Abstract
The greenhouse effect of SF6 increasingly limits its application in various gas insulated equipment. C6F12O combines the advantages of insulation resistance, safety and environmental protection. When mixed with buffer gas, C6F12O is considered to have potential application prospects in medium and low voltage equipment. In this paper, about the partial discharge characteristics of the mixed gas, an experimental study was carried out. The partial discharge initiation voltage and discharge extinction voltage of mixed gas under power frequency voltage are measured and compared with the breakdown voltage. The results show that the breakdown voltage is greatly improved after adding C6F12O, with the increase of mixing ratio, the partial discharge initiation voltage and extinction voltage of mixed gas gradually increase, and the effect of gas pressure on high mixing ratio is obvious. The difference between the partial discharge inception voltage and the breakdown voltage is larger than that of pure N2. The research in this paper can provide an important reference for the application, operation and protection of C6F12O mixed gas in medium and low voltage equipment.
Similar content being viewed by others
Introduction
Gas insulated equipment is widely used in power systems due to its miniaturization and stable insulation performance. Its main insulating medium is SF6, which has a high Global Warming Potential (GWP). The GWP value of SF6 is approximately 23,500 times that of CO21, limiting its use is the current environmental demand. Using new environmental protection gas as insulation medium has become a hot spot in the current research of the global power industry2.
C6F12O of ketones fluoride is also a substance with excellent insulating properties. This substance is non-flammable, non-explosive and non-toxic. It has a boiling point of 49 ℃ under standard conditions and a molecular weight of 316. It is used as a cover gas for fire extinguishing agents, magnesium treatment, and two-phase immersion cooling. The GWP value of the substance is 1, which has no destructive effect on the ozone layer, and the dielectric strength is 1.7 times higher than SF63. The GIS electrical performance test of 145 kV shows that adding C6F12O to the air can achieve the same insulation strength as SF64. The molecular formula of this substance is shown in Fig. 1.
The basic parameters of C6F12O are shown in Table 1. It is worth noting that the liquefaction temperature of C6F12O is much higher than SF6, which is also the main reason for limiting its scope of application to medium and low pressure equipment. Therefore, it is necessary to determine the pressure value and mixing ratio that can be used in the equipment according to its saturated vapor pressure parameters.
Mantilla et al. in Switzerland found that when the mixture of C5F10O, C6F12O and air was proportional to a certain proportion, the power frequency AC breakdown voltage of the mixture can reach three times that of air and carbon dioxide. Although these are not as high as the breakdown voltage of SF6 under the same conditions, it can reach the level of SF6 at a lower pressure by increasing the pressure of the mixture. At the same time, he also observed that the mixed gas obtained by adding a small amount of C6F12O to the air has significantly improved the withstand voltage level of the air under the lightning impulse voltage5. Zhao et al. studied the decomposition products of C6F12O/N2 gas mixture and C6F12O/air mixture after corona discharge, and concluded that C6F12O/N2 mixture gas decomposes more products after corona discharge and may produce CF3CN toxic substances6. It is found that the breakdown voltage of 3% C6F12O/N2 mixture gas at atmospheric pressure is 1.7 times higher than that of pure N2 breakdown voltage, which is equivalent to the breakdown voltage of 10% SF6/N2 mixture gas, and there is no decreasing trend of breakdown voltage after 100 times breakdown experiments. CF4, C2F6, C3F6 and other fluorocarbons were obtained by analyzing the decomposition products of the mixture gas after breakdown7. The liquefaction temperature of C6F12O is high, which needs to be mixed with buffer gas when used as insulating material. Therefore, it can be considered to mix the substance with a single conventional gas to improve the insulation characteristics of the conventional gas.8,9,10Due to the stable chemical nature of N2, we need to further study the insulating properties of C6F12O/N2 gas mixture, and give full play to its greater application potential of the mixed gas in low-voltage equipment such as gas insulated switchgear and ring network cabinets.
Gas insulation equipment often has various kinds of insulation defects such as impurity residue and metal protrusions, which makes the local electric field become non-uniform and cause partial discharge11. In this paper, the partial discharge of C6F12O mixed gas at power frequency is studied. Simultaneously measure the breakdown voltage value of the mixed gas under different conditions, and compare with the partial discharge voltage to summarize the partial discharge characteristics of the mixed gas.
