Abstract
One of the most studied aspects of animal communication is the acoustic repertoire difference between populations of the same species. While numerous studies have investigated the variability of bottlenose dolphin whistles between populations, very few studies have focused on the signature whistles alone and the factors underlying differentiation of signature whistles are still poorly understood. Here we describe the signature whistles produced by six distinct geographical units of the common bottlenose dolphin (Tursiops truncatus) in the Mediterranean Sea and identify the main determinants of their variability. Particularly, the influence of the region (proxy of genetic distance), the geographic site, and the environmental (sea bottom-related) and demographical (population-related) conditions on the acoustic structure of signature whistles was evaluated. The study provides the first evidence that the genetic structure, which distinguishes the eastern and western Mediterranean bottlenose dolphin populations has no strong influence on the acoustic structure of their signature whistles, and that the geographical isolation between populations only partially affected whistle variability. The environmental conditions of the areas where the whistles developed and the demographic characteristics of the belonging populations strongly influenced signature whistles, in accordance with the “acoustic adaptation hypothesis” and the theory of signature whistle determination mediated by learning.
Similar content being viewed by others
Introduction
Acoustic signals are widely used in animals, from insects to mammals, and can be involved in a variety of contexts such as sexual selection, social cohesion, individual and group recognition, resource defence and competition1. Cetaceans rely on acoustic communication for orientation, locating food, reproduction and avoiding predators2. Consequently, they have a varied and complex acoustic repertoire, used to transmit information related to the sender identity (individual, social group, population, species), sender position and behavioural contexts3. Some of the most studied cetacean species produce individual signals aimed to identify the emitter, as in Tursiops spp.4,5, or have acoustic repertoires able to identify family group or population, as in killer whales (Orcinus orca) and sperm whales (Physeter macrocephalus)6,7,8.
One of the most studied aspects of animal communication is the acoustic difference between closely related species or between populations of the same species1,9. Several processes have been proposed as determinants in the acoustic difference among populations of the same species: (i) the acoustic traits of the environment where the populations live mediate the acoustic signals’ evolution due to different sound propagation and ambient noise (the “acoustic adaptation hypothesis”10; (ii) social and cultural selection, especially when vocal learning is involved in the development of acoustic behaviour1; (iii) genetic causes, which may be further related to the geographic isolation between populations11,12.
The bottlenose dolphin, Tursiops spp., lives in fission–fusion societies13 where individual recognition, contact maintenance, and group coordination are mediated by frequency-modulated, narrow-band acoustic signals, called “whistles”14,15. Those whistles characterized by a stereotyped frequency modulation pattern (or contour) and used to identify the emitter are known as “signature whistles”14. They are primarily produced when an animal is separated by the conspecifics4,5,23,65 and to ensure social cohesion14. In fact, in captivity signature whistles are mainly produced when animals are isolated from the rest of the group5,23,26, while in wild dolphins, signature whistles account for 38–70% of the whole whistles’ repertoire16,17,18.
The signature whistle develops during the first year of a dolphin’s life and seems to be modelled hearing whistles from conspecifics19,20,21,22 and through vocal production learning14,23. Data from both captivity and the wild have partially excluded a strict genetic determination of signature whistle structure19,22,24,28.
The contour remains stable for decades24, even if a few situations are known to be responsible for its changes: (i) males can change their whistles contour in the attempt to resemble those of their alliance partners25; (ii) subtle changes (such as in the start frequency or bandwidth) may occur as consequence of motivational states. Thus, in addition to the information of emitter’s identity, the signature whistle may transmit context-related information26.
Since signature whistles are likely developed through vocal production learning14, as a young animal learns its vocalizations from its neighbours, a geographic variation between the signature whistles of different populations can be assumed. Attempts to characterise the signature whistle repertoire of bottlenose dolphin sub-populations have been done only in a few areas, such as Florida17,27, Scotland28,29, Namibia30, and Portugal31. In recent years the number of this kind of studies has increased thanks to development and application of the SIGnature IDentification method (SIGID51), based on the temporal patterning and stereotyped structure of the signature whistles14,29,30,68. While numerous studies have investigated the variability of the bottlenose dolphin whistles between populations32,33,34,35,36, with no distinction between signature and non-signature whistles, very few studies have focused on the signature whistles variability alone30. Thus, the factors underlying differentiation of signature whistles are still poorly understood and the investigation of signature whistles variability between populations may clarify the development process and evolution of this call type. The aim of this study was to describe the signature whistles produced by distinct geographical units of common bottlenose dolphin (Tursiops truncatus) in different Mediterranean sites and identify the determinants of their variability. Variability among populations may arise due to their relative geographic isolation and/or genetic distance. A genetic differentiation between the western and the eastern Mediterranean bottlenose dolphin populations exists37 and could influence signature whistles. Furthermore, when acoustic signal development is mediated by vocal learning, differences in social structure, population size, and connection between adjacent populations may affect acoustic variability. In the end, the environmental conditions related to water depth, substrate type and habitat, may influence acoustic signals through their effect on sound transmission38. Then, to disentangle the potential causes of variability and identify the main determinants, the acoustic structure of signature whistles (in terms of fundamental frequencies, frequency modulation and duration) was investigated and the influence of the Mediterranean region (as a proxy of genetic distance), the geographic site, and the environmental (sea bottom-related) and demographical (population-related) conditions were evaluated.
