Abstract
Coral reefs experience phase shifts from coral- to algae-dominated benthic communities, which could affect the interplay between processes introducing and removing bioavailable nitrogen. However, the magnitude of such processes, i.e., dinitrogen (N2) fixation and denitrification levels, and their responses to phase shifts remain unknown in coral reefs. We assessed both processes for the dominant species of six benthic categories (hard corals, soft corals, turf algae, coral rubble, biogenic rock, and reef sands) accounting for > 98% of the benthic cover of a central Red Sea coral reef. Rates were extrapolated to the relative benthic cover of the studied organisms in co-occurring coral- and algae-dominated areas of the same reef. In general, benthic categories with high N2 fixation exhibited low denitrification activity. Extrapolated to the respective reef area, turf algae and coral rubble accounted for > 90% of overall N2 fixation, whereas corals contributed to more than half of reef denitrification. Total N2 fixation was twice as high in algae- compared to coral-dominated areas, whereas denitrification levels were similar. We conclude that algae-dominated reefs promote new nitrogen input through enhanced N2 fixation and comparatively low denitrification. The subsequent increased nitrogen availability could support net productivity, resulting in a positive feedback loop that increases the competitive advantage of algae over corals in reefs that experienced a phase shift.
Similar content being viewed by others
Introduction
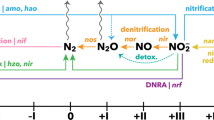
Nitrogen (N) is vital for all living organisms and is required for primary production and the production of biomass. Among the key elements required for life (i.e., N, carbon, phosphorus [P], oxygen and sulphur1), N in the form of dinitrogen (N2) gas has the greatest total abundance in the environment2. Ironically, however, N2 gas is the least accessible for flora and fauna1. In oligotrophic marine ecosystems such as coral reefs, primary production is limited by low amounts of bioavailable N forms such as ammonium (NH4+) or nitrate (NO3-)3,4,5. Yet, coral reefs belong to the most productive ecosystems on earth and are regarded as oases in an oceanic desert6,7,8. In this context, microbial N cycling plays a key role by introducing, recycling and removing N from coral reefs9. Particularly, biological N2 fixation, i.e., the conversion of atmospheric N2 into bioavailable NH4+ by prokaryotic microbes (diazotrophs), can alleviate N limitation for coral reef primary producers10. In addition, the recycling of de novo bioavailable N via nitrification4,11 may serve as a mechanism to prevent the loss of N12. In contrast, denitrification (i.e., the conversion of nitrate to atmospheric N2 by microbes) may remove bioavailable N in times of high environmental N availability12,13,14. Likewise, fixed N can be transformed into atmospheric N2 via anaerobic ammonium oxidation (ANAMMOX), a pathway functioning as an additional N removing mechanism in coral reef sponges15, and hypothetically in other coral reef associated organisms12. Whereas N influxes to coral reefs via N2 fixation are comparably well-studied10,16,17, knowledge about N efflux via denitrification is limited to some coral reef substrates (such as reef sediments)13,14, and is just starting to be generated for other coral reef organisms (e.g., hard corals)18,19,20,21. Corals reefs and their main ecosystem engineers, scleractinian corals, are adapted to nutrient-poor environments22,23. Under these conditions, both the import of bioavailable N via N2 fixation as well as the removal via denitrification may essentially contribute to maintaining a stable, low N availability and, hence, ecosystem functioning22. Further N cycling processes have been detected or hypothesised in coral reef environments12. For example, nitrification, i.e., the oxidation of NH4+ to nitrite (NO2-) and NO3-, has been measured in coral reef environments4,24, and may function as an internal recycling mechanism with nitrate serving as a substrate for coupled denitrification12.
Coral reefs not only belong to one of the most productive but also to the most threatened ecosystems on the planet. Global and local change associated stressors such as ocean warming and acidification25,26, eutrophication27, and overfishing28 undermine the health of coral reefs and can eventually lead to coral mass mortality29. The remaining coral skeletons offer substrates30,31,32 for fast-growing, highly competitive algae assemblages33, which may lead to a transition from coral-dominated to algae-dominated reef states. Due to the stability of these novel communities, these transitions have been interpreted as phase-shifts34,35,36. Although the effects of these phase-shifts on ecosystem services37,38,39,40,41 and functioning42,43 have received some attention, Williams and Graham44 emphasise our yet rudimentary understanding of alterations in coral reef functional ecology. Although we already observe different ecosystem states (e.g., coral-dominated or alternative states on coral reefs)45, our knowledge about their functioning is still in its infancy. Additionally, functioning likely differs between coral- and algae-dominated communities46,47.
N cycling is critical for the stability of coral reef ecosystems; however, it has not been investigated yet how gain (via N2 fixation) and loss (via denitrification) terms of bioavailable N differ quantitatively between coral- and algae-dominated reef states. For this study, we hypothesised differences between coral- and algae-dominated reef states in the amount of total fixed and denitrified N, based on differences in N2 fixation and denitrification activities of different benthic categories10,48,49. Hypothetically, an altered reef community structure associated with phase-shifts could lead to different total N2 fixation and denitrification budgets in coral- and algae-dominated reef areas. Further, we hypothesised that N cycling processes may have the potential to amplify and catalyse phase-shifts through the proliferation of turf algae as prominent N2 fixers50. The role of denitrification in reef communities is mostly unknown, as well as the interplay of both N2 fixation and denitrification in coral- and algae-dominated reefs. Understanding N cycling patterns in baseline scenarios (i.e., coral-dominated) and alternative reef states (i.e., algae-dominated) is, hence, of paramount interest to gain a holistic understanding of these dynamic systems, which then can be used as a basis to address, elaborate, and expand future management strategies.
In the present study, we carried out acetylene-based incubations (i) to identify the key players that import and/or remove nitrogenous compounds into/from the reef system; and (ii) to provide a relative budget for two counteracting N cycling processes (i.e., N2 fixation and denitrification) in a comparative framework that covers the main species of six key benthic categories that together account for > 98% of the benthic cover on a central Red Sea coral reef with two distinct reef areas.
Results
Benthic community composition
Two distinct reef community states characterised by contrasting relative cover of benthic categories were identified (Fig. 1A, Fig. S1) and pre-defined as “algae-” and “coral-dominated reef areas” (according to Roth and others51). A detailed overview of the benthic communities can be found in Table S1. Briefly, algae-dominated areas displayed approximately twice as much turf algae-cover compared to coral-dominated areas (Mann–Whitney U p = 0.007), whereas hard coral cover was threefold lower (Mann–Whitney U p < 0.001). Pre-defined differentiation between algae- and coral-dominated reef areas were confirmed visually by Principle Coordinate Analysis (PCO, Fig. 1B) and ANOSIM (R = 0.605, p = 0.001). Hard corals and turf algae were the major drivers of separation and explained 64.2% of the dissimilarities between reef areas (DistLM).
(A) Relative benthic cover in algae- and coral-dominated areas. Benthic cover is presented as mean proportional cover of major benthic categories assessed by photo quadrats (n = 10 in algae-dominated areas, n = 12 in coral-dominated areas). Adapted from Roth et. al 51. (B) Principle Coordinate Analysis (PCO) of benthic community cover at the algae-dominated (green) and coral-dominated (blue) areas. Vector overlays shows correlations > 0.4 based on Pearson ranking, green triangles display replicates of algae-dominated areas (n = 10), blue triangles display replicates of coral-dominated areas (n = 12).
Nitrogen fluxes of individual benthic categories
Turf algae and coral rubble showed highest N2 fixation rates per substrate surface area, being approximately 5 to 6-fold higher than those of biogenic rock, 10 to 12-fold higher than reef sands, 32 to 39-fold higher than soft coral, and approximately two orders of magnitude higher than hard corals (Fig. 2A, Table 1 and S2).
(A) Dinitrogen (N2) fixation rates and (B) denitrification potential of investigated benthic categories and hard coral species. Organisms and substrates (turf algae n = 5, coral rubble n = 4, soft coral n = 5, biogenic rock n = 5, reef sands n = 5, hard corals n = 13) were sampled randomly from both reef areas. Rates and potential for hard corals consist of mean values of P. verrucosa (PV, n = 5), A. hemprichii (AH, n = 4), S. pistillata (SP, n = 4). Letters above bars indicate significant differences if different, or non-significance if shared. Y-Axis labels for imbedded hard coral data plots are analogue to respective N2 fixation rate or denitrification potential plot. Note different scales for N2 fixation and denitrification.
