898 (2015).
de Vries, S. G. et al. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 28, 47–64 (2014).
Allan, K. J. et al. Epidemiology of leptospirosis in Africa: A systematic review of a neglected zoonosis and a paradigm for ‘One Health’ in Africa. PLoS Negl. Trop. Dis. 9, 1–25 (2015).
Biggs, H. M. et al. Estimating leptospirosis incidence using hospital-based surveillance and a population-based health care utilization survey in Tanzania. PLoS Negl. Trop. Dis. 7, e2589 (2013).
Dreyfus, A. et al. Leptospira seroprevalence and risk factors in health centre patients in Hoima district, Western Uganda. PLoS Negl. Trop. Dis. 10, e0004858 (2016).
Millán, J. et al. Serosurvey of dogs for human, livestock, and wildlife pathogens, Uganda. Emerg. Infect. Dis. 19, 680 (2013).
Maze, M. J. et al. Risk factors for human acute leptospirosis in northern Tanzania. PLoS Negl. Trop. Dis. 12, e0006372 (2018).
Croda, J. et al. Leptospirosis pulmonary haemorrhage syndrome is associated with linear deposition of immunoglobulin and complement on the alveolar surface. Clin. Microbiol. Infect. https://doi.org/10.1111/j.1469-0691.2009.02916.x (2010).
Jayathilaka, P. G. N. S. et al. An outbreak of leptospirosis with predominant cardiac involvement: A case series. BMC Infect. Dis. 19, 1–8 (2019).
Yang, C. W., Wu, M. S. & Pan, M. J. Leptospirosis renal disease. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/16.suppl_5.73 (2001).
Araujo, E. R. et al. Acute kidney injury in human leptospirosis: An immunohistochemical study with pathophysiological correlation. Virchows Arch. https://doi.org/10.1007/s00428-010-0894-8 (2010).
Lubwama, M. et al. Bacteremia in febrile cancer patients in Uganda. BMC Res. Notes 12, 1–6 (2019).
Chirathaworn, C., Inwattana, R., Poovorawan, Y. & Suwancharoen, D. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian Pac. J. Trop. Biomed. 4, S162 (2014).
WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects—WMA—The World Medical Association. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
Biggs, H. M. et al. Leptospirosis among hospitalized febrile patients in Northern Tanzania. Am. J. Trop. Med. Hyg. 85, 275 (2011).
Dreyfus, A. et al. Leptstern ugandaospira seroprevalence and risk factors in health centre patients in Hoima district, We. PLoS Negl. Trop. Dis. https://doi.org/10.1371/journal.pntd.0004858 (2016).
Liegeon, G., Delory, T. & Picardeau, M. Antibiotic susceptibilities of livestock isolates of Leptospira. Int. J. Antimicrob. Agents 51, 693–699 (2018).
Chakraborty, A., Miyahara, S., Villanueva, S. Y. A. M., Gloriani, N. G. & Yoshida, S. I. In vitro sensitivity and resistance of 46 Leptospira strains isolated from rats in the Philippines to 14 antimicrobial agents. Antimicrob. Agents Chemother. 54, 5403–5405 (2010).
Suut, L. et al. Serological prevalence of leptospirosis among rural communities in the Rejang Basin, Sarawak, Malaysia. Asia-Pacific J. Public Health 28, 450–457 (2016).
Jayasundara, D. et al. Optimizing the microscopic agglutination test (Mat) panel for the diagnosis of leptospirosis in a low resource, hyper-endemic setting with varied microgeographic variation in reactivity. PLoS Negl. Trop. Dis. 15, 1–15 (2021).
Alinaitwe, L., Kankya, C., Namanya, D., Pithua, P. & Dreyfus, A. Leptospira seroprevalence among Ugandan slaughter cattle: Comparison of sero-status with renal Leptospira infection. Front. Vet. Sci. 7, 106 (2020).
Dreyfus, A. et al. Cross-sectional serological survey for Leptospira spp. in beef and dairy cattle in two districts in Uganda. Int. J. Environ. Res. Public Health 14, 1421 (2017).
Héry, G. et al. Massive intra-alveolar hemorrhage caused by Leptospira serovar djasiman in a traveler returning from Laos. J. Travel Med. 22, 212–214 (2015).
Søndergaard, M. M., Tursunovic, A., Thye-Rønn, P., Bang, J. C. & Hansen, I. M. J. Leptospirosis-associated severe pulmonary hemorrhagic syndrome with lower back pain as an initial symptom. Am. J. Case Rep. 17, 883 (2016).
Maier, A., Kaeser, R., Thimme, R. & Boettler, T. Acute pancreatitis and vasoplegic shock associated with leptospirosis—A case report and review of the literature. BMC Infect. Dis. 19, 1–5 (2019).
Author information
Authors and Affiliations
Contributions
R.W. conducted research and wrote up the initial draft of the manuscript. W.W. and L.M. gave technical advise for the study and supported in revision of the manuscript. J.M. supported data analysis and the flow of the study. S.A. supported drafting of the manuscript and its submission to the journal. L.M. provided laboratory requirements for sample collection and analysis in this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wambi, R., Worodria, W., Muleme, J. et al. Prevalence of leptospirosis among patients attending renal and general outpatient clinics in Mulago Hospital, Kampala, Uganda. Sci Rep 12, 8391 (2022). https://doi.org/10.1038/s41598-022-12544-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12544-3
This article is cited by
-
Leptospirosis in humans and selected animals in Sub-Saharan Africa, 2014–2022: a systematic review and meta-analysis
BMC Infectious Diseases (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.