Results
A total of 188 h of recordings were collected, from which we overall extracted 2036 good quality signature whistle contours (SWs). Particularly, 101 SWs from Port Cros (PC) (corresponding to 11 SW-IDs), 166 from Ostia-Fiumicino (FI) (corresponding to 17 SW-IDs), 925 from Alghero (AL) (corresponding to 58 SW-IDs), 406 from Lampedusa (LA) (corresponding to 37 SW-IDs), 83 from Gulf of Corinth (GC) (corresponding to 12 SW-IDs), and 346 from Cres-Lošinj (CL) (corresponding to 33 SW-IDs) (see Supplementary Table S1 and Fig. S1 online).
Similarity between SWs
SW structure was affected by all the factors considered as indicated by the nMDS (stress = 0.15; Fig. 1). This is confirmed by one-way non-parametric similarity analyses (Anosim) that identified a significant effect of region (p = 0.004, R = 0.081), site (p = 0.001, R = 0.14), sea bottom (p = 0.001, R = 0.15) and population demography (p = 0.032, R = 0.057).
Collinearity among SW variables
The seven SW acoustic characteristics were highly collinear, thus a PCA was applied. The first principal component (PC1) was negatively correlated with max and end frequencies (and explained 36% of the variance); the second principal component (PC2) was positively correlated with min and start frequencies (and explained 26% of the variance); the third principal component (PC3) was positively correlated with duration and number of inflection points and negatively correlated with frequency range (and explained 21% of the variance) (Fig. 2).
Influence of region, site, sea bottom and population demography
We used GLMMs on PC1, PC2 and PC3 to investigate the influence of region, site, sea bottom and population demography with separated models.
Only PC2 and PC3 were significantly associated with region (Table 1). PC2 and PC3 were both higher in the SOUTH compared to the EAST and WEST (Fig. 3). Particularly, min and start frequencies were higher in the SOUTH compared to WEST and EAST, while frequency range was lower. Duration and number of inflection points did not change (Table 1, Fig. 3).
Only PC2 and PC3 were significantly associated with site, while no differences were found on PC1 (Table 2, Fig. 4). PC2 (min and start frequencies) were higher in PC and LA and lower in GC. PC3 was higher in LA, thus the negatively correlated frequency range was lower, while duration and number of inflection points did not change (Table 2, Fig. 4).
The cluster analysis grouped the sea bottom variables (habitat type and depth range) into three clusters: (i) H1 grouped together the sites (PC and LA) characterized by Posidonia bed, infralittoral sand and circalittoral coarse sediment bottoms not exceeding 150 m of depth; (ii) H2 grouped together the sites (AL, CL and FI) characterized by circalittoral coastal terrigenous muds and coarse sediment and muddy detritic bottoms not exceeding 100 m of depth; (iii) H3 corresponded with GC, characterized by open-sea detritic bottoms on shelf-edge, offshore circalittoral coastal terrigenous muds and bathyal mud, with a depth up to 500 m. Only PC2 and PC3 were significantly associated with sea bottom (Table 3). PC2 (min and start frequencies), were higher in H1 and lower in H3 (Fig. 5). PC3 was lower in H2 compared to H1 and H3 (Fig. 5). Particularly, frequency range was higher in H2 than in H1 and H3, while the number of inflection points was higher in H3.
Based on population demography, the cluster analysis grouped the six sites into four clusters: (i) P1 corresponded to LA, a quite large open population, with small group size and mostly transient individuals; (ii) P2 included AL and GC, the smallest sized populations with medium group size and mainly resident individuals; (iii) P3 corresponded to CL, an intermediate sized and isolated population, with large group size and mostly resident individuals; (iv) P4 included FI and PC, large sized open populations, with large group size and mostly transient individuals. All PCs were significantly associated with population demography (Table 4, Fig. 6). PC1 was higher in P1 respect to the others, thus max and end frequencies were both lower. PC2, thus min and start frequencies, were higher in P1. PC3 was higher in P1 and lower in P3. Particularly, frequency range and duration were lower in P1 while the number of inflection points was higher in P2 (Table 4, Fig. 6).