Due to NO3- addition during incubations, we present denitrification as potentials instead of rates (see Method section). The soft coral displayed the highest denitrification activity, being 3- to 4-fold higher than that of reef sands, biogenic rock and coral rubble, and 6-fold higher than denitrification potentials of hard corals and turf algae (Fig. 2B, Table 1 and S2). Among hard corals (i.e., data above comprised the average potentials across P. verrucosa, A. hemprichii, and S. pistillata), A. hemprichii showed the highest denitrification potential that was 5-fold higher than that of P. verrucosa (pair-wise PERMANOVA t = 3.407, p = 0.01) and 26-fold higher than S. pistillata (pair-wise PERMANOVA t = 5.696, p < 0.001).
Nitrogen fluxes in different benthic categories referred to reef areas
We used individual rates/potentials of N2 fixation and denitrification from all measured organisms and substrates (see Table S2) to calculate budgets for communities of the assessed reef areas dominated either by corals or turf algae. Cumulated fixed N was 2-fold higher in the algae-dominated compared to the coral-dominated area (Friedman’s aligned rank test p = 0.24), whereas denitrification was similar (Friedman’s aligned rank test p = 0.19; Fig. 3). Turf algae assemblages contributed most to N2 fixation in both algae-dominated (70.2%) and coral-dominated areas (79.8%), followed by coral rubble (28.4% in algae-dominated and 11.3% in coral-dominated area, respectively; Fig. 4). In contrast, hard and soft corals combined accounted for 75.5% and 52.4% of denitrification activity in coral- and algae-dominated areas, respectively (Fig. 4).
Total fixed (via dinitrogen fixation) and removed N (via denitrification) in both reef areas (calculated as sum of means of extrapolated rates of individual benthic categories ± standard propagated error; according to supplementary material SM3; no significant differences were observed by applying Friedman’s aligned rank test).
Discussion
Stable and low N availability is of central importance to the health and resilience of coral holobionts12,52, and consequently, of coral reef ecosystems in general22. Thus, processes that introduce or remove bioavailable N, such as N2 fixation and denitrification, respectively, may play a key role in the functioning of coral reefs22. Our study is—to the best of our knowledge—the first to provide a comparative overview of denitrification activities of major coral reef related benthic categories. By investigating two N cycling process, we were able to extend previous work of Cardini and others10, who showed a link between reef-wide primary production and N2 fixation. We related benthic category-specific N2 fixation rates and denitrification potentials (per 2D substrate surface area, Table S2) to their relative contribution on a 3D level (Table S3), and likewise extrapolated metabolic processes to both coral- and algae-dominated reef communities (per 3D benthos surface area, Fig. 3, Table S1). This may be of particular importance, as algal dominance on coral reefs will likely increase as a consequence of frequently occurring mass coral bleaching events53,54 and reduced time available for recovery55 that diminish the return to coral-dominated reef states56.
N2 fixation and denitrification by key benthic categories on coral reefs
Individual N2 fixation rates of benthic categories are in line with10,20,48,49,57 or lower10,58,59 than rates reported in previous studies (see Table 2) from the Red Sea that were obtained with similar measurement techniques (i.e., acetylene-based assays) and sampling intervals (t0 and t24). Slightly lower N2 fixation rates in the present study may a) be due to an underestimation caused by an initial lag phase associated with acetylene-based assays60,61 (see limitations), which was omitted from rate calculations of other studies59 but included here; or b) due to varying sampling times: benthic categories of the present study were incubated in March 2018, which is the winter season. It has been demonstrated that N2 fixation activity in winter is generally lower than in summer10,49,62, which could explain lower N2 fixation rates of the present study compared to previously reported rates (see Table 2). The effect of seasonality on the denitrification potential of our benthic categories remains unknown.
Our results suggest a clear distinction between key benthic categories, in which the most active N2-fixers showed lowest denitrification potentials and vice versa. Turf algae and coral rubble were identified as the largest N2-fixers, confirming previous findings by Cardini and others10; however, they play a minor role in reducing N availability via denitrification. Reef sands, as active sites of microbial N transformations in coral reefs14, played only a marginal role in both N2 fixation and denitrification in the present study, compared to the other benthic categories. Our comparatively low N2 fixation and denitrification rates in reef sands may have been a result of well-oxygenated top sediment layers63, which could inhibit these anaerobic processes64,65. Even though reef sand N cycling rates were likely underestimated in the studied reef areas (as top layers were sampled; see limitations section), reef sands solely cover a minor fraction of the benthic cover.
From an ecological perspective, turf algae are pioneers33 and opportunists66,67, which can form extensive mats even under oligotrophic conditions68,69. Furthermore, they can rapidly take over bare substrate due to high growth rates33,66,70,71. The high N demand needed to fuel metabolism and biomass production in filamentous turf algae can, to a large extent, be satisfied by high N2-fixing activities33,49,72,73. A recent study showed that accumulated N in the form of turf algal biomass can be exported to the wider reef33. N2 fixation activities of coral rubble were similar to those of turf algae, which confirms findings of a recent study74. Coral rubble N2 fixation activity measured here is in the range of previous studies75,76 and may, thus, be driven by microbial communities inhabiting coral rubble surfaces, as suggested by Davey and others75, who observed significantly higher N2 fixation activity in coral rubble compared to living hard corals.
Among all benthic categories, corals (both hard and soft corals) showed lowest N2 fixation activities, being in a similar range10,48,77 or significantly lower59 than rates measured previously with acetylene reduction techniques. Lower N2 fixation rates for hard corals of the present study may have occurred due to a phosphate limitation of N2 fixation13,78,79, as the estimated N:P ratio of 83:1 in the incubation water (see Methods) ranged above the canonical Redfield ratio of 16:180. Additionally, discrepancies could also result from differing surface area determination techniques used here and in other studies. Previous studies determined the surface area of investigated benthic categories, such as hard corals, using the aluminium foil method81 or advanced geometry49,58, but potentially lack accuracy82 and, hence, potentially underestimate the surface area of hard corals and, thus, overestimate N fluxes. The soft coral displayed highest denitrification activity. The interplay of N2 fixation and denitrification, favouring low internal N availability, may be vital for the stability between the coral host and the associated photosynthetic algal endosymbiont of the family Symbiodiniaceae (sensu Rädecker and others12). Low N2 fixation and comparatively high denitrification activity may promote the host’s control over symbiotic algae83 by keeping N availability limited5,84. As such, seasonal fluctuations 85 or environmental stressors, like eutrophication or ocean warming, may influence both processes12, leading to shifts from N to P or another micronutrient (e.g., iron) limitation86,87,88, which can result in a breakdown of the coral-Symbiodiniaceae symbiosis87. Furthermore, Tilstra and others20 hypothesised a negative correlation between heterotrophic capacity and denitrification activity in coral holobionts, as previously suggested for diazotrophs77. The hard coral species used in the present study are all on the autotrophic end of the mixotrophic spectrum89,90,91,92. As such, these coral species rely mostly on the Symbiodiniaceae for their energy. As denitrifying microbes are heterotrophic93,94, it is likely that photosynthates translocated from the Symbiodiniaceae are their main source of energy. Thus, a differing heterotrophic capacity of the coral could potentially influence denitrification potentials. This hypothesised link may also explain the high denitrification activity found in the investigated soft coral of the family Xeniidae, which is a functional autotroph95.
Finally, denitrification is an anaerobic process. Potentially, observed denitrification potential differences among the hard coral species and Xenia sp. occurred due to their different capacity in releasing and producing mucus96,97, with thick mucus layers favouring high anaerobic potentials that might facilitate denitrification96. It has been demonstrated recently that soft coral mucus does not provide a favourable habitat for associated diazotrophs97, which in return could explain observed comparatively low N2 fixation rates.
Implications for coral- and algae-dominated reefs
Extrapolated reef-wide fixed N via N2 fixation of both coral- and algae-dominated reef areas (350.01 ± 97.87 and 722.91 ± 241.26 µmol N m−2 d−1, respectively; Fig. 3) is in line with previously calculated reef-wide N2 fixation budgets of Larkum and others17 and Cardini and others10, who have reported an annual average N2 fixation activity of 156–1330 µmol N m−2 d−1 and 546 ± 69 µmol N m−2 d−1, respectively.
