Abstract
Although dual antiplatelet therapy is essential for patients who undergo percutaneous coronary interventions, the risk of bleeding remains an unsolved problem, and there is limited information on the potential relationship between genetic variants and major bleeding. We analyzed the correlations between four major single nucleotide polymorphisms (CYP2C19, ABCB1, PON1, and P2Y12 G52T polymorphisms) and clinical outcomes in 4489 patients from a prospective multicenter registry. The primary endpoint was major bleeding, defined as a Bleeding Academic Research Consortium ≥ 3 bleeding event. The allelic frequencies of ABCB1, PON1, and both individual and combined CYP2C19 variants did not differ significantly between patient groups with and without major bleeding. However, the allelic frequency of the P2Y12 variant differed significantly between the two groups. Focusing on the P2Y12 G52T variant, patients in the TT group had a significantly higher rate of major bleeding (6.4%; adjusted hazard ratio [HR] 2.51; 95% confidence interval [CI] 1.08–5.84; p = 0.033) than patients in the other groups (GG [2.9%] or GT [1.9%]). Therefore, the TT variant of the P2Y12 G52T polymorphism may be an independent predictor of major bleeding.
Trial registration: NCT02707445 (https://clinicaltrials.gov/ct2/show/NCT02707445?term=02707445&draw=2&rank=1).
Similar content being viewed by others
Introduction
Dual antiplatelet therapy (DAPT) is essential for reducing the occurrence of ischemic events in patients undergoing percutaneous coronary intervention (PCI)1. However, the risk of bleeding associated with DAPT remains an unsolved problem, especially in patients with a high bleeding risk (HBR)2. The current guidelines recommend short-term DAPT for patients at HBR, although the current evidence is insufficient3,4. A recently reported consensus statement has established that customized DAPT should be considered for patients at HBR5,6. However, there is a lack of studies on genetic factors that affect bleeding risk.
To address this issue, we evaluated the associations of the single nucleotide polymorphisms of four genes (CYP2C19, ABCB1, PON1, and P2Y12 G52T) with major bleeding; these four genes are known to be involved in the modulation of clopidogrel absorption, metabolic activation, and biologic activity7,8,9,10. We evaluated these associations in patients who underwent PCI and DAPT over a 1-year follow-up period.
Results
Characteristics of the enrolled patients
A total of 4489 patients were enrolled in the current study. The mean loading dose of clopidogrel was 600 mg before the index PCI, and 93.3% of patients continued to receive DAPT for at least 6 months. A total of 122 (2.7%) patients showed major bleeding during the follow-up period. Among the patients showing major bleeding events, 99 patients (81.1%) had non-procedure-related bleeding and 23 patients (18.9%) had periprocedural bleeding. The rates of the individual secondary endpoints of all-cause mortality, cardiac death, non-fatal myocardial infarction, stent thrombosis, target lesion revascularization, and stroke were 1.4%, 0.9%, 0.8%, 0.5%, 5.0%, and 0.6%, respectively. Patients who had major bleeding were more likely to be female; to be older; and to have a medical history of hypertension, diabetes mellitus, hypercholesterolemia, anemia, or chronic kidney disease than patients who did not have major bleeding. The usage of aspirin, clopidogrel, cilostazol, proton-pump inhibitors, calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta-blockers was similar between patients with and without major bleeding. However, patients who had major bleeding were less likely to receive statins and short-term DAPT than those who did not (Table 1).
Allelic frequencies according to major bleeding
The allelic frequencies of ABCB1, PON1, and both the individual and combined CYP2C19 variants did not differ significantly between patients with and without major bleeding (Table 2). However, the allelic frequency of the P2Y12 variant differed significantly between patients with and without major bleeding (Table 2).
P2Y12 G52T polymorphisms
The patients were divided into three groups according to the P2Y12 G52T variant observed (GG, GT, and TT). The baseline characteristics of patients in all three groups showed similar trends, including HBR factors such as chronic kidney disease and anemia. The prevalence of the other genetic polymorphisms (CYP2C19, PON1, and ABCB1) did not differ among the three groups (Table 3). The number of treated vessels, the total number of stents, minimal stent size, length of the stent, P2Y12 reaction units, DAPT duration, and discharge medication were also not significantly different among the three groups (Supplementary Table 1).