Discussion
SWs from the six studied populations were homogeneous within but distinct between sites, and the dissimilarity was most evident between the whistles from LA and those from the other sites. This pattern is also evident when the similarity is grouped by region: SWs from the south Mediterranean appeared more dissimilar than those from the west and the east, which instead overlapped. If the SWs’ dissimilarity was mainly related to the geographic isolation between individuals from the six sites and/or the genetic distance between eastern and western populations of the basin, a greater difference in PCs would have been observed. Instead, the largest difference concerns the SWs coming from LA in the South, with the only exception of PC2, and the related min and start frequencies which were lower in CL and GC in the East compared to PC and AL in the West. The highest difference found in the SWs from LA is consistent with the closest acoustic structure between whistles from LA and those from the Atlantic Ocean, compared to those from the western Mediterranean populations, found in a previous study35. Given the geographical position in the middle of the Strait of Sicily, a most frequent or recent contact between individuals from LA with those of the neighbouring populations that inhabit the waters of the Atlantic35 could explain the difference in SWs between the South and the other regions of the Mediterranean Sea. The case in which SWs dissimilarity was not related to a greater genetic distance between populations was already described by Gridley28, who found a similar pattern in the SWs from different African Tursiops spp. populations. Although it is known that the development of SWs does not have a strict genetic determination, but rather a determination mediated by learning19,24, the definitive understanding of how the genetic distance and the isolation between populations affect the acoustic variability of SWs is still unknown. However, our study provides the first evidence that the genetic structure which distinguishes the eastern and western Mediterranean bottlenose dolphin populations has no strong influence on the acoustic structure of their SWs. Furthermore, even the geographical isolation between populations of the investigated sites only partially influenced the SWs variability. Stronger evidence was obtained by associating the SWs to the type of sea bottom in which they developed and the demographic characteristics of the belonging populations.
The highest dissimilarity was found between SWs from H1 and H3. In the environment characterized by the presence of Posidonia bed (H1, corresponding to LA and PC), the SWs had the highest start and min frequencies and were shorter and less modulated compared to the SWs from H2 and H3. Since signature whistles are cohesion call, which are used to maintain group cohesion and facilitate mother-calf reunion, lower frequency and less modulated whistles could be preferred since they transmit further in the marine environment57. However, Quintana-Rizzo and Mann58 found that min frequency whistle attenuated up to seven times more in seagrass areas than in areas with other bottom type (mud or sandy-mud). Coherently, in H3 (GC), characterized prevalently by muddy and detritic bottom, min and start frequency were the lowest recorded and duration and number of inflection points were the highest. Few studies have been conducted to understand the role of depth in dolphin whistles, however, Buckstaff16 found lower minimum frequencies in deeper habitat and Gridley28 found longer whistles related to higher depth. These findings are coherent with the SWs characteristics of GC. Unfortunately, no other conclusion can be derived for the other sites, since they all have similar depth condition, thus this aspect needs further investigation.
The strongest influence on the variability of SWs was related to the population demography. The SWs of P1 (LA) and P4 (FI and PC) were the most dissimilar. These populations have the largest size and are composed of mostly transient individuals. A high number of sounds from conspecifics in large open populations can lead to the development of widely distinctive SWs to enhance recognition57. In P1, SWs had quite distinct acoustic characteristics: the lowest max and end frequencies, frequency range, duration, and number of inflection points. These characteristics are likely influenced by the combined effect of several factors (region and bottom type), rather than by the type of population alone, and this makes the interpretation of the results more complex.
In P2 (corresponding to AL and GC, the smallest sized populations), SWs had the highest number of inflection points. Further, GC had the highest variability in duration. In small populations, where the probability to meet the same individuals is high, different SWs duration and higher number of inflection points can enhance identity coding59,60, even if this pattern was found elsewhere, but in a larger population28.
Some methodological limitations need to be considered when interpreting the results of this study. Firstly, the sample size used for the analysis should be taken into consideration, since it may be not fully representative of the variability of SWs, especially in some sites. In fact, even if the 13 SW-IDs recorded in GC can be considered sufficiently representative of the SW repertoire of this population (composed of 38 individuals on average), the SW-IDs collected in PC and FI correspond to less than 5% of individuals in these large-sized populations.
Further, a limited number of the potential factors associated with the acoustic environment and whistle variability were considered in this study. For example, data on ambient noise and vessel traffic were not available for all sites, thus these factors could not be included. High noise levels caused by vessels can have a strong influence on whistle structure36,61,62 due to the need of making the signal more efficient in terms of transmission in noisy environments and to contrasting masking phenomena. However, a recent study36 compared the effect of noise on the whistles (both signature and variant) of two populations considered in the present study (AL and CL), showing different acoustic response to the increase of Sound Pressure Levels (in the 125, 500 and 1000 Hz octave bands) and boat presence between the two sites. This finding suggests that ambient noise levels alone are not sufficient to explain the variation in whistle acoustic structure. In fact, all the factors influencing local sound propagation together with the characteristics of boat traffic (in terms of quantity, type and size of boats) and the interaction of these factors with the behavior of dolphin groups and physiology of individuals should be considered. Here, only general, broad-scale environmental characteristics were used. Sound transmission in shallow water is highly variable and depends on bottom sediments, depth and slope38, but also on tidal events, temperature gradients, freshwater inputs, obstacles in the sound path58 and the interactive effect between the sediment and plants (such as seagrass meadows) and/or animals (like benthic in-fauna) that live on the bottom63. In the end, SW convergence between alliance members can reduce diversity in whistle types25,64. In the present study no data were available about the sex of the SW emitters neither on the association patterns between individuals. Thus, a most accurate description of the acoustic environment, the identity of the SW emitters and the social relationship between them should deserve attention in future studies to further understand their effect on the development of SWs.