At the community level, the relative contribution of key benthic categories varied when compared to N fluxes related to substrate surface area. Together, turf algae and coral rubble represent 91% of overall N2 fixation in coral-dominated and 99% in algae-dominated reef areas. Hard and soft corals are key players in coral-dominated reef areas, in which both benthic categories together account for 52% of benthic cover (in the investigated Abo Shosha reef area) and contributed equally to the 78% of overall denitrification. This is surprising, as both benthic categories showed lowest (hard corals) or highest (soft coral) denitrification activity. While all investigated hard coral species showed similar N2 fixation patterns, findings regarding their denitrifying activity showed a large variability (Fig. 2). Based on our results, we hypothesise that reefs with a higher Acropora hemprichii cover (compared to Stylophora pistillata or Pocillopora verrucosa) display higher capacities to remove bioavailable N via denitrification and could, thus, be more resilient to higher N availability than those of S. pistillata or P. verrucosa dominance. However, we have considered only a small selection of hard corals with a branching morphology that are considered as autotrophs89,90,91,92. It remains speculative how other hard coral species, such as those of mounding and plating morphologies, or heterotrophic corals77,98 with different mass transfer characteristics99,100 contribute to potential reef resilience.
Besides being most abundant in coral-dominated areas, hard corals (especially branching hard corals, represented by the species selected in the present study) contribute most to the three-dimensional structure (i.e., spatial complexity or rugosity) of coral reef ecosystems100,101,103, which increases the relative importance of hard corals for N2 fixation and particularly denitrification in both reef areas in the present study. A reduction of hard coral cover and, thus, spatial complexity (i.e., a loss of cryptic 3D area) also leads to a reduced contribution to overall denitrification (Fig. 4), even though denitrification potentials for hard corals are similar to those of reef sands and turf algae when related to 2D substrate surface area (Fig. 2). Even in the algae-dominated reef area, both hard and soft corals still contributed to more than 50% of overall denitrification (Fig. 4), despite covering only ~ 22% of the seafloor. The mentioned reduction or loss of spatial complexity is commonly associated with coral-phase shifts104. Whereas the importance of structural complexity and consequences of its loss in coral reefs has been recognised on multi-fold levels before, e.g., on fish communities103,104,105,106,107, invertebrate diversity108,109 and ecosystem services41,102,110,111, results of the present study also suggest substantial consequences on N cycling dynamics. The most striking result was the difference in total N import via N2 fixation in both reef states, as well as changes in the relative contribution of coral-associated denitrification in the respective reef areas. Turf algae were identified as key N2-fixers and their higher abundance in benthic coverage leads to a 100% increase of N2 fixation in algae-dominated compared to coral-dominated areas (Fig. 3). At the same time, denitrification, a process that may alleviate coral reef environments from excess N12,13,14,15,20, remained stable in the algae-dominated part of the reef. Future studies should determine the role of ANAMMOX in coral reefs, as ANAMMOX may play a vital role in removing bioavailable N from coral reef environments12. However, key ANANMMOX-performing players have not been identified in coral reefs yet, nor were they included in the present study (see limitations). Nevertheless, a higher N availability (i.e., higher fixed N inputs with stable denitrification activity) in algae-dominated reefs could have multiple consequences resulting in a positive feedback loop. Just as eutrophication promotes turf algae growth on coral reefs112, higher N accumulation from N2 fixation could relieve N limitation and cause algae to proliferate in nutrient-poor waters113,114. This can result in high abundances of benthic algae that in turn deter herbivorous fish that successively control algal proliferation by grazing115. Jessen and Wild116 have described this feedback loop before, and we here append that higher N availability (via N2 fixation) in algae-dominated reefs can be further utilised for algal growth or metabolism. Subsequently, increased N availability could facilitate the release of algal exudates such as dissolved organic nitrogen (DON)117 from benthic algae during active growth or decomposition. Ultimately, this feedback loop could turn a reef from a previously net sink of DON into a DON source, which similarly occurs during eutrophication events16,118.
Implications for alternative reef states
In the present study, we evaluated two distinct reef communities and the implications of their varying benthic composition. Microbial communities associated with the benthic categories likely not only vary between coral- and algae-dominated reefs119, but also between investigated benthic categories119,120,121,122. Although the microbial community composition was not considered in this study (see limitations), varying microbial communities and their interactions might have implications for N2 fixation and denitrification activities and should be investigated in future studies. Discontinuous shifts123, shifts of varying intensity124, or community shifts from reefs dominated by corals to assemblages other than benthic (turf) algae have been reported (reviewed in Norström and others45). Aside from algae-dominated reefs, alternative reef states can be dominated by corallimorphia, sponges, ascidians, or soft corals45. Particularly, soft coral dominance is common in the Red Sea125,126 and other regions such as Taiwan, East Pacific127 and at the Great Barrier Reef, Australia128, where the soft coral genus Xenia attains as much as 80% of benthic cover after disturbance45,129. Moreover, climate change associated stressors such as ocean acidification might induce community shifts from hard to soft coral dominance130. Bednarz and others48 propose that soft corals may become an important player in N cycling due to their increasing dominance in benthic cover in the northern Red Sea. Based on the results of the present study, we carefully speculate that a high soft coral cover may alleviate degraded reefs, or reefs in a transitional state, from excessive N. The extent to which Xenia sp., as the main denitrifying organism, can decelerate coral reef degradation remains to be determined in future studies. Hypothetically, N limitation could be exacerbated in Xenia sp. dominated reef areas, as more N is removed via denitrification than in hard coral or algae-reef dominated areas. This hypothesis is supported by findings of Pupier and others97, who suggest a significant decrease in N2 fixation and subsequent N limitation in soft coral-dominated reefs.
Coral rubble-dominated areas, particularly reef flats, can be the result of fragmentation and erosion processes of nearby reef areas such as leeward slopes, resulting in mobile fragments that are unsuitable for coral colonisation131. Findings of the present study confirm previous hypotheses, in which the potential of coral rubble as highly active N2-fixers can aggravate N influxes to a destroyed or vulnerable reef52,75. At the same time, our findings indicate that the denitrifying capacity of rubble-dominated reefs is presumably lower than in intact coral-dominated areas (i.e., due to lower structural complexity and relatively lower denitrification activity compared to soft corals), which suggests that this reef state could compound N influxes, resulting in N accumulation at the ecosystem scale.
We demonstrated that N influxes via N2 fixation could lead to an aggregation of N in algae-dominated communities, while differences regarding the bioavailable N removal via denitrification were not observed between coral- and algae-dominated reef areas. Potentially, N2 fixation rates10,49,58 as well as denitrification potentials (85) experience seasonal fluctuations that might cause feedback responses in the extrapolated fixed or removed N. The range of these feedback responses remains to be determined.
N cycling processes in coral reefs, including anaerobic ammonium oxidation (ANAMMOX, transformation of fixed NH4+ and NO2− to elemental N2), that potentially remove fixed N from the system132,133 have been identified in coral microbiomes134 and coral-reef sponges15. The extent to which ANAMMOX can serve as a N removal process in shifting reefs remains to be determined. Further, cryptic habitats in coral reefs harbouring high abundances of sponges135 may reduce bioavailable N via ANAMMOX and should be prioritised in future studies, as they were not considered in the present study (see limitations). The biogeochemical significance of stressor-induced phase shifts and the resulting loss of structural complexity (i.e., cryptic habitats) in reef N cycling remain unknown and should be the subject of future studies.
Material and methods
Study site and benthic community composition
The Abu Shosha reef in the Jeddah Region (22° 18′ 15″ N, 39° 02′ 56″ E) on the west coast of Saudi Arabia in the central Red Sea was chosen due to the co-occurrence of both coral- (i.e., > 40% hard coral cover) and algae- (< 15% hard coral cover, > 40% turf algae cover) dominated areas within the same reef (Fig. S1)51. Both areas are approx. 50 m2 in size, were located at the same water depth (~ 5 m) and were solely used as a base for relating the respective N cycling rates to the reef area. Specimens were sampled from the total reef area (see next paragraph). The selected reef displays a small-scale heterogeneity of communities and varying degrees of community composition with both target reef areas being less than 30 m apart from each other.
