Abstract
Distinct patient characteristics have been proposed for ischaemic stroke in the anterior versus posterior circulation. However, data on functional outcome according to stroke territory in patients with acute stroke treatment are conflicting and information on outcome predictors is scarce. In this retrospective study, we analysed functional outcome in 517 patients with stroke and thrombolysis and/or thrombectomy treated at the University Hospital Zurich. We compared clinical factors and performed multivariate logistic regression analyses investigating the effect of outcome predictors according to stroke territory. Of the 517 patients included, 80 (15.5%) suffered a posterior circulation stroke (PCS). PCS patients were less often female (32.5% vs. 45.5%, p = 0.031), received thrombectomy less often (28.7% vs. 48.3%, p = 0.001), and had lower median admission NIHSS scores (5 vs. 10, p < 0.001) as well as a better median three months functional outcome (mRS 1 vs. 2, p = 0.010). Predictors for functional outcome were admission NIHSS (OR 0.864, 95% CI 0.790–0.944, p = 0.001) in PCS and age (OR 0.952, 95% CI 0.935–0.970, p < 0.001), known symptom onset (OR 1.869, 95% CI 1.111–3.144, p = 0.018) and admission NIHSS (OR 0.840, 95% CI 0.806–0.876, p < 0.001) in ACS. Acutely treated PCS and ACS patients differed in their baseline and treatment characteristics. We identified specific functional outcome predictors of thrombolysis and/or thrombectomy success for each stroke territory.
Similar content being viewed by others
Introduction
Stroke is the second leading cause of death worldwide1, with 80–87% of strokes being ischaemic2,3,4. Ischaemic strokes can be further specified according to their vascular territory as anterior (ACS, from internal carotid arteries) and posterior circulation ischaemic stroke (PCS, from vertebral arteries). About 20% of the cerebral blood flow is directed through the posterior cerebral circulation, resulting in approximately one fifth of ischaemic strokes being PCS2. Usually PCS and ACS are associated with clinically distinct symptoms5. This is intuitively clear since different brain regions are affected. However, in addition, several previous studies comparing PCS and ACS have proposed different patient characteristics regarding demographics, cardiovascular risk factors and stroke aetiology specific for the stroke territory5,6,7,8,9. Overall, patients suffering a posterior circulation ischaemic stroke tend to be younger6,7,8,10 and more often male compared to ACS patients5,6,7,8,11. Diabetes was more frequent in PCS, whereas atrial fibrillation was more common in ACS6,7,8,11. Stroke aetiologies have been reported to be unevenly distributed in PCS and ACS, though results vary a lot between studies5,8,9. The reasons for these differences remain elusive.
Acute ischaemic stroke can be treated with thrombolysis and thrombectomy to restore perfusion of the brain. Both methods are safe and effective in ACS12 as well as in PCS13,14,15,16. However, the success of both treatments depends on patient factors, stroke severity as well as time since symptom onset17. Yet, the National Institutes of Health Stroke Scale (NIHSS) which is commonly used to quantify stroke severity, incompletely depicts stroke symptoms of the posterior circulation18. Therefore, this scale may not be a suited tool to judge symptom severity or predict treatment success. The modified Rankin Scale (mRS) has been established to evaluate the degree of disability and functional outcome after stroke19,20. Comparisons of functional outcome after acute stroke treatment between PCS and ACS yielded conflicting results, with most studies proposing similar outcomes for both stroke territories5,13,14,21,22,23,24 and some finding a higher rate of disability in either PCS8 or ACS25. Since patients with PCS seem to be different from those with ACS, this implies that outcome may be determined by different factors for these vascular territories. However, information on specific outcome predictors according to stroke territory is scarce14,15,21.
The aim of this analysis was to search for different patient profiles in patients with PCS versus ACS subjected to thrombolysis and/or thrombectomy and therefore deemed significantly affected in an early time window. One goal was to identify specific predictors for the affected vascular territory by stroke. Additionally, we wanted to investigate if specific outcome predictors exist for the different vascular stroke territories.