Methods
Study areas
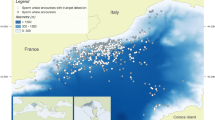
Based on physiographic, oceanographic, and biogeographic conditions, the Mediterranean Sea is divided into west and east basins, connected by the Strait of Sicily. To characterise the SWs of Mediterranean bottlenose dolphins we analysed acoustic data recorded in six different sites: Port Cros (French Riviera), Alghero (Sardinian Sea), and Ostia-Fiumicino (Tyrrhenian Sea), for the western basin, Cres-Lošinj (Adriatic Sea) and Gulf of Corinth (Ionian Sea), for the eastern basin, and Lampedusa (Strait of Sicily) in the southern Mediterranean Sea (Fig. 7).
Map of the six study sites in the Mediterranean Sea. PC (Port Cros); Al (Alghero); FI (Ostia-Fiumicino); LA (Lampedusa); GC (Gulf of Corinth); CL (Cres and Losjni). The original map was downloaded from the free source https://d-maps.com/ and modified by Preview in Mac Os.
Port Cros (PC)
The acoustic recordings were collected in an area of approximately 13 km2 in the French Riviera, characterized by three main habitat types: Posidonia bed, circalittoral coarse sediment and infralittoral sand, with a bottom depth not exceeding 150 m. The bottlenose dolphin population inhabiting these waters has an estimated size of 223 individuals (95% CI = 152–385)66,67. The population is connected to adjacent populations of the Gulf of Lion and is mainly composed of transient individuals66,67. The mean group size is 16 individuals, with a range between 1 and 5566,67 (Table 5).
Alghero (AL)
The acoustic recordings were collected in an area of about 450 km2 that includes Posidonia bed, circalittoral coarse sediment, muddy detritic bottoms, with a bottom depth up to 115 m. The bottlenose dolphin population inhabiting these waters has an estimated size of 76 individuals (95% CI = 61–118)39. Among the 122 photo-identified dolphins, at least 50% of them show a high level of site fidelity40 and they were sighted repeatedly every year and in different seasons. Nevertheless, the population seems neither closed nor isolated40,41. The mean size of the recorded groups is 7 individuals, with a range between 1 and 17 (Table 5).
Ostia-Fiumicino (FI)
The acoustic recordings were collected in an area of approximately 1300 km2 of the Tyrrhenian Sea, characterized by three main habitat types: circalittoral coastal terrigenous muds, muddy detritic bottoms and circalittoral coarse sediment, with a bottom depth up to 100 m42. Here, three distinct groups were distinguished (resident, part-time, and transient), based on progressively lower degrees of site fidelity43. The part time and transient individuals accounts for most of the photo-identified individuals (78%). The estimated population size ranged between 77 (95% CI = 65–91) for the resident dolphins to 354 (95% CI = 288–312) for the transient ones, leading to a total of 529 individuals (95% CI = 456–614) for the whole population43. The mean group size is 15 individuals, with a range between 1 and 6543 (Table 5).
Lampedusa Island (LA)
The acoustic recordings were collected in a 48 km2 area around Lampedusa Island located on the northern African continental shelf of the Strait of Sicily. The area includes Posidonia bed, infralittoral sand and circalittoral coarse sediment, with a bottom depth not exceeding 110 m. The estimated bottlenose dolphin population is 249 individuals (CI = 162–449)44, but likely these dolphins are part of a larger population44. The mean group size is 4, with a range between 1 and 2045 (Table 5).
Gulf of Corinth (GC)
The acoustic recordings were collected along the northern coast of the Gulf where waters are characterized by open-sea detritic bottoms on shelf-edge, offshore circalittoral coastal terrigenous muds and bathyal mud, and bottom depth of 500 m (although the maximum depth considering the entire Gulf is 900 m). Here, a population of 38 individuals (95% CI = 32–46) was estimated46. Most individuals show high resighting frequency, but some dolphins are known to move in and out from the Gulf47,48. The mean group size is 8, with a range between 1 and 2848 (Table 5).
Cres and Lošinj (CL)
The acoustic recordings were collected in an area of about 2000 km2 in the north-eastern Adriatic Sea. These waters are characterized by numerous uninhabited small islands and islets, infralittoral mud, circalittoral coastal terrigenous muds and circalittoral coarse sediment, with an average bottom depth of 70 m. Here, the population size was estimated to 184 individuals (95% CI = 152–250)49. The high sighting frequency of known individuals indicate their long-term fidelity to the region50. The mean size of the recorded groups is 22, with a range between 2 and 4636 (Table 5).
Acoustic data collection
Acoustic recordings were collected with different methods and equipment, in different years and by different research groups (see Table 6). When the recordings were obtained by means of PAM (Passive Acoustic Monitoring) devices deployed on the sea bottom, as in LA, species identification was not visually confirmed. Nevertheless, the depths (< 40 m) and distances from the coast (< 1.5 km) were chosen to ensure that only bottlenose dolphins were recorded even in the absence of visual identification35. Moreover, no other dolphin species are present in the area. The recordings were collected with different sampling rates (from 44 to 192 kHz). However, since the recordings collected with the lowest sampling rate (44 kHz) did not contain whistles with frequencies higher than the Nyquist frequency (22 kHz), the different sampling rate did not affect the results.