Experiment
Experimental platform
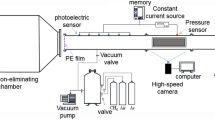
Figure 2 shows the circuit diagram of the power frequency AC experiment.
The main equipment is introduced as follows:
-
Induction voltage regulator model TEDGC-25, working voltage 380 V, output voltage range 0–400 V, rated power 50 kVA.
-
Corona free experimental transformer model YDTW-50VA/100 kV, connected with inductive voltage regulator, transformation ratio of 1:250 and output voltage of 0–100 kV.
-
Resistor R = 10 k Ω, which plays a protective role in the circuit.
-
Capacitive voltage divider C1/C2 = 2 nF/1 µ F, which converts the experimental voltage with high amplitude into the value within the range that can be directly measured by the voltmeter.
-
Discharge chamber install electrodes and fill the experimental gas, and make the experimental gas partial discharge or breakdown under different electrodes by applying voltage, so as to detect the gas insulation performance.
-
Coupling capacitance and non inductive detection impedance partial discharge signal is measured by pulse current method.
-
Digital storage oscilloscope Tektronix DPO7104, with a bandwidth of 1 GHz, 4 acquisition channels and a sampling rate of 20 GS/s.
This experiment uses a needle plate electrode to simulate metal protrusion defects in the device. The needle plate electrode is made of brass material, the tip of needle electrode is a sphere with a radius of 0.3 mm, the radius of the plate electrode is 50 mm and the thickness is 8 mm. The gap distance between the needle tip and the upper surface of the plate electrode is set to be 15 mm constant and fixed to the closed air chamber. The electrode is shown in Fig. 3.
Experimental process
The main measurement data of this experiment include partial discharge inception voltage, partial discharge extinction voltage11 and breakdown voltage. Partial discharge inception voltage (PDIV) refers to the voltage value when the partial discharge phenomenon occurs when the voltage gradually increases. Partial discharge extinguishing voltage (PDEV) means that when partial discharge occurs, the voltage continues to rise and a severe partial discharge begins to appear, at this time, the voltage value is gradually reduced, when the discharge amount is less than a certain value, the partial discharge phenomenon has just disappeared, record the voltage value at this time PDEV. Figure 4 shows typical pd signals measured during the test. The red line in Fig. 4 is the waveform of the UHF detection discharge signal, the peak of which represents the discharge signal appearing here.
Experimental results and analysis
PDIV
The case where the PDIV of the mixed gas changes with the pressure under different mixing ratios and the case where the PDIV of the mixed gas changes with the mixing ratio under different pressures are shown in Figs. 5 and 6, respectively. Each experiment adopts the step-by-step voltage division method, and the voltage is applied slowly at a constant speed from 0 kV. After the voltage is stable, observe the signal of the oscilloscope. When the voltage is high, it will cause the pin-board electrode to break down. At this time, the breakdown voltage is recorded, the voltage regulator is self-protected, the voltage drops to zero, and it is turned off. Then repeat the above operation for 5 tests, and take the average of all test results as the final result under this condition.
It can be seen from the figure that the PDIV value of the gas increases with the increase of the pressure and the gas mixing ratio. The pure N2 has a PDIV value of 5.9 kV at 0.1 MPa and 7.2 kV at 0.2 MPa, with an increase of 1.3 kV. The PDIV value of the 2% mixed gas was 6.7 kV at 0.1 MPa and 10.1 kV at 0.2 MPa, with an increase of 3.4 kV. The PDIV values of the mixture of 4% and 6% mixed gas increased by 4 kV and 4.6 kV respectively in this changing pressure. It can be concluded that the mixed gas of the high mixing ratio increases the PDIV value faster as the pressure changes.
The relationship between the starting voltage and the pressure is fitted by the formula
UPDIV is the PDIV value, A is the slope reflecting the rate of change of PDIV value with pressure, and P is the pressure. The PDIV value of the mixed gas exhibits a positive correlation with the pressure. The larger the value of A, the more obvious the PDIV value of the mixed gas is affected by the pressure. The results calculated after fitting are: A0% = 12.14; A2% = 32.43; A4% = 40; A6% = 46.86. From this, it can be concluded that the higher the mixing ratio, the greater the influence of the pressure on the gas mixture.
In Fig. 6, when 2% C6F12O is added, the PDIV value of the gas increases rapidly. After adding 4% C6F12O gas, the gas PDIV value increases slowly. And the higher the pressure, the faster the PDIV value increases. It can be concluded that the PDIV value will increase faster after N2 is added with C6F12O under high pressure, but as the mixing ratio increases the speed becomes slower at a certain pressure.