Results
Patient characteristics
3594 patients with suspected stroke treated between 2014 and 2017 were screened from the Swiss Stroke Registry at the University Hospital of Zurich. Out of these patients, 583 fulfilled the inclusion criteria of ischaemic stroke treated with thrombolysis and/or thrombectomy and either written informed consent or no objection to data research according to the ethics protocol (KEH-ZH-Nr. 2014-0304). 21 patients were excluded due to missing information on three months mRS, another 26 patients due to concurrent stroke in both the posterior and anterior cerebral territory, and 19 patients due to stroke treatment after > 24 h (n = 13) or in-hospital events (n = 6). In the end, 517 patients were included in this data analysis. Out of the 517 patients, 80 (15.5%) had PCS and 437 (84.5%) had ACS.
Demographic data and information on the patients’ medical history are shown in Table 1. There was no difference in age between PCS and ACS patients (69.4 years, IQR 24 vs. 72.3 years, IQR 20, p = 0.187). The proportion of female patients was significantly lower in PCS compared to ACS (32.5% versus 45.5%, p = 0.031). PCS patients less often suffered from diabetes (5.0% vs. 14.4%, p = 0.021) and atrial fibrillation (16.3% vs. 29.7%, p = 0.013). Other cardiovascular risk factors including previous cardiovascular events as well as pre-stroke medication did not differ between the two groups.
Stroke aetiologies were similar in both patient groups. The type of acute stroke treatment was significantly different between PCS and ACS (p = 0.005). As expected, PCS patients less often received mechanical thrombectomy (28.7% vs. 48.3%, p = 0.001). Treatment times including onset-to-door time (ODT), onset-to-treatment time (OTT) or last-time-seen-normal-to-door and last-time-seen-normal-to-treatment time, for unknown symptom onset respectively, as well as the door-to-treatment time (DTT) were similar for both stroke territories except for patients with known symptom onset who were treated with intravenous thrombolysis. In this subgroup, PCS patients had a longer OTT compared to ACS patients (median time 151.5 min, IQR 98 vs. 128 min, IQR 68, p = 0.014) (Table 2).
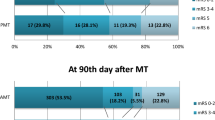
There were no differences regarding vital parameters on admission between PCS and ACS. PCS patients presented with a lower NIHSS on admission (median NIHSS 5, IQR 8 vs. 10, IQR 11, p < 0.001) and had a lower NIHSS after 24 h (median NIHSS 2, IQR 5 vs. 5, IQR 9, p < 0.001). The pre-stroke modified Rankin Scale was similar for both groups (median pre-mRS, 0 IQR 0 vs. 0 IQR 0, p = 0.974). PCS patients had a lower mRS at 90 days (median mRS90d 1, IQR 2 vs. 2, IQR 4, p = 0.010) and good outcome, defined as mRS90d ≤ 2, was more frequent among PCS patients (76.3% vs. 62.9%, p = 0.022). There was no difference in the duration of hospital stay or in the occurrence of complications within the 90 days follow-up period between the two stroke territories (Table 3).
Vascular results including occlusion or stenosis in the suspected ischaemic territory did not differ between PCS and ACS (Table 4).
Predictors for posterior versus anterior circulation stroke territory
We investigated the influence of age, sex, diabetes, atrial fibrillation, hypertension and prior stroke or TIA on the stroke territory to seek out potential predictors for PCS versus ACS. The factors were selected due to the observed differences in their occurrence in PCS vs. ACS in previous studies, their overall frequency in the population and their prognostic impact. In the multivariate binary logistic regression analysis, both diabetes (OR 0.349, 95% CI 0.122–0.994, p = 0.049) and atrial fibrillation (OR 0.496, 95% CI 0.263–0.936, p = 0.030) made PCS less likely than ACS (Table 5).
Predictors for good functional outcome in posterior and anterior circulation stroke
Age, sex, medical history of diabetes, atrial fibrillation, hypertension, dyslipidaemia, smoking, previous stroke or TIA, the admission NIHSS, knowledge of symptom onset and the onset-to-treatment time were examined for their predictive impact on good functional outcome defined as ≤ 2 on the mRS at 90 days after stroke, for each stroke territory respectively.