This work is based on the observation of dolphins and does not foresee any direct experiment on them. All procedures performed in the study were in accordance with the ethical standards of the involved institutions.
Acoustic data analysis
Signature whistles were defined as “a learned, individually distinctive whistle type in a dolphin's repertoire that broadcasts the identity of the whistle owner”14. Thus, signature whistles of a same individual are characterized by the same frequency modulation pattern (called contour—SW). The SWs can be produced in loops (repetitions of the same elements), usually separated by intervals less than 250 ms27, and can also have an introductory and/or final loop14 distinct from the central pattern. We considered any single or multiple-loop whistle, connected or disconnected, as the unit of analysis27. To classify a whistle as a SW, the SIGID method14,51 was applied, following a step-by-step procedure. First, each whistle was graded depending on the quality and the signal-to-noise ratio (SNR) as follows: (i) whistle fairly audible and with contour not clearly discernible; (ii) whistle audible and clearly visible from the beginning to the end of the contour; (iii) whistle predominant. Weak whistles, whistles overlapping with other sounds, whistles with no good definition of the contour and with no clear start and end points (graded as 1) were discarded from the sample35,36,52. Following Kriesell et al.30, the whistles present in any recording session were distinguished as repeated element whistle type (REWT—those whistles with the same contour that are present at least twice within the range of 0.25–10 s during a recording section), and other whistle (OW—variant whistles that did not respect the previous classification rule). A catalogue containing all the REWTs was constructed, assigning to each a unique identification code and a minimum of two good images of the relative contours. Then, the recordings were inspected a second time and whistles were compared with those in the catalogue. A whistle was classified as a SW if a minimum of four stereotyped contours were present in a recorded session and 75% of them occurred within 1–10 s of at least one other51. When a SW was identified, a distinctive individual code was assigned (SW-ID) and any whistle with the same contour was assigned to the same SW-ID (Fig. 8).
For each SW, minimum (min) frequency, maximum (max) frequency, start frequency, end frequency, frequency range, number of inflection points and duration (as defined in La Manna et al.36) were measured by visual inspection of the spectrogram [512/1024-point fast Fourier transform (FFT), frequency resolution of 135 Hz, Hann window, 50% overlap] under Raven 1.5 software (Cornell Laboratory of Ornithology, Ithaca, NY, USA).
Region, geographic site, sea bottom and population demography
Four types of potential factors influencing SW structure were considered: region, geographic site, sea bottom type and dolphin population demography.
From a genetic point of view, a differentiation exists between the bottlenose dolphin populations of the western and eastern Mediterranean regions37. Based on this knowledge, the whistles collected in the six sites were assigned to three regions as follows: (i) PC, AL and FI to the western Mediterranean Sea (WEST); (ii) CL and GC to the eastern Mediterranean Sea (EAST); (iii) LA to the southern Mediterranean Sea (SOUTH). ‘Region’ is therefore a factor with three levels (WEST, EAST, SOUTH) and accounts for the effect of the genetic distance on the structure of SWs. Whistles from LA were classified as SOUTH for two reasons: (i) the Strait of Sicily where Lampedusa is located is the transition zone between the island of Sicily and the African coast which separates the east and west Mediterranean Sea; (ii) there are no genetic data of the bottlenose dolphins off LA, but a previous study found that this population is acoustically closer to the Atlantic populations, compared to the western Mediterranean populations35.
The six populations studied live hundreds of kilometres apart and are assumed to be isolated from each other. ‘Site’ is a factor with six levels (PC, AL, FI, LA, GC, CL) that consider the effect of geographical isolation on the structure of SWs.
Different sea bottom type (substrate, habitat, and depth) can affect the acoustic environment and the sound propagation38, thus they can also influence SWs. Data on depth range and preferential habitat types of the studied populations were extracted by EMODnet platform (European Marine Observation and Data Network; https://emodnet.ec.europa.eu/en). The prevalent habitat types were classified based on the EUNIS 2021 classification system (Table 5). To evaluate the similarity between the six sites based on the sea bottom type, a hierarchical cluster analysis (using the ward linkage method, Euclidian distance) was performed. Thus, ’sea bottom’ is a factor with three levels (H1, H2 and H3—see “Results” section) that accounts for the influence of depth range and habitat on SW structure.
At the end, we extrapolated six demographic variables from existing literature: mean and range of the population size, mean and range of the group size, residency pattern (prevalence of resident or transient individuals) and connection with adjacent populations (Table 5). A hierarchical cluster analysis (using the complete linkage method, Euclidian distance) was performed to evaluate the demographic similarity between the six populations. Thus, population demography is a factor with four levels (P1, P2, P3 and P4—see “Results” section) that accounts for the influence of population characteristics on SW structure.
Statistical analysis
First, the similarity in SW repertoire among regions, sites, sea bottom and population demography types were estimated. Thus, a non-metric multidimensional scaling ordination (nMDS) was produced from the sample similarity matrix. The mean values of the acoustic characteristics of each SW were used and data were fourth root transformed before calculating the Bray–Curtis similarity. Then, a one-way non-parametric similarity analysis (Anosim) was applied on the same matrix to test the null hypothesis that there was no difference in SWs between the levels of each factor (region, site, sea bottom and population demography). To perform this analysis, the functions metaMDS and anosim of the R package Vegan53 were used.