Benthic community composition of the Abu Shosha reef was determined for an earlier study51 by photo quadrats136, providing a two-dimensional (2D) planar reef coverage of each benthic category. Briefly, a PVC quadrat (50 × 50 cm, 0.25 m2) was randomly placed on the reef surface (12 × in coral-dominated area, 10 × in the algae-dominated area), and a photograph was taken from approx. 1 m distance to the substrate. Photographs were then analysed with the software Coral Point Count with Excel extension (CPCe) 4.1137. With the help of the software, 48 randomly located points were overlaid on the photographs, resulting in 576 and 480, respectively, data points per study area. The underlying benthos for each data point was determined to the lowest possible taxon. The major benthic categories of the investigated reef were (Fig. 1A): filamentous turf algal assemblages (hereafter termed turf algae), coral rubble, soft coral (i.e., Xenia sp.), biogenic coral rock (hereafter termed biogenic rock), carbonate reef sands (hereafter termed reef sands), hard corals, macroalgae, and the giant clams (Tridacna sp.). Hard corals were identified to the genus level. Turf communities consisted of a heterogeneous assemblage of different filamentous algae and cyanobacteria. Examples from the Northern Red Sea have shown that turf algae, as defined for the present study, account for the highest fraction (up to 90%) of benthic algal cover138. Areas of bare coral rock, that were not covered with any of the other benthic categories but associated with endolithic algae and crustose coralline algae were defined as biogenic coral rock139 (hereafter termed biogenic rock). Coral rubble was defined as dislodged parts of framework builders with its associated microbial community according to Rasser and Riegl131.
Sample collection and maintenance
All benthic categories, i.e. hard corals Pocillopora verrucosa (n = 5), Acropora hemprichii (n = 4), Stylophora pistillata (n = 4), soft coral of the Xenia genus (n = 5), biogenic rock (n = 5), coral rubble (n = 4), reef sands (n = 5) and turf algae (n = 5), were collected randomly from the overall reef area (i.e., regardless whether from coral- or algae-dominated areas; Fig. 5—Step 1) and immediately incubated after sampling in March 2018. Due to feasibility, the three most abundant hard coral species were chosen as they represent the most abundant species of the Abo Shosha reef. To increase readability and comprehensiveness, we refer to “hard corals” from here on. The aforementioned benthic categories were selected as they comprised more than 98% of the benthic cover in both coral- and algae-dominated parts of the reef51. Where necessary, fragments were collected with hammer and chisel. P. verrucosa, A. hemprichii, S. pistillata and turf algae fragments (limestone covered with turf-algae) were approx. 10 cm long. Hard coral fragments were sampled from different coral colonies (> 10 m distance between each other) to account for genetic diversity. Individual coral colonies of Xenia sp. were collected with a small piece of anchoring rock (< 0.5 cm diameter) to prevent tissue damage. Reef sands were sampled using a Petri dish (polystyrene, 5.5 cm diameter, 1.4 cm depth) which was pushed carefully into the sand. Reef sands were then fixed to the dish from underneath so that upper sand “cores” with a max. sediment depth of 14 mm were sampled, covering a similar depth as reported previously10,58. All fragments and Petri dishes containing reef sand samples were immediately transferred to recirculation aquaria on the boat after sampling, each filled with ambient seawater. Fragments of P. verrucosa, Xenia sp., turf algae, coral rubble, biogenic rock as well as sediment samples were kept at ambient water temperature and light conditions until the experimental incubations started within 3 h after sampling. For P. verrucosa, it has been demonstrated successfully that freshly collected fragments can be utilised for physiological quantifications140. Fragments of A. hemprichii and S. pistillata were sampled two weeks prior all other specimens. After being transferred to the wet lab facilities of the Coastal and Marine Resources (CMOR) Core Lab at KAUST, fragments were distributed randomly into four independent replicate 150 L flow-through tanks (flow-through rate 300 L h−1) for two weeks to allow for acclimation and healing of tissue damage. Each aquarium was constantly supplied with ambient, sediment-filtered reef water from inshore reefs located 1.5 km off KAUST, and ambient light conditions, i.e., a photon flux of ~ 200 µM quanta m−2 s−1, representing the daytime average photon flux of the studied reef and water depth during this period of the year.
Stepwise illustration of sampling, data acquisition and analysis. Step I illustrates sampling of specimens randomly from the overall reef area (i.e., regardless of coral- or algae-dominated area). Afterwards (step II), all specimens were incubated according to the COBRA method74 with the displayed replication. Gas samples were taken for ethylene (C2H4) and nitrous oxide (N2O) measurements via gas chromatography (step III) for N cycling rate quantifications. Obtained data were used for extrapolation to N fluxes to either coral- or algae-dominated reef areas (step IV).
Nitrogen cycle fluxes
Incubations were performed using a COmbined Blockage/Reduction Acetylene assay (hereafter COBRA; Fig. 5—Step 2) modified after El-Khaled and others74. Briefly, COBRA incubations were performed in gastight 1 L glass chambers (800 mL seawater + 200 mL headspace). As acetylene inhibits the production of NO3- via nitrification141,142, seawater (ambient NO3- concentrations ranged between 0.09 and 0.34 µM)21,112 was supplemented with nitrate to a final concentration of 5 µM as a substrate for the denitrification pathway to counteract substrate limitation (see supplementary material SM 5). Incubations with nitrate amended seawater have been performed successfully in previous studies141,142,143,144,145. Potentially, the addition of nitrate suppresses N2 fixation146, particularly as nitrate uptake has been reported for various benthic categories147,148. Theoretically, this could alter microbial functioning of benthic categories, as the acquisition of N via uptake is less cost-intensive149. Additionally, this results in an estimated NO3-:P ratio ranging between 5.09:0.06 and 5.34:0.07 in the incubation water, which is well above the canonical Redfield ratio of 16:1 (DIN:DIP)80. However, persistent N2 fixation rates in the presence of nitrate of up to 30 µM have been reported149. Furthermore, El-Khaled and others74 conclude that the technique provides sufficient information about the relative importance of different benthic categories by accounting for relative changes in N cycling rates (both N2 fixation and denitrification). COBRA provides denitrification “potentials” as artificially provided nitrate during the incubations drives denitrification above natural levels74. Acetylene was added to both incubation water and headspace at a concentration of 10%. This saturated acetylene concentration in the gastight incubation chambers leads to the preferential reduction of acetylene to ethylene (C2H4) instead of N2 to NH4+ by the key enzyme nitrogenase150,151. Moreover, acetylene blocks the nitrous oxide (N2O) reductase activity in the denitrification pathway leading to an accumulation of N2O152,153. Replicate samples were incubated and two additional chambers without specimens served as controls to correct for planktonic background activity. All incubations lasted for 24 h with a 12:12 h dark/light cycle and a photon flux of ~ 200 µM quanta m−2 s−1, representing the daytime average photon flux of the studied reef and water depth during this period of the year. Incubation chambers were submerged in a temperature-controlled water bath at 27 °C (resembling the ambient seawater temperature measured at the reef in 5 m depth during sampling) and constantly stirred (500 rpm) to ensure sufficient exchange between the water body and headspace. Gas samples were taken at the start (t0) and the end (t24) of each incubation, and analysed targeting C2H4 (as a proxy for N2 fixation) and N2O (as a proxy for denitrification) by gas chromatography and helium pulsed discharge detector (Agilent 7890B GC system with HP-Plot/Q column, lower detection limits for both target gases were 0.3 ppm). A detailed description of N2 fixation and denitrification rate/potential calculation can be found in the supplementary (SM 6). Briefly, results were normalised to incubation time, corrected for the seawater control signal, related to incubation volume, and normalised to the surface area of the organisms/substrates. Surface areas of incubated organisms/substrates were determined photometrically using cloud-based 3D models (Autodesk Remake v19.1.1.2)154,155 of P. verrucosa, A. hemprichii, S. pistillata, Xenia sp., biogenic rock, coral rubble and turf algae fragments. Reef sand surface areas were calculated using dimensions of Petri dishes that were utilised for sand core sampling (surface area = π * radius2). Notably, sediment depth and, hence, oxygenation status as well as pore-water movement through the sediment matrix affect the biogeochemical cycling, with potentially higher N cycling activity due to anaerobic milieus provided in deeper sand layers63. Oxygen fluxes were quantified parallel with identical benthic categories to validate that neither hypoxia nor hyperoxia (conditions that, e.g., are detrimental to organisms evoking alteration of physiological responses) conditions occurred during N cycling incubations156. We refer to supplementary material SM 7 for further information.