In the multivariate binary logistic regression analysis, the admission NIHSS remained the only predictor for functional outcome in PCS with a higher NIHSS making a good functional outcome less likely (OR 0.864, 95% CI 0.790–0.944, p = 0.001). For ACS, the multivariate binary logistic regression analysis showed that an increase in age (OR 0.952, 95% CI 0.935–0.970, p < 0.001) and an increase in the admission NIHSS (OR 0.840, 95% CI 0.806–0.876, p = 0.000) made a good functional outcome less likely. On the other hand, a known symptom onset was associated with a good functional outcome (OR 1.869, 95% CI 1.111–3.144, p = 0.018) (Table 6).
Discussion
Stroke location and affected vascular territory crucially determine clinical symptoms. However, in addition to these anatomical differences, our results support the hypothesis of different patient baseline characteristics for PCS versus ACS, even for acute stroke patients all selected for thrombolysis and/or thrombectomy. In our descriptive analysis, we found differences in patients with PCS versus ACS. Multivariate logistic regression analyses revealed that the presence of diabetes and atrial fibrillation made an ACS more likely than a PCS. Furthermore, specific predictors of favourable outcome were different in ACS (younger age, lower admission NIHSS, known symptom onset) and PCS (lower admission NIHSS).
In our cohort of patients with ischaemic stroke receiving acute stroke therapy, 15.5% of strokes occurred in the posterior cerebral circulation. This proportion is similar to observations in treatment-only cohorts13,15,22. PCS patients in our cohort were more often male, less often suffering from diabetes or atrial fibrillation, had lower admission NIHSS scores compared to ACS and less often underwent mechanical thrombectomy. Moreover, we observed a better three months functional outcome after acute stroke therapy in PCS. Our findings are in line with a recent data analysis from the SITS-MOST (Safe Implementation of Thrombolysis in Stroke Monitoring Study) registry, which had intracerebral haemorrhage (ICH) after intravenous thrombolysis as primary outcome, and found less ICH in patients with PCS strokes, along with a higher proportion of patients reaching functional independence (mRS 0–2) at 3 months16.
We could not confirm the observation of PCS patients being younger than ACS patients8,13,15,26. Similar to others, we found more men among patients suffering a PCS5,8,9,15,26,27. The reason for this sex discrepancy is unclear. Vavilala et al.’s findings of a lower autoregulation capacity in the basilar artery in teenage boys compared to girls might to some extent offer an explanation of the male preponderance in posterior circulation stroke28. Atrial fibrillation was previously found more frequently in patients with ACS5,8,9,15. On the other hand, the lower incidence of diabetes in PCS contrasts with previous observations which indicated an equal prevalence of diabetes in PCS and ACS5,13,15,21,22,26,27 or even a higher prevalence in PCS patients7,8,9,29. While we did not find any differences between PCS and ACS patients concerning pre-stroke medication, other studies suggest a greater use of antihypertensives5 and platelet inhibitors in ACS10,22. In our descriptive analysis, there were no statistically significant differences in stroke aetiology between the two stroke territories. Previous studies detected a higher incidence of cardiac stroke in ACS and small artery disease in PCS5,22,27. These discrepancies are likely due to different patient cohorts, since we considered only patients receiving acute stroke treatments, thus excluding very mild strokes.
The greater frequency of thrombectomy in ACS patients was expected. IAT is safe and effective in PCS and in ACS14. The longer onset to treatment time (OTT) in PCS patients with known symptom onset who received thrombolysis was related to the onset-to-door (ODT) rather than the door-to-treatment time (DTT). Several studies observed longer OTT in PCS compared to ACS receiving IVT14,15,22,26 and elongated ODT in PCS regardless of acute treatment or knowledge of symptom onset5,30. Possible explanations for the pre-hospital time delay in PCS are less obvious stroke symptoms (such as vertigo and double vision compared to hemiplegia or aphasia) and less severe neurological symptoms as judged from the lower admission NIHSS in PCS. However, even though a subgroup of PCS patients arrived later at the hospital, subsequently treated with longer OTT, there was no disadvantage regarding functional outcome at three months detectable in our analysis.