Table 2 shows the ratio of the PDIV value at different pressures of each group of mixed gases to the PDIV value of N2, reflecting the increase in the partial discharge starting voltage of the mixed gas obtained after the addition of C6F12O. It can be seen from the table that as the pressure and the mixing ratio increase, the ratio of the PDIV value of each mixed gas to the PDIV value of N2 is higher. At the same mixing ratio, as the pressure increases, the ratio of the PDIV value of the mixed gas to the PDIV value of N2 gradually increases. From Table 2, the ratio of 2% mixed gas to pure N2 increases from 1.14 times at 0.1 MPa to 1.40 times at 0.2 MPa with the increase of pressure, at the same time, when the mixing ratio is 6%, the ratio of PDIV of the mixed gas to PDIV of pure N2 increases from 1.20 times of 0.1 MPa to 1.63 times of 0.2 MPa. So the mixed gas of high mixed ratio increases the PDIV value more obviously with the increase of pressure, which consistent with the results of the previous linear. At the same pressure, as the mixed ratio increases, the ratio of the PDIV value of the mixed gas to the PDIV value of N2 also gradually increases. When the pressure is 0.1 MPa and the mixing ratio of the mixed gas increases from 2 to 6%, the ratio of the PDIV value of the mixed gas to the PDIV value of N2 is from 1.14 to 1.20, an increase of 5.3%; and when the pressure is 0.2 MPa, the data changes from 1.40 to 1.63, an increase of 16.4%. From this it can be concluded that the addition of C6F12O to N2 will result in a more significant increase in the PDIV value of the mixed gas at higher pressure.
In summary, when the pressure is higher than 0.16 MPa and the mixing ratio is higher than 2%, the PDIV value of the mixed gas is increased by 50% or more compared to pure N2.
PDEV
In this experiment, the measurement results of the partial discharge extinction voltage according to the experimental procedure are shown in Figs. 7 and 8. The figures show the change of PDEV value with pressure under different mixing ratios and the change of PDEV value with mixing ratio under different pressures.
In Figs. 7 and 8, similar to the change of gas PDIV value, as the mixing ratio and pressure increase, the gas PDEV value gradually increases. For pure N2, its PDEV value is 5.8 kV at 0.1 MPa, and its PDEV value is 6.9 kV at 0.2 MPa, an increase of 1.1 kV. For 2% C6F12O, its PDEV value is 6.2 kV at 0.1 MPa, and its PDEV value is 9.2 kV at 0.2 MPa, an increase of 3 kV. When the mixing ratio is 4%, the PDEV value of the mixed gas at 0.1 MPa is 6.4 kV, which is not significantly improved compared to the 2% mixed gas. When it reaches 0.2 MPa, it increases to 10.3 kV and increases by 3.9 kV. When the mixing ratio reaches 6%, the PDEV value of the mixed gas is 7 kV at 0.1 MPa, which is a relatively large increase compared to the ratio of 2% and 4%. At 0.2 MPa, the PDEV value of the mixed gas is 10.6 kV, which increases 3.6 kV. Therefore, the addition of C6F12O increases the PDEV value of N2, while the sensitivity to pressure increases slightly.
According to the formula (2), linearly fit each curve in Fig. 7 to study the linear relationship between the PDEV value of various mixed gases and the pressure.
where UPDEV is the partial discharge quenching discharge voltage value, and A′ is the slope. The values of A′ calculated for each curve are: A′0% = 11, A′2% = 30.71, A′4% = 36.71, A′6% = 37.71. The values of the four curves A′ are all positive values, that is, the PDEV voltage value of each group of gases is positively correlated with the pressure. After adding C6F12O, the influence of pressure on the PDEV value of the mixed gas increases sharply, and the higher the mixing ratio of the mixed gas, the greater the PDEV value is affected by the pressure. The PDEV value of the mixed gas with a mixing ratio of 4% and 6% is approximately the same under the influence of pressure.
From Fig. 8, adding 2% C6F12O will increase the PDEV value of N2, and as the pressure is increased, the magnitude of the increase also increases accordingly. However, the PDEV value of the mixed gas with a mixing ratio of 4% relative to the mixed gas of 2% has not increased much. At 0.14 MPa and 0.18 MPa, the PDEV values of the two mixed gases are almost equal. That is, as the mixing ratio continues to increase, the increasing trend of the PDEV value of the mixed gas gradually slows down.