When mean values of SWs are used as units of analysis, in order to respect the independence between samples, the magnitude of variability decreases. Thus, to investigate the association between the SW structure and each factor, all the SWs collected were used, and Generalized Linear Mixed Models (GLMMs) with Gaussian distribution were applied. The GLMMs are an extension of Generalized Linear Models that allow for the inclusion of random effects, by modelling the covariance structure that is generated by the grouping of data54. They are used when the data are not independent, such as when a variable is measured more than once from the same individuals55 as the SWs.
Since the seven whistle characteristics were highly collinear, before running the GLMMs, a principal component analysis (PCA) was used to reduce them into three independent variables. The assumptions (linear relation between variables, sampling adequacy, and absence of outliers) were verified and some outliers were removed from the sample. The first three components (PC1, PC2, PC3) explained 84% of the total variance (see the “Results” section) and were retained because eigenvalues for the remaining four components were all < 1 (Kaiser's criterion). To perform PCA, the function prcomp of the R package Rstats53 was used. Thus, with the aim to investigate the association between PC1, PC2, and PC3 (the response variables) and each factor separately, four groups of models were built using region, site, sea bottom and population demography as fixed terms, while SW-ID was considered as a random factor in the models. The best model was validated by means of graphical inspection of residuals (i.e., residuals vs. fitted values plots to verify homogeneity; Q–Q plots of the residuals for normality; and plots of residuals vs. each explanatory variable to check for independence). To perform the GLMMs, the function lme of the R package nlme56 was used.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wilkins, M. R., Seddon, N. R. & Safran, R. J. Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol. Evol. 28, 156–166 (2013).
Wei, C. Sound production and propagation in cetacean. In Neuroendocrine Regulation of Animal Vocalization (eds Rosenfeld, C. S. & Hoffmann, F.) 267–291 (Academic Press, 2021).
Nakakara, F. Social functions of cetacean acoustic communication. Fish. Sci. 68, 298–301 (2002).
Caldwell, M. C. & Caldwell, D. K. Vocalization of naive captive dolphins in small groups. Science 159, 1121–1123 (1968).
Caldwell, M. C., Caldwell, D. K. & Tyack, P. L. Review of the signature-whistle-hypothesis for the Atlantic bottlenose dolphin. In The bottlenose dolphin (eds Leatherwood, S. & Reeves, R. R.) 199–234 (Academic Press, 1990).
Ford, J. B. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can. J. Zool. 69, 1454–1483 (1991).
Weilgart, L. & Whitehead, H. Group-specific dialects and geographical variation in coda repertoire in South Pacific sperm whales. Behav. Ecol. Sociobiol. 40, 277–285 (1997).
Deeck, V. B., Ford, J. K. B. & Spong, P. Dialect change in resident killer whales: implications for vocal learning and cultural transmission. Anim. Behav. 60, 629–638 (2000).
Chen, Z. & Wiens, J. J. The origins of acoustic communication in vertebrates. Nat. Commun. 11, 369 (2020).
Morton, E. S. Sources of selection on avian sounds. Am. Nat. 109, 17–34 (1975).
Irwin, D. E., Thimgan, M. P. & Irwin, J. H. Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): A strong role for stochasticity in signal evolution?. J. Evol. Biol. 21, 435–448 (2008).
Campbell, P. et al. Geographic variation in the songs of Neotropical singing mice: Testing the relative importance of drift and local adaptation. Evol. 64, 1955–1972 (2010).
Connor, R. C., Wells, R. S., Mann, J. & Read, A. J. The bottlenose dolphin: Social relationships in a fission-fusion society. In Cetacean societies: Field studies of dolphins and whales (eds Mann, J. et al.) 91–126 (University of Chicago Press, Chicago, 2000).
Janik, V. M. & Sayigh, L. S. Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A https://doi.org/10.1007/s00359-013-0817-7 (2013).
MacFarlane, N. et al. Signature whistles facilitate reunions and/or advertise identity in Bottlenose Dolphins. JASA 141, 3543 (2017).
Buckstaff, K. C. Effects of watercraft noise on the acoustic behaviour of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mam. Sci. 20, 709–725 (2004).
Cook, M. L. H., Sayigh, L. S., Blum, J. E. & Wells, R. S. Signature-whistle production in undisturbed free-ranging bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. Lond. B. 271, 1043–1049 (2004).
Watwood, S. L., Owen, E. C. G., Tyack, P. L. & Wells, R. S. Signature whistle use by temporarily restrained and free-swimming bottlenose dolphins, Tursiops truncatus. Anim. Behav. 69, 1373–1386 (2005).
Sayigh, L. S., Tyack, P. L., Wells, R. S., Scott, M. D. & Irvine, A. B. Sex difference in signature whistle production of free-ranging bottle-nosed dolphins, Tursiops-truncatus. Beh. Ecol. Soc. 36, 171–177 (1995).