Data treatment of nitrogen fluxes
Production rates of C2H4 and N2O were converted to N fluxes using conservative molar ratios of N2O:N2 = 1 and C2H4:N2 = 4157. Extrapolations for total fixed or removed N (via N2 fixation and denitrification, respectively) were performed according to formulas in supplementary material SM 8. Briefly, rates were extrapolated by multiplication according to reef benthos 3D area considering the respective 2D to 3D conversion factor (Table S4, according to Cardini and others10). Then, these benthic category-specific rates were used to account for the relative cover (i.e., 2D planar coverage obtained from cover assessments described previously) of each benthic category in the respective reef area (i.e., coral- and algae-dominated, resp.), which provides cumulative N fluxes related to 3D reef area (Fig. 4; expressed as fixed or denitrified µmol N m−2 benthos 3D area d−1, resp.).
Statistical analysis
Statistical analyses were performed using Primer-E v6158 with the PERMANOVA + extension159. Differences in the N cycling processes among benthic categories were tested for significance using permutational analysis of variance (PERMANOVA) on a Bray–Curtis similarity matrix of square-root transformed data. In case significant differences occurred, pairwise t-tests with parallel Monte Carlo tests were performed. Type III (partial) sum of squares was used with an unrestricted permutation of raw data (999 permutations). Unless mentioned otherwise, hard coral data consists of pooled replicates of the three investigated hard coral species (i.e., P. verrucosa, A. hemprichii, S. pistillata) of which mean rates and standard error of means were calculated.
Normality (Shapiro–Wilk test) and differences between benthic category coverage in both reef areas (e.g., hard coral cover in coral-dominated versus hard coral cover in algae-dominated areas) were tested using SigmaPlot (Version 12.0). Two-tailed t-tests were used if data were distributed normally whereas Mann–Whitney-Rank-sum tests were used if data were not normally distributed. Differences in the benthic composition among reef areas (i.e., coral vs. algae-dominated) were visualised using a principal coordinate analysis (PCO). Total fixed and removed N in both reef areas were calculated based on the sum of means of extrapolated rates/potentials of individual benthic categories and the respective standard propagated error, with Friedman’s aligned rank test checking for significant differences among respective reef areas (using R v4.0.4160 with the interface Rstudio v1.0.153161). A one-way analysis of similarities (ANOSIM; 999 permutations) was used to describe the dissimilarities between both reef areas. Furthermore, a distance-based linear model (DistLM; 999 permutations) using a step-wise selection procedure with AICc as a selection criterion was used to calculate which benthic category(ies) coverage explained visualised dissimilarities best158,159.
Limitations
Typical budget uncertainties include i) deviations from theoretical molar ratios (i.e., N2O:N2 and C2H4:N2) in different benthic categories (Wilson and others (2012) and references therein162), ii) methodological underestimation of N cycle processes74,163,164, iii) environmental alterations by benthic categories as an effect of benthic primary productivity119, iv) underestimations of N cycling rates for benthic categories that could not be assessed in their entirety such as reef sands, and v) reef-wide underestimations/omission of metabolic processes in cryptic habitats, such as cracks and crevices within the natural reef matrix, that harbour specific organisms (e.g., sponges, bryozoan, and tunicates). These organisms are generally not included in ex situ budget derivations. Nevertheless, the results of the present study are comparable to reefs of similar character and structural complexity. However, N cycling activity may vary substantially in reefs of differing structure, with more/fewer cracks and crevices in the reef matrix with inhabiting species that can remarkably contribute to metabolic processes in coral reefs165,166.
Furthermore, all specimens were sampled randomly from the overall reef area, thus, disregarding potential differences in the microbial communities of benthic categories between reef areas. These differences are likely to occur between coral- or algae-dominated reef areas119, and may influence N cycling processes. Potential effects of these differences, however, are rather insignificant as they would not allow for identification of differences in N cycling activities between benthic categories. Hence, we assume that shifts in the community composition are likely more relevant for the overall N fluxes than changes of N cycling activities within single benthic categories.
We, thus, consider the major results of the present study as solid and reliable, especially as the investigated benthic categories of investigated species cover > 98% in both reef areas. Nevertheless, we consider discussed and presented data as conservative estimates. Furthermore, aforementioned underestimation of N fluxes result from initial lag phases in the evolution of C2H460,74, and from potential incomplete blockages of the denitrification pathway163. Due to potential underestimations of both N2 fixation and denitrification, we, thus, refrained from directly comparing both pathways.
References
Galloway, J. N. et al. The nitrogen cascade. Bioscience 53, 341–356 (2003).
Mackenzie, F. T. Our Changing Planet: An Introduction to Earth System Science and Global Environmental Change (1998). https://downloads.globalchange.gov/ocp/ocp1998/ocp1998.pdf
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: How can it occur?. Biogeochemistry 13, 87–115 (1991).
Webb, K. L., DuPaul, W. D., Wiebe, W., Sottile, W. & Johannes, R. E. Enewetak (Eniwetok) Atoll: aspects of the nitrogen cycle on a coral reef. Limnol. Oceanogr. 20, 198–210 (1975).
Lesser, M. P. et al. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 346, 143–152 (2007).
Hoegh-Guldberg, O. Environmental and economic importance of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866 (1999).
Bell, P. R. F. Eutrophication and coral reefs-some examples in the Great Barrier Reef lagoon. Water Res. 26, 553–568 (1992).
Sorokin, Y. I. Microbiological Aspects of the Productivity of Coral Reefs. In Biology and Geology of Coral Reefs (eds. Jones, O. A. & Endean, R.) 17–46 (Academic press, Inc., 1973).
O’Neil, J. M. & Capone, D. G. Nitrogen Cycling in Coral Reef Environments. In Nitrogen in the Marine Environment 949–989 (2008). https://doi.org/10.1016/B978-0-12-372522-6.00021-9
Cardini, U. et al. Budget of primary production and dinitrogen fixation in a highly seasonal red sea coral reef. Ecosystems 19, 771–785 (2016).
Scheffers, S. R., Nieuwland, G., Bak, R. P. M. & Van Duyl, F. C. Removal of bacteria and nutrient dynamics within the coral reef framework of Curaçao (Netherlands Antilles). Coral Reefs 23, 413–422 (2004).
Rädecker, N., Pogoreutz, C., Voolstra, C. R., Wiedenmann, J. & Wild, C. Nitrogen cycling in corals: the key to understanding holobiont functioning?. Trends Microbiol. 23, 490–497 (2015).
Koop, K. et al. ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar. Pollut. Bull. 42, 91–120 (2001).
Capone, D. G., Dunham, S. E., Horrigan, S. G. & Duguay, L. E. Microbial nitrogen transformations in unconsolidated coral reef sediments. Mar. Ecol. Prog. Ser. 80, 75–88 (1992).
Hoffmann, F. et al. Complex nitrogen cycling in the sponge Geodia barretti. Environ. Microbiol. 11, 2228–2243 (2009).
Wiebe, W. J., Johannes, R. E. & Webb, K. L. Nitrogen fixation in a coral reef community. Science 188, 257–259 (1975).
Larkum, A. W. D., Kennedy, I. R. & Muller, W. J. Nitrogen fixation on a coral reef. Mar. Biol. 98, 143–155 (1988).
Kimes, N. E., Van Nostrand, J. D., Weil, E., Zhou, J. & Morris, P. J. Microbial functional structure of Montastraea faveolata, an important Caribbean reef-building coral, differs between healthy and yellow-band diseased colonies. Environ. Microbiol. 12, 541–556 (2010).
Yang, S., Sun, W., Zhang, F. & Li, Z. Phylogenetically diverse denitrifying and ammonia-oxidizing bacteria in corals Alcyonium gracillimum and Tubastraea coccinea. Mar. Biotechnol. 15, 540–551 (2013).
Tilstra, A. et al. Denitrification aligns with N2 fixation in red sea corals. Sci. Rep. 9, 19460 (2019).
El-Khaled, Y. et al. In situ eutrophication stimulates dinitrogen fixation, denitrification, and productivity in Red Sea coral reefs. Mar. Ecol. Prog. Ser. 645, 55–66 (2020).
O’Neil, J. M. & Capone, D. G. Nitrogen cycling in coral reef environments. Nitrog. Mar. Environ. https://doi.org/10.1016/B978-0-12-372522-6.00021-9 (2008).