In line with our analysis, the admission NIHSS is often lower in PCS5,9,11,13,21,22,26,27, since this score is designed to assess ACS-induced deficits18. In our cohort, the pre-stroke mRS, which describes functional impairment, was equally low in PCS and ACS. Interestingly, the mRS after 90 days was lower in PCS, and functional independence defined as mRS ≤ 2 after 90 days was significantly more frequent in PCS patients (76.3% vs. 62.9%, p = 0.010). Complications within three months follow-up including recurrent strokes and symptomatic intracranial haemorrhage (ICH) occurred with similar frequencies in PCS and ACS.
We confirmed differences in patient characteristics and treatment variables between ACS and PCS. However, one major goal of our study was to explore variables that would favour the occurrence of ACS versus PCS, and subsequently, to find variables that would indicate 3-months disability as a measure of treatment success for either territory.
Some studies have reported that atrial fibrillation occurs more frequently in ACS, especially in women above the age of 80 years31, while the evidence regarding diabetes as well as further predictors for stroke territory—such as sex, cardiovascular risk factors and stroke mechanism—is conflicting5,9. We found that both diabetes and atrial fibrillation favoured the presence of ACS to PCS. There was no evidence that age, sex, hypertension and prior stroke or TIA had an effect on the presence of ACS vs. PCS.
In addition, predictors for 3-months disability were different for patients suffering ACS vs. PCS. The NIHSS on admission was the only predictor for functional outcome in PCS. In contrast, in ACS, in addition to the admission NIHSS, known symptom onset and age predicted outcome after stroke and acute stroke therapy. The correlation of the admission NIHSS with outcome in acutely treated patients has been described before for PCS14,32 as well as for ACS patients21,33. Many other factors have been proposed to influence outcome after stroke, but either no distinction was made between stroke territories13,24,29 or acutely treated and untreated patients were investigated as one entity8. In our analysis, we additionally considered sex, medical history of diabetes, hypertension, dyslipidaemia, smoking, previous stroke or TIA and onset to treatment time, which, however, showed no significant effect on functional outcome neither in ACS nor in PCS patients.
It is interesting that the admission NIHSS independently predicted outcome in PCS, although its validity for the assessment of stroke severity in PCS has been questioned34. We only considered patients with acute stroke treatment in our analysis. Therefore, the median admission NIHSS score may have been higher compared to non-treated or mixed cohorts, which could have influenced its predictive value for PCS patients. A known symptom onset facilitates a faster identification of stroke symptoms and prompt therapeutic interventions, ideally resulting in a better functional outcome. However, the OTT did not prove to be an independent outcome predictor in either PCS or ACS. For ACS, knowledge on symptom onset may have outweighed the influence of the OTT on the mRS in multivariate regression analysis. For PCS, the OTT has been shown to be less crucial in thrombolysis35 which might explain the lack of influence of known symptom onset and OTT on functional outcome in PCS patients.
Our findings imply that differences in patient characteristics between PCS and ACS exist in stroke patients that qualify for acute stroke treatment. Fortunately, the familiar issue of elongated time intervals in the workup of PCS was not very pronounced in our analysis. Although the NIHSS might not entirely capture stroke severity in PCS, its assessment might have prognostic value. Against our expectations and in contrast to Dornak et al.’s findings of a negative correlation of age and outcome in thrombolysed PCS patients15, in our analysis older age indicated worse outcome in ACS patients, while age did not correlate with functional outcome in PCS. While an existing influence could be concealed by the limited number of patients, this observation should be considered in assessing the eligibility for acute stroke treatment for PCS. In our analysis, we only considered ischaemic stroke patients receiving acute stroke treatment to evaluate outcome of these treatments in patients with PCS versus ACS. We did not compare treated with untreated patient groups, as our goal was not to reaffirm the efficacy of thrombolysis and thrombectomy in ACS and PCS12,13,14,15,16.
One limitation of this analysis is its relatively small number of patients, especially in the subgroup of PCS. This might have contributed to the fact that less outcome predictors were identified in multivariate regression analysis for PCS compared to ACS. In addition, our analysis only included patients from a single centre and the data analysis was performed retrospectively. The strength of our analysis lies in the large number of variables that were compared between the two patient groups, including pre- and post-stroke as well as treatment-specific factors.
