Abstract
Limited data are available, linking the plant-based diets to breast cancer (BC). We examined the association of overall plant-based diet index (PDI), hypothesized healthful (hPDI) and unhealthful versions of a plant-based diet index (uPDI) with BC in Iranian women. This population-based case–control study included 350 cases with newly diagnosed BC and 700 age-matched apparently healthy controls. We collected dietary data using a validated, Willett-format semi-quantitative food frequency questionnaire. Using these data, we generated a PDI by dedicating positive scores to plant foods, and reverse scores to animal foods, hPDI by assigning positive scores to healthy plant foods and reverse scores to less healthy plant foods and animal foods, and finally uPDI in which positive scores were assigned to less healthy plant foods and reverse scores to healthy plant foods and animal foods. After controlling for potential confounders, individuals in the highest quartile of PDI had 67% lower odds of BC than those in the lowest quartile (OR 0.33; 95% CI 0.22–0.50). Individuals with the greatest adherence to hPDI were 36% less likely to have BC than those with the lowest adherence, in the fully adjusted model (OR 0.64; 95% CI 0.43–0.94). In terms of uPDI, women in the top quartile had a 2.23 times greater chance of BC than those in the bottom quartile (OR 2.23; 95% CI 1.48–3.36). Greater adherence to PDI and hPDI was inversely associated with the risk of BC, whereas uPDI was associated with an increased risk.
Similar content being viewed by others
Introduction
Breast cancer (BC) ranks as the second leading cause of cancer mortality worldwide1. The World Health Organization reports an upward trend of 1.8–2.0% for this cancer each year across the globe2. According to global statistics, annually, nearly 1.4 million new cases of BC are detected, with a mortality rate of about 450,000 worldwide3. In Iran, based on the report of the National Cancer Registry (NCR), BC has accounted for 24.4% of various types of cancers with age-standardized rate (ASR) of 23.1 per 100,0004. Modifiable lifestyle factors might explain the geographic difference in BC’s incidence rate5, highlighting the importance of preventive approaches as promising measures to control the disease.
Heredity factors can explain less than 10% of BC etiology, while environmental, reproductive, and lifestyle factors are the main drivers of this malignancy6. Mounting evidence from epidemiological studies documented the effectiveness of lifestyle modifications, particularly diet on the risk of BC7. The majority of evidence about diet–BC associations have focused on assessing individual nutrients, food items, or food groups8,9,10. It must be noted that dietary constituents represent complex inter-relationships, and through applying defined dietary patterns, which can potentially capture the joint effect of dietary ingredients, we can rule out co-linearity drawbacks11,12,13. In this context, adherence to prudent dietary pattern7, Mediterranean diet14,15 and dietary approaches to stop hypertension eating pattern16 has been linked with documented benefits on BC risk. On the other hand, the Western dietary pattern7 has been positively associated with the risk.
Plant-based diets are a diverse family of dietary patterns defined as infrequent consumption of animal foods along with frequent intake of plant-based foods in usual diet. Vegetarian diets are a subset of plant-based diets, in which some or all animal foods have been eliminated entirely17. In the clinical setting, different quality of plant-based diets yielded different outcomes with respect to diet–disease relationships18. This highlighted the need to develop a continuous scoring system as a rigorous approach for evaluation of adherence to such dietary patterns19. Based on this scoring method, an overall plant-based diet index (PDI), reflecting consumption of all plant foods, was developed. In addition, a healthy plant-based diet index (hPDI), representing intake of high-quality plant foods with documented benefit on clinical outcomes, and an unhealthy plant-based diet index (uPDI), demonstrating the consumption of less nutritive plant foods with a detrimental impact on the risk of chronic conditions, were also suggested18. Earlier studies examining the link between these dietary patterns and the risk of chronic conditions have provided evidence indicating the predictive value of these eating habits in several conditions, including non-alcoholic fatty liver disease20, psychological disorder21, gestational diabetes mellitus22 and cancer23.
Despite several studies originating from Western nations that assessed PDI in relation to the risk of BC24,25, we are aware of no report in Middle-Eastern countries in this regard. This assessment in the under-studied region is of fundamental importance owing to having a traditional diet characterized by a high proportion of refined grains and detrimental fats1, and lower consumption of fruits, vegetables, and whole grains26. Considering the documented association between individual plant-based foods and breast malignancy in earlier studies8,27,28,29,30,31,32,33, it seems that the whole plant-based diet might be associated with BC. Iranian diet, which is mainly composed of plant-based foods, provides a unique opportunity to look at this association closely. Given the aforementioned points, this study was undertaken to assess the association between PDI, hPDI, and uPDI and the risk of BC in Iranian women.