Muscatine, L. & Porter, J. W. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460 (1977).
Wafar, M., Wafar, S. & David, J. J. Nitrification in reef corals. Limnol. Oceanogr. 35, 725–730 (1990).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting coral reef futures under global warming and ocean acidification. Science (80-) 333, 418–422 (2011).
Hughes, T. P. et al. Coral reefs in the anthropocene. Nature 546, 82–90 (2017).
Fabricius, K. E. Factors determining the resilience of coral reefs to eutrophication: a review and conceptual model. In Coral Reefs: An Ecosystem in Transition (eds. Dubinsky, Z. & Stambler, N.) 493–505 (2011). https://doi.org/10.1007/978-94-007-0114-4_28.
Bellwood, D. R., Hughes, T. P., Folke, C. & Nyström, M. Confronting the coral reef crisis. Nature 429, 827–833 (2004).
Lapointe, B. E., Brewton, R. A., Herren, L. W., Porter, J. W. & Hu, C. Nitrogen enrichment, altered stoichiometry, and coral reef decline at Looe Key, Florida Keys, USA: a 3-decade study. Marine Biology 166, (Springer, 2019).
Hughes, T. P. et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365 (2007).
Williams, I. D., Polunin, N. V. C. & Hendrick, V. J. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar. Ecol. Prog. Ser. 222, 187–196 (2001).
Mumby, P. J., Hastings, A. & Edwards, H. J. Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101 (2007).
Roth, F. et al. High rates of carbon and dinitrogen fixation suggest a critical role of benthic pioneer communities in the energy and nutrient dynamics of coral reefs. Funct. Ecol. https://doi.org/10.1111/1365-2435.13625 (2020).
Done, T. J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247, 121–132 (1992).
Hughes, T. P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science (80-) 265, 1547–1551 (1994).
McManus, J. W. & Polsenberg, J. F. Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog. Oceanogr. 60, 263–279 (2004).
Moberg, F. & Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233 (1999).
White, A. T., Vogt, H. P. & Arin, T. Philippine coral reefs under threat: the economic losses caused by reef destruction. Mar. Pollut. Bull. 40, 598–605 (2000).
McClanahan, T. R., Hicks, C. C. & Darling, E. S. Malthusian overfishing and efforts to overcome it on Kenyan coral reefs. Ecol. Appl. 18, 1516–1529 (2008).
Nyström, M. et al. Confronting feedbacks of degraded marine ecosystems. Ecosystems 15, 695–710 (2012).
Woodhead, A. J., Hicks, C. C., Norström, A. V., Williams, G. J. & Graham, N. A. J. Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 33, 1023–1034 (2019).
McClanahan, T., Polunin, N. & Done, T. Ecological states and the resilience of coral reefs. Conserv. Ecol. 6 (2), 18, (2002).
Munday, P. L. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Chang. Biol. 10, 1642–1647 (2004).
Williams, G. J. & Graham, N. A. J. Rethinking coral reef functional futures. Funct. Ecol. 33, 942–947 (2019).
Norström, A. V., Nyström, M., Lokrantz, J. & Folke, C. Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 293–306 (2009).
Brandl, S. J. et al. Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front. Ecol. Environ. 17, 445–454 (2019).
Roth, F. et al. High summer temperatures amplify functional differences between coral- and algae-dominated reef communities. Ecology https://doi.org/10.1002/ecy.3226 (2020).
Bednarz, V. N., Cardini, U., Van Hoytema, N., Al-Rshaidat, M. M. D. & Wild, C. Seasonal variation in dinitrogen fixation and oxygen fluxes associated with two dominant zooxanthellate soft corals from the northern Red Sea. Mar. Ecol. Prog. Ser. 519, 141–152 (2015).
Rix, L. et al. Seasonality in dinitrogen fixation and primary productivity by coral reef framework substrates from the northern Red Sea. Mar. Ecol. Prog. Ser. 533, 79–92 (2015).
den Haan, J. et al. Nitrogen fixation rates in algal turf communities of a degraded versus less degraded coral reef. Coral Reefs 33, 1003–1015 (2014).
Roth, F. et al. Coral reef degradation affects the potential for reef recovery after disturbance. Mar. Environ. Res. 142, 48–58 (2018).
Holmes, G. & Johnstone, R. W. The role of coral mortality in nitrogen dynamics on coral reefs. J. Exp. Mar. Biol. Ecol. 387, 1–8 (2010).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science (80-) 318, 1737–1742 (2007).
Van Hooidonk, R. et al. Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 6, 1–8 (2016).
Osborne, K. et al. Delayed coral recovery in a warming ocean. Glob. Chang. Biol. 23, 3869–3881 (2017).
Graham, N. A. J., Jennings, S., MacNeil, M. A., Mouillot, D. & Wilson, S. K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97 (2015).
Pogoreutz, C. et al. Sugar enrichment provides evidence for a role of nitrogen fixation in coral bleaching. Glob. Chang. Biol. 23, 3838–3848 (2017).
Bednarz, V. N. et al. Dinitrogen fixation and primary productivity by carbonate and silicate reef sand communities of the Northern Red Sea. Mar. Ecol. Prog. Ser. 527, 47–57 (2015).
Shashar, N., Feldstein, T., Cohen, Y. & Loya, Y. Nitrogen fixation (acetylene reduction) on a coral reef. Coral Reefs 13, 171–174 (1994).
Patriquin, D. G. & McClung, C. R. Nitrogen accretion, and the nature and possible significance of N2 fixation (acetylene reduction) in a Nova Scotian Spartina alterniflora Stand. Mar. Biol. 47, 227–242 (1978).
Shieh, W. Y. & Lin, Y. M. Nitrogen fixation (acetylene reduction) associated with the zoanthid Palythoa tuberculosa Esper. J. Exp. Mar. Biol. Ecol. 163, 31–41 (1992).
Bednarz, V. N. et al. Contrasting seasonal responses in dinitrogen fixation between shallow and deep-water colonies of the model coral Stylophora pistillata in the northern Red Sea. PLoS ONE 13, 1–13 (2018).
Schöttner, S. et al. Drivers of bacterial diversity dynamics in permeable carbonate and silicate coral reef sands from the Red Sea. Environ. Microbiol. 13, 1815–1826 (2011).
Zumft, W. G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 (1997).
Compaoré, J. & Stal, L. J. Effect of temperature on the sensitivity of nitrogenase to oxygen in two heterocystous cyanobacteria. J. Physcol. 46, 1172–1179 (2010).
Littler, M. & Littler, D. The nature of turf and boring algae and their interactions on reefs. Smithson. Contrib. to Mar. Sci. 213–217 (2013).
Rosenberg, G. & Ramus, J. Uptake of inorganic nitrogen and seaweed surface area: volume ratios. Aquat. Bot. 19, 65–72 (1984).
Fong, P., Rudnicki, R. & Zedler, J. B. Algal community response to nitrogen and phosphorus loading in experimental mesocosms: Management recommendations for southern California lagoons. (1987).
Fong, C. R., Gaynus, C. J. & Carpenter, R. C. Complex interactions among stressors evolve over time to drive shifts from short turfs to macroalgae on tropical reefs. Ecosphere 11(5), e03130 (2020).
Roth, F., Stuhldreier, I., Sánchez-Noguera, C., Morales-Ramírez, T. & Wild, C. Effects of simulated overfishing on the succession of benthic algae and invertebrates in an upwelling-influenced coral reef of Pacific Costa Rica. J. Exp. Mar. Bio. Ecol. 468, 55–66 (2015).
Stuhldreier, I., Bastian, P., Schönig, E. & Wild, C. Effects of simulated eutrophication and overfishing on algae and invertebrate settlement in a coral reef of Koh Phangan, Gulf of Thailand. Mar. Pollut. Bull. 92, 35–44 (2015).
Yamamuro, M., Kayanne, H. & Minagawa, M. Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol. Oceanogr. 40, 617–621 (1995).
Tilstra, A. et al. Seasonality affects dinitrogen fixation associated with two common macroalgae from a coral reef in the northern Red Sea. Mar. Ecol. Prog. Ser. 575, 69–80 (2017).
El-Khaled, Y. C. et al. Simultaneous measurements of dinitrogen fixation and denitrification associated with coral reef substrates: advantages and limitations of a combined acetylene assay. Front. Mar. Sci. 7, 411 (2020).