Overall, our analysis of acutely treated stroke patients supports the hypothesis of different patient profiles for posterior versus anterior circulation strokes. We identified predictors for the stroke territory as well as predictors for functional outcome for each vascular territory respectively. Knowledge about specific characteristics of patients with ACS and PCS including outcome predictors of thrombolysis and thrombectomy in these patients may aid in improving treatment decisions in acute stroke.
Methods
Patients
This retrospective data analysis contains patient data from the Swiss Stroke Registry of the University Hospital Zurich (USZ) from 2014 until the end of 2017. The Swiss Stroke Registry enlists every patient presenting with suspected stroke (< 7 days before admission) at the USZ. Data were collected by the treating physician. Stroke treatments at the USZ Stroke Center were performed according to the current guidelines of the European Stroke Organization36. Accordingly, intravenous thrombolysis was performed within 4.5 h of symptom onset, and thrombectomy within 6 h of symptom onset in patients without contraindications. In some patients, treatment windows were extended based on advanced imaging such as small core and/or mismatch. All data was reviewed from the electronic patient files of the clinical information system and corrected if missing or inconclusive. Inclusion criteria were ischaemic stroke and acute stroke therapy with intravenous thrombolysis or intra-arterial therapy, i.e. mechanical thrombectomy. Haemorrhagic strokes and transient ischaemic attacks were excluded. Patients with onset-to-treatment times greater 24 h, in-hospital events and patients with missing information on three months functional outcome were excluded. Consent to research was obtained as written informed consent from all participants and/or their legal guardians or waived according to the ethics protocol KEH-ZH-Nr. 2014-0304 “Bildgebungsprädikatoren für die Erholung nach Schlaganfall (PREDICT)”, approved by the ethics committee Kantonale Ethikkommission Kanton Zürich (KEK ZH); and all methods were carried out in accordance with relevant guidelines and regulations.
Patients were divided into two groups according to their stroke territory in posterior (PCS) versus anterior circulation ischaemic stroke (ACS) by clinical and radiological findings. ACS was defined as a stroke in the territory supplied by the anterior choroidal arteries, the medial cerebral arteries and the anterior cerebral arteries together with their supplying vascular territory. PCS was likewise defined as a stroke in the vascular territory of the vertebral arteries, the basilar artery and the posterior cerebral arteries. To obtain homogenous comparable groups, patients with concurrent posterior and anterior circulation strokes were excluded. All patients received acute stroke therapy by either intravenous thrombolysis (IVT) or intra-arterial therapy (IAT) or a combination of both treatments. Stroke severity was assessed by the National Institutes of Health Stroke Scale (NIHSS) which captures the degree of neurological deficits in acute ischaemic stroke patients, ranging from 0—no neurological deficits—to a total of 42 points37. In the Swiss Stroke Registry, the NIHSS is assessed on admission and after 24 h. Both pre-stroke disability and functional outcome were quantified by the modified Rankin Scale (mRS)38 which assesses functional independence, ranging from 0—no constraint—to 6—death. In the Swiss Stroke Registry, the mRS is assessed retrospectively on admission and three months after stroke. A mRS ≤ 2 represents functional independence. Stroke aetiology was assessed according to ASTRAL, the Acute STroke Registry and Analysis of Lausanne5, which is based on the TOAST classification39.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 25. The two patient groups ACS and PCS were compared regarding demographic and clinical characteristics. Continuous variables are shown as median and interquartile range. Nominal variables are expressed as fractions and percentages. The Mann–Whitney U test for two independent samples was performed to compare continuous variables between the two groups. The Chi-squared test and the Fisher’s Exact test (for expected values less than five) were conducted to compare nominal variables. Binary logistic regression analyses were performed including variables in a stepwise backward manner to identify predictors for posterior versus anterior circulation stroke territory and to investigate predictors for functional outcome (mRS 90d) for each stroke territory. The mRS was dichotomized and good outcome was defined as a score of ≤ 2 on the mRS at 90 days. Results are expressed as odds ratios and the 95% confidence interval (CI) of the odds ratios. A two-sided significance level of alpha = 0.05 was considered. Data are shown in tables.