Table 3 lists the ratio of the PDEV value of each group of mixed gas to the PDEV value of pure N2, further showing the partial discharge extinction voltage characteristics of the mixed gas. In the case where the mixing ratio is constant, the ratio of the PDEV value of the mixed gas to the PDEV value of N2 gradually increases as the pressure increases. When the mixing ratio is 2% and the pressure is from 0.1 to 0.2 MPa, the ratio of the PDEV value of the mixed gas to the PDEV value of N2 increases from 1.07 to 1.33; At a mixing ratio of 6%, this value increased from 1.21 to 1.54. So the higher the mixing ratio, the higher the ratio of the PDEV value of the mixed gas to the PDEV value of N2. At a fixed pressure, the increase of the mixing ratio for the increase of the N2’s PDEV value is as follows. At 0.1 MPa, the ratio of the PDEV value of the mixed gas to the PDEV value of N2 changes from 1.07 to 1.21 as the mixing ratio increases. At 0.2 MPa, the ratio of the PDEV value of the mixed gas to the PDEV value of N2 increases from 1.33 to 1.54 as the mixing ratio increases. It can be concluded that the higher the pressure, the higher the ratio of the PDEV value of the mixed gas to the PDEV value of pure N2, and the greater the increase in the PDEV value of the mixed gas relative to the pure N2. The PDEV value of 6% of the mixed gas at 0.18 MPa and above can reach more than 1.5 times of N2 under the same conditions.
Comparison of partial discharge voltage and breakdown voltage of mixed gas
This section compares the partial discharge characteristics and breakdown characteristics of the gas. Tables 4 and 5 respectively list the ratio of partial discharge inception voltage and partial discharge extinction voltage to breakdown voltage of the mixed gas under different mixing ratios and different pressure conditions.
Table 4 shows that the ratio of the pure N2’s PDIV value to the breakdown voltage value is concentrated around 0.6 at 0.1–0.2 MPa, when the mixing ratio is 2%, the ratio of the mixed gas’s PDIV value to the breakdown voltage is about 0.4 at 0.1–0.2 MPa, so the partial discharge voltage of the mixed gas is already smaller than the breakdown voltage value under the same conditions. When the mixing ratio is 4% and 6%, the ratio of the mixed gas’s PDIV value to the breakdown voltage value is about 0.34 at 0.1–0.2 MPa. It shows that under this mixing ratio condition, the partial discharge starting voltage is great smaller than the breakdown voltage. It can be concluded that when the pure N2 is partially discharged, the voltage value may be relatively close to the breakdown value. After adding C6F12O, the difference between the partial discharge voltage and the breakdown voltage of the gas increases significantly.
It can be seen from Table 5 that the ratio of the pure N2’s PDEV value to the corresponding breakdown voltage value is 0.59 at 0.1–0.2 MPa, which is relatively close to the above-mentioned PDIV value. When the mixing ratio is 2%, the ratio of the mixed gas’s PDEV value to the breakdown voltage value under the corresponding conditions is about 0.35 at 0.1–0.2 MPa. When the mixing ratio is 4% and 6%, this value is 0.31 and 0.32 respectively. The addition of C6F12O mixed gas can reduce the ratio of the pure N2’s partial discharge extinction voltage to the breakdown voltage under corresponding conditions.
Application analysis of C6F12O mixed gas insulation equipment
The smaller the partial discharge of the insulating equipment, the better the performance. The current standard stipulates that the level of partial discharge is mainly considering the usage time under the current common process conditions and under normal operating conditions. Through the experimental data analysis of the partial discharge characteristics of the C6F12O mixed gas in this paper, the results show that the discharge amount of the C6F12O mixed gas is small. However, the long-term partial discharge will cause destructive effects on the insulating material, and eventually lead to insulation equipment failure. Therefore, for new equipment, the discharge capacity should not exceed the specified value. When the discharge amount exceeds l times of the standard, although the impact on the equipment is very small, it cannot be ignored. When the discharge amount exceeds 1–4 times of the standard, it is necessary to analyze the possible causes and monitor the operation. If it exceeds 10 times the standard value or even greater, it means that there may be serious hidden faults in the insulation equipment. Faults are usually exposed between 2 months and 2 years, and various invisible faults are often unable to be detected by other insulation tests. Measuring the partial discharge experimental data of C6F12O mixed gas can provide data for monitoring equipment, which can be found and solved in the early stage of partial discharge of electrical equipment, so as to prevent equipment failure caused by long-term partial discharge of electrical equipment. At the same time, the best gas mixing ratio and pressure data can also be determined, which lays a theoretical foundation for practical engineering application. Therefore, the partial discharge experiment of C6F12O mixed gas can provide technical reference for preventing electrical equipment faults. And it can ensure the safe operation of the equipment, which has a certain engineering reference significance12.