Methods and participants
Study population
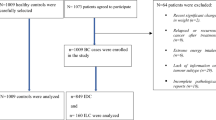
This population-based case–control study was carried out on women aged > 30 years in Isfahan, Iran, between July 2013 and July 2015. Cases were selected using a convenience sampling method from those who were referred to hospitals or private clinics. Their disease was confirmed based on physical examination, mammography, and pathological verification. Required sample size for the current work was calculated based on the hypothesis of 1.5 times increased odds of BC by unhealthy dietary pattern. Therefore, considering type I error of 5%, the study power of 80%, the common ratio of 0.25, and the ratio of controls to cases as 2, we needed 350 cases and 700 controls for this project. Patients with BC, who had a prior history of surgical resection or chemotherapy or radiotherapy or all of them, were admitted to participate in the study. BC was identified by primary incident malignant breast tumors with invasive nature in medical records. Patients with a history of any type of neoplastic lesion or cysts (except BC) and those with a prior history of any hormone replacement therapy were not included in our work. Similarly, those who were on special diets during the year before the interview were not qualified. Controls were selected randomly from healthy women. Cases and controls were matched in terms of age (± 5 years) and socioeconomic status. Inclusion criteria for control subjects were as follows: (a) being female, (b) having Iranian nationality, (c) lacking a history of any cancer, cysts, and pathological disease. The exclusion criteria for controls included adherence to special diets and having a history of hormone replacement therapy. Finally, 350 cases and 700 controls were qualified to participate in our study. Informed written consent was taken from all case and control subjects after making them aware of the study methodology. Ethical Committee of Tehran University of Medical Sciences approved the study protocol.
Dietary intake assessment
Details on dietary intake assessment have been previously reported16. Briefly, to assess habitual dietary intakes (over the past year) of study participants, a 106-item Willett-format semi-quantitative dish-based food frequency questionnaire (FFQ) which was designed specifically for Iranian adults, was applied. This questionnaire included commonly-used food items and dishes in Iranian culture. Although, a classic validation study has not been conducted for this FFQ so far, several established relationships between dietary factors and several diseases using this questionnaire have been reported34,35,36,37. Such findings can be interpreted as qualitative support for the validity of the questionnaire38. A trained nutritionist conducted face-to-face interviews with participants to inquire about the average consumption frequency of foods and dishes in the FFQ. The questionnaire encompassed five sets of foods and dishes, including (1) mixed dishes (cooked or canned, 29 items); (2) carbohydrate-based foods (different sorts of bread, cakes, biscuits, and potato, 10 items); (3) milk-derived products (dairies, butter, and cream, 9 items); (4) fruits and vegetables (22 items); and (5) accessory food items and beverages (including sweets, fast foods, nuts, desserts and beverages, 36 items). Participants specified their frequency consumption for each food item and mixed dishes considering nine multiple-choice frequency response categories, ranging from “never or less than once a month” to “12 or more times per day”. In this questionnaire, for seldom-used foods, the number of multiple-choice options reduced, whereas, for frequently-used foods, more multiple-choice categories were assigned. The daily value for each item was estimated based on food composition, an average of reported frequency, and given standard size units. A trained group of dietitians used nutritionist IV software (modified for Iranian foods) to derive the average daily intake of energy and nutrients.