Tyack, P. L. & Sayigh, L. S. Vocal learning in cetaceans. In Social influences on vocal development (eds Snowdon, C. T. & Hausberger, M.) 208–233 (Cambridge University Press, 1997).
Miksis, J. L., Tyack, P. & Buck, J. R. Captive dolphins, Tursiops truncatus, develop signature whistles that match acoustic features of human-made model sounds. JASA 112, 728–739 (2002).
Fripp, D. et al. Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim. Cogn. 8, 17–26 (2005).
Janik, V. M. & Slater, P. J. B. Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim. Behav. 56, 829–838 (1998).
Sayigh, L. S., Tyack, P. L., Wells, R. S. & Scott, M. D. Signature whistles of free-ranging bottlenose dolphins, Tursiops truncatus: mother offspring comparisons. Behav. Ecol. Sociobiol. 26, 247–260 (1990).
Watwood, S. L., Tyack, P. L. & Wells, R. S. Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav. Ecol. Sociobiol. 55, 531–543 (2004).
Janik, V. M., Dehnhardt, G. & Todt, D. Signature whistle variations in a bottlenosed dolphin, Tursiops truncatus. Behav. Ecol. Sociobiol. 35, 243–248 (1994).
Esch, H. C., Sayigh, L. S. & Wells, R. S. Quantifying parameters of bottlenose dolphin signature whistles. Mar. Mam. Sci. 24, 976–986 (2009).
Gridley, T. Geographic and species variation in bottlenose dolphin (Tursiops spp.) signature whistle types. PhD Thesis Biology. University of St Andrews (2011).
King, S. L. & Janik, V. M. Bottlenose dolphins can use learned vocal labels to address each other. Proc Natl Acad Sci USA 110, 13216–13221 (2013).
Kriesell, H., Elwen, S. H., Nastasi, A. & Gridley, T. Identification and characteristics of signature whistles in wild bottlenose dolphins (Tursiops truncatus) from Namibia. PLoS ONE 9, e106317 (2014).
Luis, A. R., Couchinho, M. N. & dos Santos, M. E. Signature whistles in wild bottlenose dolphins: Long term stability and emission rates. Acta Ethol. https://doi.org/10.1007/s10211-015-0230-z (2015).
Wang, D. W., Würsig, B. & Evans, W. E. Whistles of bottlenose dolphins: Comparisons among populations. Aquatic Mam. 21, 65–77 (1995).
May-Collado, L. J. & Wartzok, D. A comparison of bottlenose dolphin whistles in the Atlantic Ocean: Factors promoting whistle variation. J. Mammal. 89, 1229–1240 (2008).
Papale, E. et al. Acoustic divergence between bottlenose dolphin whistles from the Central-Eastern North Atlantic and Mediterranean Sea. Acta Ethol. 17, 155–165 (2014).
La Manna, G., Rako-Gospić, N., Manghi, M., Picciulin, M. & Sarà, G. Assessing geographical variation on whistle acoustic structure of three Mediterranean populations of common bottlenose dolphin (Tursiops truncatus). Beh. 154, 583–607 (2017).
La Manna, G. et al. Whistle variation in Mediterranean common bottlenose dolphin: The role of geographical, anthropogenic, social, and behavioral factors. Ecol. Evol. 00, 1–7 (2020).
Natoli, A., Birkun, A., Aguilar, A., Lopez, A. & Rus Hoelzel, A. Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus) based on microsatellite and mitochon-drial DNA analyses. Proc. R. Soc. Lond. B. 272, 1217–2122 (2005).
Richardson, W. J., Greene, C. R., Malme, C. I. & Thomson, D. H. Marine mammals and noise (Academic Press, London, 1995).
Gnone, G., et al. TursioMed: An international project to assess the conservation status of the bottlenose dolphin in the Mediterranean Sea. Final Report (2019).
La Manna, G. & Ronchetti, F. Relazione sul monitoraggio della presenza e distribuzione del tursiope Tursiops truncatus nell’area del nord Sardegna comprendente l’Area Marina Protetta Capo Caccia - Isola Piana. Report AMP, 42 (2018).
La Manna, G., Ronchetti, F., Sarà, G., Ruiu, A. & Ceccherelli, G. Common bottlenose dolphin protection and sustainable boating: species distribution modeling for effective coastal planning. Front. Mar. Sci. 7, 542648 (2020).
Pace, D. S. et al. An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquat. Conserv. 29, 1302–1323 (2019).
Pace, D. S. et al. Capitoline Dolphins: Residency patterns and abundance estimate of Tursiops truncatus at the Tiber River Estuary (Mediterranean Sea). Biology 10, 275 (2021).
Pulcini, M., Pace, D. S., La Manna, G., Triossi, F. & Fortuna, C. M. Distribution and abundance estimates of bottlenose dolphins (Tursiops truncatus) around Lampedusa Island (Sicily Channel, Italy). Implications for their management. J. Mar. Biol. Assoc. UK 6, 1175–1184 (2013).
La Manna, G., Ronchetti, F. & Sarà, G. Predicting common bottlenose dolphin habitat preference to dynamically adapt management measures from a Marine Spatial Planning perspective. Ocean Coast. Manag. 130, 317–327 (2016).