Conclusion
-
(1)
The partial discharge inception voltage and partial discharge extinction voltage of the C6F12O mixed gas gradually increases with the increase of the mixed gas and the mixing ratio. The partial discharge characteristic of mixed gas grows slowly with the increase of mixing ratio, and the pressure has a greater influence on the gas with a higher mixing ratio.
-
(2)
The breakdown voltage of pure N2 is greatly improved after adding C6F12O, when the mixing ratio is 4% at a pressure of 0.18 MPa or the mixing ratio is 6% at a pressure of 0.16 MPa, the breakdown voltage can be achieved 2.5 times or more than the pure N2.
-
(3)
When comparing the partial discharge voltage with the breakdown voltage value, it was found that the partial discharge voltage value and the breakdown voltage value of the mixed gas after the addition of C6F12O were smaller than that of pure N2.
-
(4)
The partial discharge of the C6F12O mixed gas phase is small, which can provide technical reference for the safe operation of the C6F12O mixed gas equipment and the prevention of electrical equipment failures, it has engineering significance.
References
Zhang, X. et al. Recent advances in decomposition of the most potent greenhouse gas SF6. Crit. Rev. Environ. Sci. Technol. 47(18), 1763–1782 (2017).
Silvant, S. et al. Detailed analysis of live tanks and GIS circuit breakers using a new environmental friendly gas. In CIGRE 2016, A3–A114 (CIGRE, 2016).
Li, Y. et al. Acute inhalation toxicity studies of gas insulating medium C4F7N. High Volt. Eng. 45(01), 109–116 (2019).
Kieffel, Y. et al. Green gas to replace SF6 in electrical grids. IEEE Power Energy Mag. 14(2), 32–39 (2016).
Mantilla, J. D. et al. Investigation of the insulation performance of a new gas mixture with extremely low GWP. In Proc. 2014 Electrical Insulation Conference, 469–473 (IEEE, 2014).
Mingyue, Z. H. A. O. et al. Decomposition by-products of C6F12O/N2 and C6F12O/air mixed gases under AC 50Hz corona discharge. Adv. Technol. Electr. Eng. Energy 37(11), 1–8 (2018).
Tian, S. et al. Breakdown characteristics and decomposition characteristics of C6F12O and N2 mixed gas under AC voltage. Proc. CSEE 38(10), 3125–3132 (2018).
Hyrenbach, M. & Zache, S. Alternative insulation gas for medium-voltage switchgear. In Petroleum & Chemical Industry Conference Europe (IEEE, 2016).
Stoller, P. C. et al. Mixtures of CO2 and C5F10O perfluoroketone for high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 24(5), 2712–2721 (2017).
Li, X. et al. Insulation performance and application of enviroment-friendly gases mixtures of C4F7N and C5F10O with CO2. High Volt. Eng. 43(03), 708–714 (2017).
Bargigia, A., Koltinowcz, W. & Pigini, A. Detection of partial discharge in gas insulated substations. IEEE Trans. Power Deliv. 7(3), 1239–1249 (1992).
Shuangshuang, T. et al. Experimental research on insulation properties of C6F12O /N2 and C6F12O/CO2 gas mixtures. IET Gener. Transm. Distrib. 13(3), 417–422 (2019).
Author information
Authors and Affiliations
Contributions
R.X., L.D. and Z.X. contributed to the conception of the study; S.Y. and L.Y. performed the experiment, contributed significantly to analysis and manuscript preparation; P. B. performed the data analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rao, X., Li, D., Zhang, X. et al. Study on partial discharge characteristics of C6F12O mixed gas. Sci Rep 12, 6265 (2022). https://doi.org/10.1038/s41598-022-05427-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05427-0
This article is cited by
-
Magnetic field modulated dynamics of partial discharges in defects of high voltage insulating materials
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.