Construction of plant-based dietary scores
We categorized plant foods into the healthy and less healthy groups based on epidemiological knowledge concerning the relationship between food items with several chronic conditions (type 2 diabetes, cardiovascular disease, and certain cancers) along with intermediate conditions (obesity, hypertension or inflammation)18. We generated 16 food groups (belonged to the whole collection of animal foods, healthy and less healthy plant foods) according to nutrient and culinary standard features. After accounting for daily values for each food item, the number of servings for entire foods incorporated in each of the 16 food groups were summed up. In our project, we generated an overall plant-based diet index (PDI) according to the algorithm developed by Martinez-Gonzalez et al.19 and two fitted versions of a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI), as suggested by Satija et al.18. Whole grains, fruits, vegetables, nuts, legumes, and vegetable oils were classified in the category of healthy plant food groups; fruit juices, sugar-sweetened beverages, refined grains, and potatoes were located in less healthy plant food groups; animal food groups encompassed the wide range of animal fats, dairy, eggs, fish/seafood, meat (poultry and red meat) and miscellaneous animal-based foods. The cut points for the quintiles were calculated for each 16 food groups with assigned score between 1 and 5 for each quintile. Regarding PDI, the score of 5 was dedicated for each plant food groups which were above the highest quintile of consumption, a score of 4 was assigned to each plant food groups which were above the second-highest quintile and below the highest quintile, and so on, at last participants received a score of 1 for each plant food groups for which they were below the lowest quintile of consumption (positive scores). In contrast, a score of 1 was allocated to each animal food groups upper the highest quintile of consumption, a score of 2 was dedicated to each animal food groups incorporated between the highest and second highest quintiles, and so on, at last a score of 5 for consumption under the bottom quintile (reverse scores). To calculate hPDI, scores of 5 and 1 were given to subjects with the highest and lowest consumption of healthy plant foods, respectively. A score of 1 for the highest consumption and 5 for the lowest consumption of unhealthy plant foods and animal food items was also given. To calculate uPDI, a score between 5 and 1 was given to the highest through the lowest consumption of unhealthy plant foods. Furthermore, subjects with the highest to lowest consumption of animal foods and healthy plant foods were given a score between 1 and 5. To achieve each participant’s indices, we added up 16 food group scores. Though we theoretically reached the range of 16 (as the lowest possible score) to 80 (as the highest possible score) for these indices, in practice the observed score ranges for PDI, hPDI, and uPDI, were 21–74, 26–77, and 31–78, respectively, across the groups. It is worth mentioning that a higher amount of all indices is indicative of a lower intake of animal foods. Alcoholic beverages were not included in our indices due to their diverse association with several health outcomes.
Assessment of breast cancer
Diagnosis of BC was made by physical examination and mammography; pathological assessments determined final confirmation of BC. Only females with newly diagnosed (maximum 6 months since diagnosis) BC (stage I–IV) with Iranian nationality were recruited into this study.
Assessment of covariates
A general information pretested questionnaire including several variables of sociodemographic status (age, marital status, residential place, and education), alcohol consumption, smoking, menopausal status, disease history, family history of BC, history of breastfeeding and supplement use was administered to collect general information of subjects. A short form of the International Physical Activity Questionnaire (IPAQ) was applied through face-to-face interviews. The acquired information from the IPAQ were stated as Metabolic Equivalent-hours per week (METs/week). Anthropometric measures were assessed, and participants’ BMI was calculated accordingly.
Statistical analysis
We classified all subjects based on the PDI, hPDI and uPDI scores into quartile ranges. The Chi-square test was applied to assess the distribution of study participants in terms of general characteristics between cases and controls. To compare the means of continuous variables, including dietary intakes between cases and controls, one-way analysis of variance (ANOVA) was used. Comparisons across quartiles of PDI, hPDI, and uPDI were performed using one-way ANOVA. We used multivariable logistic regression to investigate the association of PDI, hPDI, and uPDI with BC. We reached the ORs and 95% CIs using three different models: i.e., Model 1: Adjusted for age and energy intake, Model 2: Adjusted for education, social-economic status, residential area, supplement use, family history of BC, disease history, physical activity, marital status, smoking status, alcohol consumption, history of breast-feeding and menopausal status, Model 3: Adjusted for body mass index(BMI). Selection of covariates was done according to the previous studies on BC that have introduced contributing factors to this condition39,40,41,42,43,44. All the statistical analyses were performed using SPSS (SPSS Inc., version 19). Significant results were characterized, considering two-sided p < 0.05.
Ethical approval
All procedures performed in our study that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Overall, 1050 participants with a mean age of 62.4 years were included. Baseline characteristics and dietary intakes of study participants separately by cases and controls are provided in Table 1. Women with BC were more likely to be older, post menopause, and have a family history of BC than control subjects. Moreover, they had lower BMI and were less likely to be married and have higher educational levels than those without BC. In terms of dietary intakes, they were more likely to have higher intakes of total energy, carbohydrate, total fat, cholesterol, SFA, MUFA, vitamin B6, calcium, magnesium, potassium, fruits, sugar-sweetened beverages, sweet dessert, animal fats, tea and coffee as well as lower intakes of PUFA, whole grains, vegetables, nuts, legumes, vegetable oils, fruit juices, potato, and meats compared with controls.