Regarding the primary endpoint, the TT group showed the highest incidence of major bleeding compared to the other groups (GG vs. GT vs. TT: 2.9% vs. 1.9% vs. 6.4%, log-rank p = 0.026) (Fig. 1). However, there were no significant differences in the secondary endpoints (any cause mortality, cardiac death, myocardial infarction, stent thrombosis, target lesion revascularization, and stroke) among the groups.
Multivariate analysis for poor prognostic factors of major bleeding
In the multivariate Cox regression analysis for major bleeding, age (per 1-year increase; adjusted hazard ratio [HR] 1.03; 95% confidence interval [CI] 1.01–1.05; p = 0.004), diabetes mellitus (adjusted HR 1.45; 95% CI 1.00–2.09; p = 0.049), chronic kidney disease (adjusted HR 2.10; 95% CI 1.24–3.55; p = 0.006), anemia (adjusted HR 4.20; 95% CI 2.79–6.34; p < 0.001), and the TT variant (adjusted HR 2.51; 95% CI 1.08–5.84; p = 0.033) were found to be poor prognostic predictors after adjusting for various factors.
Discussion
We investigated the clinical impact of genetic variants on major bleeding in patients after PCI. The two key findings are as follows: (1) The P2Y12 G52T polymorphism was a predictor of HBR in patients who underwent PCI and received DAPT; and (2) patients with the TT variant had a higher incidence of major bleeding than those with other variants. Furthermore, the TT variant was found to increase the bleeding risk after PCI as per multivariate analysis.
In a recent consensus statement from the Academic Research Consortium, several factors, such as renal failure, liver failure, and anemia, have been suggested as potential contributors to HBR. In patients with HBR, short-term DAPT is recommended5,6; however, the current guidelines provide insufficient information regarding the adjustment of the DAPT duration based on genetic information. In a recent report, the genotype-guided DAPT group showed a significant decrease in the incidence of bleeding events without differences in the total combined clinical outcomes compared to the standard-treatment DAPT group11. These findings suggest that genotype-guided therapy and precision medicine may improve clinical outcomes in this patient group. In this study, the TT variant of the P2Y12 G52T polymorphism was an independent predictor of major bleeding. Multivariate analysis showed the same tendency as that reported by the current consensus on HBR in terms of renal disease and anemia, suggesting that a novel factor (the TT variant) should be considered to achieve better clinical outcomes in HBR patients.
Previous studies that evaluated the association between genetic variations and clinical outcomes have mainly focused on ischemic outcomes such as cardiac death, myocardial infarction, stent thrombosis, and target lesion revascularization12. CYP2C19 is the most commonly studied gene, and several studies on this gene have shown that the gain-of-function group had a higher occurrence of bleeding events than the loss-of-function group7,13. However, the prevalence of gain-of-function in Asians with the CYP2C19 variant is very low compared to that in Caucasians with the CYP2C19 variant8,14,15. Another study on the Asian population found no association between the gain-of-function allele and bleeding16. In addition, ABCB1, PON1, and P2Y12 showed a trend for poor clinical outcomes in terms of ischemic event. However, similar to that for CYP2C19, there was a lack of evidence regarding the association with poor clinical outcomes in terms of major bleeding for these genes8,9,17,18.
Our results are similar to those of previous studies that showed that CYP2C19, ABCB1, and PON1 variants were not associated with significant differences in major bleeding. However, our study revealed that the P2Y12 G52T variant had a significant relationship with major bleeding; this is important since studies on the association between P2Y12 and major bleeding have rarely been reported.