Davey, M., Holmes, G. & Johnstone, R. High rates of nitrogen fixation (acetylene reduction) on coral skeletons following bleaching mortality. Coral Reefs 27, 227–236 (2008).
Larkum, A. W. D. High rates of nitrogen fixation on coral skeletons after predation by the crown of thorns starfish Acanthaster planci. Mar. Biol. 97, 503–506 (1988).
Pogoreutz, C. et al. Nitrogen fixation aligns with nifH abundance and expression in two coral trophic functional groups. Front. Microbiol. 8, 1–7 (2017).
Arrigo, K. K. Marine microorganisms and global nutrient cycles. Nature 437, 349–355 (2004).
Mills, M. M., Ridame, C., Davey, M., La Roche, J. & Geider, R. J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294 (2004).
Redfield, A. C. The biological control of chemical factors in the environment. Am. Sci. 46, 205–221 (1958).
Porter, J. W., Muscatine, L., Dubinsky, Z. & Falkowski, P. G. Primary production and photoadaptation in light- and shade-adapted colonies of the symbiotic coral, stylophora pistillata. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 222, 161–180 (1984).
Veal, C. J., Holmes, G., Nunez, M., Hoegh-Guldberg, O. & Osborn, J. A comparative study of methods for surface area and three dimensional shape measurement of coral skeletons. Limnol. Oceanogr. Methods 8, 241–253 (2010).
Falkowski, P. P. G., Dubinsky, Z., Muscatine, L. & McCloskey, L. Population control in symbiotic corals. Bioscience 43, 606–611 (1993).
Eyre, B. D., Glud, R. N. & Patten, N. Mass coral spawning: a natural large-scale nutrient addition experiment. Limnol. Oceanogr. 53, 997–1013 (2008).
Tilstra et al. Relative abundance of nitrogen cycling microbes in coral holobionts reflects environmental nitrate availability, Royal Society Open Science, https://doi.org/10.1098/rsos.201835 (2021).
D’Angelo, C. & Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 7, 82–93 (2014).
Wiedenmann, J. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 3, 160–164 (2013).
Ferrier-Pagès, C., Godinot, C., D’Angelo, C., Wiedenmann, J. & Grover, R. Phosphorus metabolism of reef organisms with algal symbionts. Ecol. Monogr. 86, 262–277 (2016).
Muscatine, L., Falkowski, P. G., Porter, J. W. & Dubinsky, Z. Fate of photosynthetic fixed carbon in light- and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc. R. Soc. B Biol. Sci. 222, 181–202 (1984).
Conti-Jerpe, I. E. et al. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 6(15), eaaz5443 (2020).
Houlbrèque, F. & Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17 (2009).
Muscatine, L., Porter, J. W. & Kaplan, I. R. Resource partitioning by reef corals as determined from stable isotope composition. Pac. Sci. 48, 304–312 (1994).
Her, J.-J. & Huang, J.-S. Influences of carbon source and C/N ratio on nitrate/nitrite denitrification and carbon breakthrough. Bioresour. Technol. 54, 45–51 (1995).
Chen, S. et al. Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ. Microbiol. 20, 980–992 (2018).
Schlichter, D., Svoboda, A. & Kremer, B. P. Functional autotrophy of Heteroxenia fuscescens (Anthozoa: Alcyonaria): carbon assimilation and translocation of photosynthates from symbionts to host. Mar. Biol. 78, 29–38 (1983).
Babbin, A. R. et al. Discovery and quantification of anaerobic nitrogen metabolisms among oxygenated tropical stony corals. ISME J. https://doi.org/10.1038/s41396-020-00845-2 (2020).
Pupier, C. A. et al. Divergent capacity of scleractinian and soft corals to assimilate and transfer diazotrophically derived nitrogen to the reef environment. Front. Microbiol. 10, 1860 (2019).
Muscatine, L. The role of symbiotic algae in carbon and energy flux in coral reefs. In Coral Reefs (ed. Dubinsky, Z.) 75–87 (1990).
van Woesik, R., Irikawa, A., Anzai, R. & Nakamura, T. Effects of coral colony morphologies on mass transfer and susceptibility to thermal stress. Coral Reefs 31, 633–639 (2012).
Patterson, M. R. & Sebens, K. P. Forced convection modulates gas exchange in cnidarians. Proc. Natl. Acad. Sci. U. S. A. 86, 8833–8836 (1989).
Jones, C. G., Lawton, J. H. & Shachak, M. Organisms as ecosystem engineers. Oikos 69, 373 (1994).
Graham, N. A. J. & Nash, K. L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326 (2013).
Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (2003).
Graham, N. A. J. et al. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. U. S. A. 103, 8425–8429 (2006).
Sano, M., Shimizu, M. & Nose, Y. Long-term effects of destruction of hermatypic corals by Acanthaster plana infestation on reef fish communities at Iriomote Island, Japan. Mar. Ecol. Prog. Ser. 37, 191–199 (1987).
Lindahl, U., Öhman, M. C. & Schelten, C. K. The 1997/1998 mass mortality of corals: effects on fish communities on a Tanzanian coral reef. Mar. Pollut. Bull. 42, 127–131 (2001).
Jones, G. P., McCormick, M. I., Srinivasan, M. & Eagle, J. V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. U. S. A. 101, 8251–8253 (2004).
Idjadi, J. A. & Edmunds, P. J. Scleractinian corals as facilitators for other invertebrates on a Caribbean reef. Mar. Ecol. Prog. Ser. 319, 117–127 (2006).
Bracewell, S. A., Clark, G. F. & Johnston, E. L. Habitat complexity effects on diversity and abundance differ with latitude: an experimental study over 20 degrees. Ecology 99, 1964–1974 (2018).
Cinner, J. E. et al. Linking social and ecological systems to sustain coral reef fisheries. Curr. Biol. 19, 206–212 (2009).
Sheppard, C., Dixon, D. J., Gourlay, M., Sheppard, A. & Payet, R. Coral mortality increases wave energy reaching shores protected by reef flats: examples from the Seychelles. Estuar. Coast. Shelf Sci. 64, 223–234 (2005).
Karcher, D. B. et al. Nitrogen eutrophication particularly promotes turf algae in coral reefs of the central Red Sea. PeerJ 8, e8737 (2020).
Adey, W. H. & Goertemiller, T. Coral reef algal turfs: master producers in nutrient poor seas. Phycologia 26, 374–386 (1987).
Fong, P. & Paul, V. J. Coral reef algae. In Coral Reefs: An Ecosystem in Transition (eds. Dubinsky, Z. & Stambler, N.) 241–272 (Springer, 2011). https://doi.org/10.1007/978-94-007-0114-4_17.
Hoey, A. S. & Bellwood, D. R. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs?. Ecol. Lett. 14, 267–273 (2011).
Jessen, C. & Wild, C. Herbivory effects on benthic algal composition and growth on a coral reef flat in the Egyptian Red Sea. Mar. Ecol. Prog. Ser. 476, 9–21 (2013).
Haas, A. F. & Wild, C. Composition analysis of organic matter released by cosmopolitan coral reef-associated green algae. Aquat. Biol. 10, 131–138 (2010).
Roth et al. Nutrient pollution enhances productivity and framework dissolution in algae- but not in coral-dominated reef communities. Marine Pollution Bulletin. 168, 112444 (2021).
Haas, A. F. et al. Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ 2013, 1–28 (2013).
Roach, T. N. F. et al. A multiomic analysis of in situ coral-turf algal interactions. Proc. Natl. Acad. Sci. U. S. A. 117, 13588–13595 (2020).
van Oppen, M. J. H. & Blackall, L. L. Coral microbiome dynamics, functions and design in a changing world. Nat. Rev. Microbiol. 17, 557–567 (2019).
Liang, J. et al. Distinct bacterial communities associated with massive and branching scleractinian corals and potential linkages to coral susceptibility to thermal or cold stress. Front. Microbiol. 8, 1–10 (2017).
Fung, T., Seymour, R. M. & Johnson, C. R. Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92, 967–982 (2011).
Bruno, J. F., Sweatman, H., Precht, W. F., Selig, E. R. & Schutte, V. G. W. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90, 1478–1484 (2009).