General characteristics and dietary intakes of participants according to quartile categories of PDI, hPDI and uPDI are described in Tables 2 and 3, respectively. Higher PDI score was associated with greater BMI, higher educational level, and younger age. Also, a lower percentage of subjects in the highest category of PDI had disease history than those in the lowest category. In addition, the highest PDI was accompanied by higher intakes of fruits, vegetables, nuts, legumes, vegetable oils, tea and coffee, refined grains, sugar-sweetened beverages, fruit juices, potato, sweet dessert, and a lower intake of total energy, carbohydrate, protein, total fat, cholesterol, SFA, MUFA, thiamine, vitamin B6, vitamin B12, calcium, magnesium, potassium, animal fats, dairy, eggs, fish/seafood, and meat. Compared with subjects in the lowest quartile, women in the top quartile of hPDI were more likely to be alcohol users and less likely to be older. They also had higher intakes of carbohydrates, dietary fiber, PUFA, thiamine, vitamin B6, folate, magnesium, potassium, whole grains, fruits, vegetables, nuts, legumes, vegetable oils, tea, and coffee and lower intakes of total energy, total fat, cholesterol, SFA, MUFA, vitamin B12, calcium, refined grains, sugar-sweetened beverages, potato, animal fats, dairy, eggs, fish/seafood, and meats. In terms of uPDI, a greater percentage of subjects in the highest category of uPDI were married and had low socioeconomic status than those in the lowest category. Higher uPDI was associated with lower BMI. Individuals in the highest category of uPDI were less likely to be university graduated, alcohol users, and live in urban areas than those in the lowest category. Moreover, they consumed higher amounts of total energy, carbohydrates, proteins, total fat, dietary fiber, SFA, MUFA, thiamine, vitamin B6, folate, calcium, magnesium, potassium, whole grains, tea and coffee, sugar-sweetened beverages, potato, sweet dessert and lower amounts of fruits, vegetables, nuts, legumes, vegetable oils, fruit juices, dairy, eggs, fish/seafood and meats than those with the lowest adherence to this index.
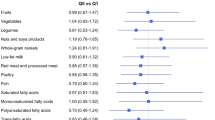
Table 4 outlines the crude and multivariable-adjusted ORs and 95% CIs for BC across quartiles of PDI, hPDI and uPDI. In the crude model, women in the top quartile of PDI had 77% lower odds of BC than those in the bottom quartile (OR 0.23; 95% CI 0.15–0.33). After adjustment for age, energy intake, and several demographic confounders, this association remained significant but slightly attenuated (OR 0.33; 95% CI 0.22–0.49). When BMI was taken into account, this association did not alter (OR 0.33; 95% CI 0.22–0.50). Such findings were also seen with regard to hPDI; such that there was a similar decreasing trend of odds ratios across increasing quartiles of hPDI, either before or after adjustment for confounders. For instance, after controlling for potential confounders, including BMI, individuals with the greatest adherence to hPDI were 36% less likely to have BC than those with the lowest adherence (OR 0.64; 95% CI 0.43–0.94). In terms of uPDI, we found evidence for increased odds for BC across increasing quartiles. Women in the top quartile of this index had a 2.12 times greater chance of BC than those in the bottom quartile (OR 2.12; 95% CI 1.46–3.08). The finding remained unchanged in the fully adjusted model (OR 2.23; 95% CI 1.48–3.36).
When we made stratified analysis by menopausal status, PDI was inversely associated with BC risk in both pre- (OR 0.23; 95% CI 0.59–0.94) and post-menopausal women (OR 0.35; 95% CI 0.22–0.56) (Table 5). There was an inverse association between hPDI and odds of postmenopausal BC (OR for the highest vs. lowest quartile 0.62; 95% CI 0.41–0.95). No significant association was found between hPDI and odds of BC among premenopausal women. A higher score of uPDI was positively associated with risk of postmenopausal BC (OR 2.42; 95% CI 1.51–3.87). No significant association was found between uPDI and odds of BC among premenopausal women.
Discussion
In the current population-based case–control study, we found a significant inverse association between PDI and hPDI scores and the likelihood of having BC, whereas there was a significant direct association between higher uPDI score and odds of BC. These associations remained significant even after considering potential confounders. Such associations were also seen in postmenopausal women. Though PDI was inversely associated with BC risk in premenopausal women, we failed to find any significant association between hPDI, uPDI and odds of premenopausal BC. To the best of our knowledge, this study is the first investigation that examined the association between adherence to plan-based dietary indices and odds of BC in the Middle East.