Data availability
All data generated or analysed during this study are included in this published article. The datasets generated during and/or analysed during the current study are available in anonymized form from the corresponding author upon reasonable request.
References
Feigin, V. L. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16, 877–897. https://doi.org/10.1016/s1474-4422(17)30299-5 (2017).
Savitz, S. I. & Caplan, L. R. Vertebrobasilar disease. N. Engl. J. Med. 352, 2618–2626. https://doi.org/10.1056/NEJMra041544 (2005).
Heuschmann, P. U. et al. Incidence of stroke in Europe at the beginning of the 21st century. Stroke 40, 1557–1563. https://doi.org/10.1161/strokeaha.108.535088 (2009).
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137, e67–e492. https://doi.org/10.1161/cir.0000000000000558 (2018).
Zürcher, E., Richoz, B., Faouzi, M. & Michel, P. Differences in ischemic anterior and posterior circulation strokes: A clinico-radiological and outcome analysis. J. Stroke Cerebrovasc. Dis. 28, 710–718. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.11.016 (2019).
Subramanian, G. et al. Risk factors for posterior compared to anterior ischemic stroke: An observational study of the Registry of the Canadian Stroke Network. Neuroepidemiology 33, 12–16. https://doi.org/10.1159/000209282 (2009).
Miyamoto, N. et al. Comparison of clinical backgrounds with anterior versus posterior circulation infarcts. J. Stroke Cerebrovasc. Dis. 19, 393–397. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.07.012 (2010).
Kim, J. T. et al. Clinical outcomes of posterior versus anterior circulation infarction with low national institutes of health stroke scale scores. Stroke 48, 55–62. https://doi.org/10.1161/strokeaha.116.013432 (2017).
Zeng, Q., Tao, W., Lei, C., Dong, W. & Liu, M. Etiology and risk factors of posterior circulation infarction compared with anterior circulation infarction. J. Stroke Cerebrovasc. Dis. 24, 1614–1620. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.03.033 (2015).
Puustjarvi, V. et al. Recognition of posterior circulation stroke. Acta Neurol. Scand. 131, 389–393. https://doi.org/10.1111/ane.12351 (2015).
Tao, W. D. et al. Posterior versus anterior circulation infarction: How different are the neurological deficits?. Stroke 43, 2060–2065. https://doi.org/10.1161/strokeaha.112.652420 (2012).
Goyal, M. et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731. https://doi.org/10.1016/s0140-6736(16)00163-x (2016).
Sarikaya, H. et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke 42, 2498–2502. https://doi.org/10.1161/strokeaha.110.607614 (2011).
Weber, R. et al. Thrombectomy in posterior circulation stroke: Differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur. J. Neurol. 26, 299–305. https://doi.org/10.1111/ene.13809 (2019).
Dornak, T. et al. Posterior vs. anterior circulation infarction: Demography, outcomes, and frequency of hemorrhage after thrombolysis. Int. J. Stroke 10, 1224–1228. https://doi.org/10.1111/ijs.12626 (2015).
Keselman, B. et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: Results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke https://doi.org/10.1161/strokeaha.119.027071 (2020).
Rabinstein, A. A. Treatment of acute ischemic stroke. Continuum (Minneap Minn) 23, 62–81. https://doi.org/10.1212/con.0000000000000420 (2017).
Kasner, S. E. Clinical interpretation and use of stroke scales. Lancet Neurol. 5, 603–612 (2006).
van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J. & van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19, 604–607. https://doi.org/10.1161/01.str.19.5.604 (1988).
Eriksson, M., Norrving, B., Terént, A. & Stegmayr, B. Functional outcome 3 months after stroke predicts long-term survival. Cerebrovasc. Dis. 25, 423–429. https://doi.org/10.1159/000121343 (2008).
De Marchis, G. M. et al. Posterior versus anterior circulation strokes: Comparison of clinical, radiological and outcome characteristics. J. Neurol. Neurosurg. Psychiatry 82, 33–37. https://doi.org/10.1136/jnnp.2010.211151 (2011).
Förster, A. et al. Thrombolysis in posterior circulation stroke: Stroke subtypes and patterns, complications and outcome. Cerebrovasc Dis 32, 349–353. https://doi.org/10.1159/000330346 (2011).