Tilot, V., Leujak, W., Ormond, R. F. G., Ashworth, J. A. & Mabrouk, A. Monitoring of South Sinai coral reefs: influence of natural and anthropogenic factors. Aquat. Conserv. Mar. Freshw. Ecosyst. https://doi.org/10.1002/aqc.942 (2008).
Riegl, B. & Piller, W. E. Coral frameworks revisited-reefs and coral carpets in the northern Red Sea. Coral Reefs 18, 241–253 (1999).
Benayahu, Y., Jeng, M. S., Perkol-Finkel, S. & Dai, C. F. Soft corals (Octocorallia: Alcyonacea) from Southern Taiwan. II. Species diversity and distributional patterns. Zool. Stud. 43, 548–560 (2004).
Ninio, R., Meekan, M., Done, T. & Sweatman, H. Temporal patterns in coral assemblages on the Great Barrier Reef from local to large spatial scales. Mar. Ecol. Prog. Ser. 194, 65–74 (2000).
Fox, H. E., Pet, J. S., Dahuri, R. & Caldwell, R. L. Recovery in rubble fields: long-term impacts of blast fishing. Mar. Pollut. Bull. 46, 1024–1031 (2003).
Inoue, S., Kayanne, H., Yamamoto, S. & Kurihara, H. Spatial community shift from hard to soft corals in acidified water. Nat. Clim. Chang. 3, 683–687 (2013).
Rasser, M. W. & Riegl, B. Holocene coral reef rubble and its binding agents. Coral Reefs 21, 57–72 (2002).
Dalsgaard, T., Thamdrup, B. & Canfield, D. E. Anaerobic ammonium oxidation (anammox) in the marine environment. Res. Microbiol. 156, 457–464 (2005).
Brunner, B. et al. Nitrogen isotope effects induced by anammox bacteria. Proc. Natl. Acad. Sci. 110, 18994–18999 (2013).
Zhang, Y. et al. The functional gene composition and metabolic potential of coral-associated microbial communities. Sci. Rep. 5, 1–11 (2015).
Richter, C., Wunsch, M., Rasheed, M., Kötter, I. & Badran, M. I. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 413, 726–730 (2001).
Hill, J. & Wilkinson, C. Methods for ecological monitoring of coral reefs. Aust. Inst. Mar. Sci. Townsv. https://doi.org/10.1017/CBO9781107415324.004 (2004).
Kohler, K. E. & Gill, S. M. Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269 (2006).
Haas, A., El-Zibdah, M. & Wild, C. Seasonal monitoring of coral-algae interactions in fringing reefs of the Gulf of Aqaba, Northern Red Sea. Coral Reefs 29, 93–103 (2010).
Bahartan, K. et al. Macroalgae in the coral reefs of Eilat (Gulf of Aqaba, Red Sea) as a possible indicator of reef degradation. Mar. Pollut. Bull. 60, 759–764 (2010).
Voolstra, C. R. et al. Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Chang. Biol. 26, 4328–4343 (2020).
Hynes, R. K. & Knowles, R. Inhibition by acetylene of ammonia oxidation in Nitrosomonas europaea. FEMS Microbiol. Lett. 4, 319–321 (1978).
Oremland, R. S. & Capone, D. G. Use of ‘specific’ inhibitors in biogeochemistry and microbial ecology. Adv. Microb. Ecol. https://doi.org/10.1007/978-1-4684-5409-3_8 (1988).
Haines, J. R., Atlas, R. M., Griffiths, R. P. & Morita, R. Y. Denitrification and nitrogen fixation in Alaskan continental shelf sediments. Appl. Environ. Microbiol. 41, 412–421 (1981).
Joye, S. B. & Paerl, H. W. Contemporaneous nitrogen fixation and denitrification in intertidal microbial mats: rapid response to runoff events. Mar. Ecol. Prog. Ser. 94, 267–274 (1993).
Miyajima, T., Suzumura, M., Umezawa, Y. & Koike, I. Microbiological nitrogen transformation in carbonate sediments of a coral-reef lagoon and associated seagrass beds. Mar. Ecol. Prog. Ser. 217, 273–286 (2001).
Falkowski, P. G. Enzymology of Nitrogen Assimilation Nitrogen in the Marine Environment (Academic Press, 1983). https://doi.org/10.1016/b978-0-12-160280-2.50031-6.
den Haan, J. et al. Nitrogen and phosphorus uptake rates of different species from a coral reef community after a nutrient pulse. Sci. Rep. 6, 28821 (2016).
Grover, R., Maguer, J. F., Allemand, D. & Ferrier-Pagès, C. Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 48, 2266–2274 (2003).
Knapp, A. N. The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front. Microbiol. 3, 374 (2012).
Dilworth, M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim. Biophys. Acta 127, 285–294 (1966).
Schöllhorn, R. & Burris, R. H. Acetylene as a competitive inhibitor of N-2 fixation. Proc. Natl. Acad. Sci. U. S. A. 58, 213–216 (1967).
Balderston, W. L., Sherr, B. & Payne, W. J. Blockage by acetylene of nitrous-oxide reduction in pseudomonas-perfectomarinus. Appl. Environ. Microbiol. 31, 504–508 (1976).
Yoshinari, T. & Knowles, R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 69, 705–710 (1976).
Lavy, A. et al. A quick, easy and non-intrusive method for underwater volume and surface area evaluation of benthic organisms by 3D computer modelling. Methods Ecol. Evol. 6, 521–531 (2015).
Gutierrez-Heredia, L., Benzoni, F., Murphy, E. & Reynaud, E. G. End to end digitisation and analysis of three-dimensional coral models, from communities to corallites. PLoS ONE 11, e0149641 (2016).
Hughes, D. J. et al. Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Chang. 10, 296–307 (2020).
Mulholland, M. R., Bronk, D. A. & Capone, D. G. Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat. Microb. Ecol. 37, 85–94 (2004).
Clarke, K. R. & Gorley, R. N. PRIMER v6: Use manual/Tutorial. PRIMER-E:Plymouth (2006).
Anderson, M., Gorley, R. & Clarke, K. PERMANOVA+ for PRIMER. Guide to software and statistical methods. (2008).
R Core Team. R: A language and environment for statistical computing. (2017).
RStudio Team. RStudio: Integrated Development for R. (2020).
Wilson, S. T., Böttjer, D., Church, M. J. & Karl, D. M. Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic north pacific ocean. Appl. Environ. Microbiol. 78, 6516–6523 (2012).
Yu, K., Seo, D. C. & Delaune, R. D. Incomplete acetylene inhibition of nitrous oxide reduction in potential denitrification assay as revealed by using 15N-Nitrate tracer. Commun. Soil Sci. Plant Anal. 41, 2201–2210 (2010).
Groffman, P. M. et al. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 (2006).
Maldonado, M., Ribes, M. & van Duyl, F. C. Nutrient Fluxes Through Sponges. Biology, Budgets, and Ecological Implications. Advances in Marine Biology Vol. 62 (Elsevier Ltd., 2012).
Roth, F. et al. An in situ approach for measuring biogeochemical fluxes in structurally complex benthic communities. Methods Ecol. Evol. https://doi.org/10.1111/2041-210X.13151 (2019).
Acknowledgements
We are grateful to Rodrigo Villalobos and João Cúrdia for their support during fieldwork. Many thanks also to Carina Kitir, Söphiä Tobler and Jän Krause for their support in developing the figures, as well as to Najeh Kharbatia for technical support with GC. We thank the anonymous reviewers for their helpful comments and suggestions. This work was funded by the German Research Association (DFG Projects: Wi 2677/9-1 and Wi 2677/16-1) to CW and King Abdullah University of Science and Technology (KAUST) baseline funding to BHJ and CRV.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Y.E.K., C.W., C.R.V., and F.R. planned the research. Y.E.K., F.R., and D.B.K. participated in the collection of data. Y.E.K., N.R., and C.W. led data analysis. Y.E.K. wrote the manuscript with significant contributions of all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Khaled, Y.C., Roth, F., Rädecker, N. et al. Nitrogen fixation and denitrification activity differ between coral- and algae-dominated Red Sea reefs. Sci Rep 11, 11820 (2021). https://doi.org/10.1038/s41598-021-90204-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90204-8
This article is cited by
-
Sedimentary nitrogen dynamics in a coastal reef area with relatively high nitrogen concentration
Acta Oceanologica Sinica (2023)
-
The widely distributed soft coral Xenia umbellata exhibits high resistance against phosphate enrichment and temperature increase
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.