Carcinoma of the breast as a major health concern represents a progressively increasing trend and is subject to decreased age at the onset, making it a major cost of the healthcare system in the future45,46. Thus, effective primary preventions have important implications for relieving the rising burden of BC. Epidemiological evidence support the role of diet in BC's pathogenesis, so that it may facilitate or increase the risk of BC47,48,49. The results of the current study suggested an inverse association between higher adherence to PDI and hPDI scores and odds of BC, while the uPDI score was positively related to the chance of BC. Such associations were also seen in postmenopausal women. Though PDI was inversely associated with BC risk in premenopausal women we failed to find any significant association between hPDI, uPDI and odds of premenopausal BC. Published studies on plant-based dietary indices and BC have found little evidence of an association, which is inconsistent with our results. In the French cohort study of 487 BC survivors, though the greater plant-based dietary score was related to decreased overall cancer recurrence, the tumor-specific assessment showed a non-significant association with BC incidence25. In contrary, Martinez-Gonzalez and colleagues found that pro-plant-based score was inversely associated with all-cause mortality among omnivorous participants, nevertheless stratified analysis revealed non-significant association for cancer deaths50. Notably, a small number of cancer deaths could undermine statistical power to detect any small association in this regard. In another cohort study of 10,812 Spanish women, the investigators reported inverse associations between moderate adherence to overall pro-vegetarian dietary patterns (PVG) and BC risk, but the healthful PVG (hPVG) and unhealthful PVG (uPVG) had no association with BC risk51. Our findings were comparable with those from several investigations evaluating the association between BC risk and: (1) overall diet, encompasses plant-based and animal components, determined by a priori (dietary patterns) or posteriori (dietary scores) procedures, or (2) vegetarian and/or vegan diets. In line with our findings, inverse associations were seen between dietary scores assessing compliance to numerous healthy dietary patterns including the Mediterranean Diet Score (MDS)14, the Healthy Eating Index (HEI)52 and the Dietary Approaches to Stop Hypertension score (DASH)53 in relation to the risk of BC. In a recent meta-analysis, individuals with greater adherence to Western dietary pattern were 14% more likely to have BC compared with those with the lowest adherence, while consumption of a prudent dietary pattern was associated with an 18% reduced risk of BC7. In a recent systematic review on dietary patterns and risk of BC, lower risk of BC was observed with a healthy dietary pattern comprising of vegetables as well as fruits, legumes and whole grains while higher risk was observed with a dietary pattern high in saturated fats and red and processed meats as well as added sugars, fried foods and refined grains54. Similarly, according to summary results of a recent publication, a higher dietary colored non-starchy vegetable intake was associated with a reduced risk for BC, while high intakes of red and processed meats, high energy dense and high glycemic foods and beverages were positively associated with the risk of BC55. For vegetarian diets existing data are limited, but a meta-analysis of cohort studies reported no significant association between exclusive vegetarian diet and BC56. In line with our results, Dinu et al., in a meta-analysis on vegan diets and multiple health outcomes found evidence that conformity to exclusive vegetarian and vegan diets was associated with reduced overall cancer risk23. Different study designs, subjects' characteristics, lack of controlling for several confounders and different sample sizes across studies might explain the differences between our findings and those of earlier studies. Overall, further research using the PDI, hPDI, and uPDI or other holistic approaches might help resolve these inconsistencies.
The biological rationale for the possible association of plant-based diet on BC risk might be as follow: higher intake of healthy plant-products including fruits, legumes, vegetables and whole grains is accompanied with higher intake of fiber, micronutrients including vitamins C, E, folate, carotenoids, trace minerals (selenium, zinc, copper, and manganese), some non-nutrient such as phytoestrogens, phenolic acids, and lignans57 and carotenoids which are known to affect cellular differentiation and apoptosis58. Dietary folate, by its role in DNA methylation, affects gene expression of critical tumor suppressors and proto-oncogenes59. Dietary fiber plays important protective role against cancer through estrogen modulatory effects60,61. In addition, dietary fiber was found to be associated with a reduced cancer risk through removing damaged cells from the digestive dilute bile acids, and thereby it can reduce cell proliferation and the likelihood of mutations62. Reducing N-nitroso compounds, enhancing immunity, and producing various anti-inflammatory cytokines involved in the initiation and progression of mammary cell growth were also attributed to the anti-carcinogenic effects of dietary fiber63. Vitamins C, E, and trace minerals such as selenium, zinc, copper, and manganese are key constituents for antioxidant enzymes29. Essential non-nutrients, such as phytoestrogens, phenolic acids, and lignans with a modulatory effect on hormonal pathways through antioxidant, antiproliferative, antiangiogenic, and apoptotic properties play important protective roles against cancer64. On the other hand, animal products, mostly red and processed meat, are rich sources of heme iron, which through the production of genotoxic free radicals, NOCs, or through lipid peroxidation might have carcinogenic effects65. Moreover, elevated IGF-1 levels resulting from higher intake of high-protein animal foods can also play a role in this regard. This factor has been linked with both obesity and the development of various types of cancer. The mitogenic, anti-apoptotic, and angiogenic properties of IGF-1, particularly in mammary cell lines, have previously been confirmed66,67.