Wollenweber, F. A. et al. Functional outcome following stroke thrombectomy in clinical practice. Stroke 50, 2500–2506. https://doi.org/10.1161/strokeaha.119.026005 (2019).
Sommer, P. et al. Is functional outcome different in posterior and anterior circulation stroke?. Stroke 49, 2728–2732. https://doi.org/10.1161/strokeaha.118.021785 (2018).
Tong, X. et al. Intravenous thrombolysis is more safe and effective for posterior circulation stroke: Data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China). Medicine (Baltimore) 95, e3848. https://doi.org/10.1097/md.0000000000003848 (2016).
Keselman, B. et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: Results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke 51, 876–882. https://doi.org/10.1161/STROKEAHA.119.027071 (2020).
von Sarnowski, B. et al. Posterior versus anterior circulation stroke in young adults: A comparative study of stroke aetiologies and risk factors in stroke among young Fabry patients (sifap1). Cerebrovasc. Dis. 43, 152–160. https://doi.org/10.1159/000454840 (2017).
Vavilala, M. S. et al. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr. Res. 58, 574–578. https://doi.org/10.1203/01.pdr.0000179405.30737.0f (2005).
Tao, W. D. et al. One-year case fatality and disability after posterior circulation infarction in a Chinese hospital-based stroke study. Cerebrovasc. Dis. 29, 376–381. https://doi.org/10.1159/000281836 (2010).
Sommer, P. et al. Prehospital and intra-hospital time delays in posterior circulation stroke: Results from the Austrian Stroke Unit Registry. J. Neurol. 264, 131–138. https://doi.org/10.1007/s00415-016-8330-x (2017).
Marini, C. et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke 36, 1115–1119. https://doi.org/10.1161/01.STR.0000166053.83476.4a (2005).
Huang, Q., Song, H. Q., Ma, Q. F., Song, X. W. & Wu, J. Effects of time delays on the therapeutic outcomes of intravenous thrombolysis for acute ischemic stroke in the posterior circulation: An observational study. Brain Behav. 9, e01189. https://doi.org/10.1002/brb3.1189 (2019).
Yoon, W., Kim, S. K., Park, M. S., Baek, B. H. & Lee, Y. Y. Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J. Stroke 19, 97–103. https://doi.org/10.5853/jos.2016.00675 (2017).
Siniscalchi, A., Sztajzel, R., Malferrari, G. & Gallelli, L. The National Institutes of Health Stroke Scale: Its role in patients with posterior circulation stroke. Hosp. Top. 95, 79–81. https://doi.org/10.1080/00185868.2017.1322888 (2017).
Dornak, T., Kral, M., Sanak, D. & Kanovsky, P. Intravenous thrombolysis in posterior circulation stroke. Front. Neurol. 10, 417. https://doi.org/10.3389/fneur.2019.00417 (2019).
Ringelstein, E. B. et al. European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke 44, 828–840. https://doi.org/10.1161/STROKEAHA.112.670430 (2013).
Brott, T. et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 20, 864–870. https://doi.org/10.1161/01.str.20.7.864 (1989).
Broderick, J. P., Adeoye, O. & Elm, J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke 48, 2007–2012. https://doi.org/10.1161/strokeaha.117.017866 (2017).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24, 35–41 (1993).
Funding
Funding was provided by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Grant no. PP00P3_170683) and Universität Zürich (Grant no. CRPP stroke).
Author information
Authors and Affiliations
Contributions
H.H. prepared the first draft of the manuscript and performed patient clinical data analysis. L.H. suggested the statistical design and gave critical input into statistical analyses. L.C. and A.R.L. participated in patient enrollment and data collection. S.W. conceived the study, contributed to data analysis and drafting of the manuscript. All authors critically reviewed the paper and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Handelsmann, H., Herzog, L., Kulcsar, Z. et al. Predictors for affected stroke territory and outcome of acute stroke treatments are different for posterior versus anterior circulation stroke. Sci Rep 11, 10544 (2021). https://doi.org/10.1038/s41598-021-89871-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89871-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.