Santostasi, N. L., Bonizzoni, S., Bearzi, G., Eddy, L. & Gimenez, O. A robust design capture-recapture analysis of abundance, survival and temporary emigration of three odontocete species in the Gulf of Corinth, Greece. PLoS ONE 11, e0166650 (2016).
Bearzi, G., Bonizzoni, S. & Gonzalvo, J. Mid-distance movements of common bottlenose dolphins in the coastal waters of Greece. J. Ethol 29, 369–374 (2011).
Bearzi, G. et al. Dolphins in a scaled-down Mediterranean: The Gulf of Corinth’s odontocetes. In Adv. Mar. Biol. Vol. 75 (eds NotarbartolodiSciara, G. et al.) 297–331 (Academic Press, 2016).
Pleslić, G. et al. The abundance of common bottlenose dolphins (Tursiops truncatus) in the former special marine reserve of the Cres-Lošinj Archipelago, Croatia. Aquat. Conserv. 25, 125–137 (2015).
Rako-Gospić, N. et al. Factor associated variations in the home range of a resident Adriatic common bottlenose dolphin population. Mar. Pol. Bul. 124, 234–244 (2017).
Janik, V. M., King, S. L., Sayigh, L. S. & Wells, R. S. Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus). Mar Mam. Sci 29, 1–14 (2013).
La Manna, G., Manghi, M., Pavan, G., Lo Mascolo, F. & Sarà, G. Behavioural strategy of common bottlenose dolphins (Tursiops truncatus) in response to different kinds of boats in the waters of Lampedusa Island (Italy). Aquat. Conserv. 23, 745–757 (2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2015).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. H. Mixed effects models and extensions in ecology with R, 579 (Springer, 2009).
Garamszegi, L. Z. A simple statistical guide for the analysis of behaviour when data are constrained due to practical or ethical reasons. Anim. Beh. 120, 223–234 (2015).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–137 (2018).
Janik, V. M. Source levels and the estimated active space of bottlenose dolphin (Tursiops truncatus) whistles in the Moray Firth, Scotland. J. Comp. Physiol. A Sens. Neural Behav. Physiol 186, 673–680 (2000).
Quintana-Rizzo, E., Mann, D. A. & Wells, R. S. Estimated communication range of social sounds used by bottlenose dolphins (Tursiops truncatus). JASA 120, 1671–1683 (2006).
Sayigh, L. S. Development and function of signature whistles of free ranging bottlenose dolphins, Tursiops truncatus. MIT/WHOI joint program (1992).
Janik, V. M., Sayigh, L. S. & Wells, R. S. Signature whistle shape conveys identity information to bottlenose dolphins. PNAS 103, 8293–8297 (2006).
Papale, E., Gamba, M., Perez-Gil, M., Martin, V. M. & Giacoma, C. Dolphins adjust species-specific frequency parameters to compensate for increasing background noise. PLoS ONE 10, e0121711 (2015).
La Manna, G., Rako-Gospić, N., Manghi, M. & Ceccherelli, G. Influence of environmental, social and behavioural variables on the whistling of the common bottlenose dolphin (Tursiops truncatus). Behav. Ecol. Sociobiol. 73, 12 (2019).
Ballard, S. M. & Lee, K. M. The acoustics of marine sediments. JASA 13, 18–18 (2017).
Smolker, R. & Pepper, J. W. Whistle convergence among allied male bottlenose dolphins (Delphinidae, Tursiops sp). Ethology 105, 595–617 (1999).
Sayigh, L. S., Esch, H. C., Wells, R. S. & Janik, V. M. Facts about signature whistles of bottlenose dolphins (Tursiops truncatus). Anim. Behav. 74, 1631–1642 (2007).
Jourdan J., et al. Distribution and abundance of bottlenose dolphin (Tursiops truncatus) along French Provençal coast. In Proceeding of the 30th European Cetacean Society Conference, Madeira (2016).
Labach, H. et al. Distribution and abundance of common bottlenose dolphin (Tursiops truncatus) over the French Mediterranean continental shelf. Mar. Mam. Sci. 2021, 1–11 (2021).
Terranova, F. et al. Signature whistles of the demographic unit of bottlenose dolphins (Tursiops truncatus) inhabiting the Eastern Ligurian Sea: characterisation and comparison with the literature. Eur. Zool. J. 88, 771–781 (2021).
Acknowledgements
The authors would like to thank all the students for their assistance and help in the fieldwork. Thanks to Alain Barcelo, Marion Peirache, Julie Jordan, the staff of the Parc National del Port-Cros, Miraceti and the Project GDEGEM.
Author information
Authors and Affiliations
Contributions
G.LM. and G.C. designed the study, wrote the main manuscript text and prepared all figures and tables. G.LM. and D.S.P. carried out acoustic data analysis. All authors participated in data acquisition and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Manna, G., Rako-Gospić, N., Pace, D.S. et al. Determinants of variability in signature whistles of the Mediterranean common bottlenose dolphin. Sci Rep 12, 6980 (2022). https://doi.org/10.1038/s41598-022-10920-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10920-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.