Several advantages of this project include adequate sample size, adjustment for a wide range of potential confounders in the analysis, reduced chance of altered usual dietary intakes by the enrollment of newly diagnosed BC cases, being the first investigation in the Middle East region and using energy-adjusted amounts of all food groups for constructing plant-based diet indices. Given these strengths, some potential limitations also need to be pointed out. The present study was carried out in a case–control design, which is prone to recall and selection biases and would not allow us to infer causality. However, this study was a population-based study in which controls were chosen from the healthy population in the community, which can in turn reduce selection bias. As in any epidemiological study, the application of FFQ might result in participants’ misclassification in terms of dietary intakes. Given the potential for existing unmeasured or unknown confounders, despite comprehensive adjustment for many potential confounders, the possibility of residual confounding cannot be ignored. We implemented sample-based scoring of plant-based diets to evaluate conformity to different types of plant-based diets; however, due to the lack of clear thresholds for absolute levels of plant and animal foods intake concerning chronic conditions, this field needs further investigation. Finally, we did not collect information on BC stage in the current study.
In conclusion, findings of the present study indicated that greater adherence to PDI and hPDI were inversely associated with risk of BC, whereas uPDI was associated with an increased risk. These findings support current recommendations for increasing the intake of healthy plant-based foods in dietary guidelines. Further studies are needed to confirm our findings, especially those with a prospective design.
References
Sasanfar, B., Toorang, F., Maleki, F., Esmaillzadeh, A. & Zendehdel, K. Association between dietary total antioxidant capacity and breast cancer: A case–control study in a Middle Eastern country. Public Health Nutr. https://doi.org/10.1017/S1368980019004397 (2020).
Marzbani, B. et al. Dietary patterns, nutrition, and risk of breast cancer: A case–control study in the west of Iran. Epidemiol. Health 41, e2019003 (2019).
DeSantis, C. E., Ma, J., Goding Sauer, A., Newman, L. A. & Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 67, 439–448 (2017).
Farhood, B., Geraily, G. & Alizadeh, A. Incidence and mortality of various cancers in Iran and compare to other countries: A review article. Iran. J. Public Health 47, 309 (2018).
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 94, 153–156 (2001).
Rojas, K. & Stuckey, A. Breast cancer epidemiology and risk factors. Clin. Obstet. Gynecol. 59, 651–672 (2016).
Xiao, Y. et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 21, 16 (2019).
Sharif, Y., Sadeghi, O., Benisi-Kohansal, S., Azadbakht, L. & Esmaillzadeh, A. Legume and nuts consumption in relation to odds of breast cancer: A case–control study. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1773874 (2020).
Dierssen-Sotos, T. et al. Fatty acid intake and breast cancer in the Spanish multicase–control study on cancer (MCC-Spain). Eur. J. Nutr. 59, 1171–1179 (2020).
Tayyem, R. F., Mahmoud, R. I. & Marei, L. S. The Intake of some nutrients is associated with the risk of breast cancer: Results from jordanian case–control study. Curr. Res. Nutr. Food Sci. J. 8, 12–24 (2020).
Jacobs, D. R. Jr., Gross, M. D. & Tapsell, L. C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 89, 1543s–1548s (2009).
Salari-Moghaddam, A. et al. Adherence to the MIND diet and prevalence of psychological disorders in adults. J. Affect. Disord. 256, 96–102 (2019).
Mousavi, S. M. et al. Adherence to the Mediterranean dietary pattern in relation to glioma: A case–control study. Clin. Nutr. 40, 313–319 (2021).
van den Brandt, P. A. & Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 140, 2220–2231 (2017).
Mourouti, N. et al. Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: A case–control study. Nutr. Cancer 66, 810–817 (2014).
Soltani, S., Benisi-Kohansal, S., Azadbakht, L. & Esmaillzadeh, A. Association between adherence to “dietary approaches to stop hypertension” eating plan and breast cancer. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1756354 (2020).
Tonstad, S. et al. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 23, 292–299 (2013).
Satija, A. et al. Plant-based dietary patterns and incidence of type 2 diabetes in us men and women: Results from three prospective cohort studies. PLoS Med. 13, e1002039 (2016).
Martínez-González, M. A. et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 100(Suppl 1), 320s–328s (2014).
Mazidi, M. & Kengne, A. P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 38, 1672–1677 (2019).
Zamani, B. et al. Association of plant-based dietary patterns with psychological profile and obesity in Iranian women. Clin. Nutr. 39, 1799–1808 (2020).
Zamani, B. et al. Association of a plant-based dietary pattern in relation to gestational diabetes mellitus. Nutr. Diet. 76, 589–596 (2019).
Dinu, M., Abbate, R., Gensini, G. F., Casini, A. & Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 57, 3640–3649 (2017).
Romanos-Nanclares, A. et al. Healthful and unhealthful provegetarian food patterns and the incidence of breast cancer: Results from a Mediterranean cohort. Nutrition https://doi.org/10.1016/j.nut.2020.110884 (2020).
Kane-Diallo, A. et al. Association between a pro plant-based dietary score and cancer risk in the prospective NutriNet-santé cohort. Int. J. Cancer 143, 2168–2176 (2018).
Aljefree, N. & Ahmed, F. Association between dietary pattern and risk of cardiovascular disease among adults in the Middle East and North Africa region: A systematic review. Food Nutr. Res. 59, 27486 (2015).
Potischman, N. et al. Increased risk of early-stage breast cancer related to consumption of sweet foods among women less than age 45 in the United States. Cancer Causes Control 13, 937–946 (2002).
Farvid, M. S. et al. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancer 144, 1496–1510 (2019).
Xiao, Y. et al. Association between whole grain intake and breast cancer risk: A systematic review and meta-analysis of observational studies. Nutr. J. 17, 87 (2018).
Farvid, M. S. et al. Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int. J. Cancer 143, 2787–2799 (2018).
Romanos-Nanclares, A. et al. Sugar-sweetened beverage consumption and incidence of breast cancer: The Seguimiento Universidad de Navarra (SUN) Project. Eur. J. Nutr. 58, 2875–2886 (2019).
Mourouti, N. et al. Whole grain consumption and breast cancer: A case–control study in women. J. Am. Coll. Nutr. 35, 143–149 (2016).
Mourouti, N. et al. Meat consumption and breast cancer: A case–control study in women. Meat Sci. 100, 195–201 (2015).
Esmaillzadeh, A. et al. Patterns of diet-related practices and prevalence of gastro-esophageal reflux disease. Neurogastroenterol. Motil. 25, 831-e638 (2013).
Esmaillzadeh, A. et al. Consumption of spicy foods and the prevalence of irritable bowel syndrome. World J. Gastroenterol. 19, 6465–6471 (2013).
Barak, F., Falahi, E., Keshteli, A. H., Yazdannik, A. & Esmaillzadeh, A. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet in relation to obesity among Iranian female nurses. Public Health Nutr. 18, 705–712 (2015).
Zaribaf, F. et al. Fish consumption is inversely associated with the metabolic syndrome. Eur. J. Clin. Nutr. 68, 474–480 (2014).
Willett, W. Nutritional Epidemiology (Oxford University Press, Oxford, 2012).
Gronwald, J. et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res. Treat. 95, 105–109 (2006).
Easton, D. F., Ford, D. & Bishop, D. T. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast cancer linkage consortium. Am. J. Hum. Genet. 56, 265–271 (1995).
Kotsopoulos, J. et al. Age at menarche and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Causes Control 16, 667–674 (2005).
Lipworth, L., Bailey, L. R. & Trichopoulos, D. History of breast-feeding in relation to breast cancer risk: A review of the epidemiologic literature. J. Natl. Cancer Inst. 92, 302–312 (2000).
Ghadirian, P. et al. Smoking and the risk of breast cancer among carriers of BRCA mutations. Int. J. Cancer 110, 413–416 (2004).
Nindrea, R. D., Aryandono, T., Lazuardi, L. & Dwiprahasto, I. Association of overweight and obesity with breast cancer during premenopausal period in Asia: A meta-analysis. Int. J. Prev. Med. 10, 192 (2019).
Sharma, G. N., Dave, R., Sanadya, J., Sharma, P. & Sharma, K. K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 1, 109–126 (2010).
Vahid, F. et al. The association between the Index of Nutritional Quality (INQ) and breast cancer and the evaluation of nutrient intake of breast cancer patients: A case–control study. Nutrition 45, 11–16 (2018).
Key, T. J., Verkasalo, P. K. & Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2, 133–140 (2001).
Pharoah, P. D., Day, N. E., Duffy, S., Easton, D. F. & Ponder, B. A. Family history and the risk of breast cancer: A systematic review and meta-analysis. Int. J. Cancer 71, 800–809 (1997).
Nelson, H. D. et al. Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann. Intern. Med. 156, 635–648 (2012).
Martínez-González, M. A. et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 100, 320S-328S (2014).
Romanos-Nanclares, A. et al. Healthful and unhealthful provegetarian food patterns and the incidence of breast cancer: Results from a Mediterranean cohort. Nutrition 79–80, 110884 (2020).
Kord- Varkaneh, H. et al. Association between Healthy Eating Index-2015 and breast cancer risk: A case–control study. Asian Pac. J. Cancer Prev. 21, 1363–1367 (2020).
Soltani, S., Benisi-Kohansal, S., Azadbakht, L. & Esmaillzadeh, A. Association between adherence to “dietary approaches to stop hypertension” eating plan and breast cancer. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1756354 (2020).
Dandamudi, A., Tommie, J., Nommsen-Rivers, L. & Couch, S. Dietary patterns and breast cancer risk: A systematic review. Anticancer Res. 38, 3209–3222 (2018).
Dreher, M. L. Dietary patterns, whole plant foods, nutrients and phytochemicals in breast cancer prevention and management. in Dietary Patterns and Whole Plant Foods in Aging and Disease 557–609 (Springer International Publishing, Cham, 2018).
Godos, J., Bella, F., Sciacca, S., Galvano, F. & Grosso, G. Vegetarianism and breast, colorectal and prostate cancer risk: An overview and meta-analysis of cohort studies. J. Hum. Nutr. Diet 30, 349–359 (2017).
Kroenke, C. H., Fung, T. T., Hu, F. B. & Holmes, M. D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 23, 9295–9303 (2005).
Prakash, P., Krinsky, N. I. & Russell, R. M. Retinoids, carotenoids, and human breast cancer cell cultures: A review of differential effects. Nutr. Rev. 58, 170–176 (2000).
Pirouzpanah, S., Taleban, F. A., Mehdipour, P. & Atri, M. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J. Mol. Med. (Berl.) 93, 917–934 (2015).
Xu, X., Duncan, A. M., Merz, B. E. & Kurzer, M. S. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol. Biomark. Prev. 7, 1101–1108 (1998).
Xu, X., Duncan, A. M., Wangen, K. E. & Kurzer, M. S. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 9, 781–786 (2000).
Mousavi, S. M. et al. Refined grains consumption is associated with a greater odds of glioma. Nutr. Neurosci. https://doi.org/10.1080/1028415X.2020.1758889 (2020).
Slavin, J. Whole grains and human health. Nutr. Res. Rev. 17, 99–110 (2004).
Slavin, J. L. Mechanisms for the impact of whole grain foods on cancer risk. J. Am. Coll. Nutr. 19, 300s–307s (2000).
Bastide, N. M. et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 75, 870–879 (2015).
Muti, P. et al. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol. Biomark. Prev. 11, 1361–1368 (2002).
Outwater, J. L., Nicholson, A. & Barnard, N. Dairy products and breast cancer: the IGF-I, estrogen, and bGH hypothesis. Med. Hypotheses 48, 453–461 (1997).
Acknowledgements
This research was supported by the School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences and Health Services, Tehran, Iran.
Funding
The financial support for this study comes from Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
L.A. and S.B.K. contributed to data collection, assisted in designing the study, conceptualized and oversaw this study. S.M.M. provided guidance on the analysis and provided substantial contributions to the editing of the paper. S.R. took primary responsibility for drafting this manuscript and provided guidance on the analysis. A.E. conducted the analysis and provided substantial contributions to the editing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rigi, S., Mousavi, S.M., Benisi-Kohansal, S. et al. The association between plant-based dietary patterns and risk of breast cancer: a case–control study. Sci Rep 11, 3391 (2021). https://doi.org/10.1038/s41598-021-82659-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82659-6
This article is cited by
-
High-protein diet scores, macronutrient substitution, and breast cancer risk: insights from substitution analysis
BMC Women's Health (2024)
-
Association of plant-based dietary patterns with the risk of type 2 diabetes mellitus using cross-sectional results from RaNCD cohort
Scientific Reports (2024)
-
Pro-vegetarian dietary pattern and risk of breast cancer: a case–control study
Breast Cancer Research and Treatment (2024)
-
The association of plant-based dietary pattern with general and abdominal obesity: a large cross-sectional study
Journal of Diabetes & Metabolic Disorders (2023)
-
Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care
EPMA Journal (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.