Fontana et al. reported that the P2Y12 G52T polymorphism is one of P2Y12 gene polymorphisms18. To investigate the correlation between P2Y12 gene polymorphisms and clinical outcomes, i-T744C (rs2046934), C34T (rs6785930), and G52T (rs6809699) were evaluated. Studies on P2Y12 gene polymorphisms have not been widely conducted as compared to studies on CYP2C19 polymorphisms; this may be because the clinical impact of the P2Y12 gene polymorphism is relatively weaker than that of the CYP2C19 polymorphism8,12,19. A recent meta-analysis reported clinical outcomes according to P2Y12 gene polymorphisms. Briefly, the study found that P2Y12 gene polymorphisms may be associated with poor clinical outcomes (specifically, ischemic events, such as stent thrombosis and non-fatal myocardial infarction) and have no significant effect on bleeding. However, the meta-analysis evaluated these associations based on studies with relatively small patient populations. In addition, the bleeding analysis only included two polymorphisms (i-T744C and C34T) among the various P2Y12 receptor gene polymorphisms. One study reported that the P2Y12 G52T variant was associated with a higher incidence of major bleeding in patients with ST-elevation myocardial infarction20.
A plausible explanation for the lack of association between P2Y12 G52T polymorphisms and major bleeding in previous studies is the low prevalence of the TT variant. In this study, the prevalence of the TT variant of P2Y12 G52T was 2.1%; previous genetic studies have shown a similar prevalence (2–3%)8,21,22,23,24. Furthermore, the prevalence of the TT variant of P2Y12 G52T was similar across different ethnic populations, unlike that of the gain-of-function allele of CYP2C198,25.
In G52T, the prevalence of the TT variant was relatively lower than that of the other allelic variants (i-T744C and C34T). In context, our findings suggest that previous studies may have underestimated the association between the TT variant and the risk of major bleeding owing to the low prevalence of the P2Y12 G52T variant. Plausible explanations of the mechanism include the following: (1) Inherited defects of the P2Y12 receptor, which has a potential role in platelet function, are related to platelet dysfunction and bleeding diathesis26; and (2) there may be differences in ADP-induced maximal aggregation according to the P2Y12 G52T variant21. Furthermore, the TT variant was related to higher ADP-induced maximal aggregation than other variants (GG or GT), which may have affected bleeding during the DAPT period. Thus, further studies are needed to address the mechanisms of major bleeding associated with the G52T variant and to confirm our results.
This study has some limitations. First, although a significantly higher occurrence of major bleeding was associated with the TT variant, there may be concerns about applying this result in real-world practice owing to the low prevalence of the TT variant. However, this study is the largest population study to elucidate the correlation between the P2Y12 G52T polymorphism and major bleeding. Second, this study aimed to determine the impact of genetic variants on major bleeding after PCI. Although evaluation of the clinical impact of tailored DAPT was beyond the scope of this study, in the subgroup analysis of patients who received DAPT for 3 months, there was no significant difference in the incidence of major bleeding according to the P2Y12 G52T variant. However, since the suggested duration of DAPT was 1 year in this study, the number of patients who used DAPT for 3 months was relatively small. Thus, we cannot rule out the possibility that the number of patients included in this subgroup analysis was insufficient to achieve adequate statistical power. Third, we evaluated clinical outcomes after a 1-year follow-up after PCI. Although a long-term investigation may provide insights into the clinical impact of P2Y12 G52T polymorphisms, major bleeding is expected to be less frequent after the discontinuation of DAPT after 1 year of PCI.
In conclusion, the TT variant of the P2Y12 G52T polymorphism might be an independent predictor of major bleeding. Therefore, short-term DAPT should be considered for patients with the TT variant to prevent major bleeding.
Methods
Study population
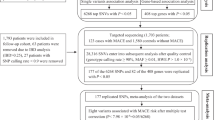
From 2012 to 2014, the GENIUS study included 5000 patients who underwent PCI for coronary artery disease in 20 tertiary hospitals and investigated the influence of various genotypes on coronary artery stenting outcomes. Among the 5000 patients, 413 patients did not meet inclusion/exclusion criteria, were lost to follow-up, withdrew consent, had missing genotyping results, or had missing platelet function test results. In addition, 98 patients with rapid metabolizers (*17) in CYP2C19 were excluded from the analysis owing to the confounding effects of these substances16. Ultimately, 4489 total patients were evaluated in the current study. DAPT was recommended for 1 year (3 months minimum) after the index PCI (Fig. 2). DAPT included aspirin (100 mg daily) and clopidogrel (75 mg daily). No other P2Y12 inhibitors, such as ticagrelor and prasugrel, or anticoagulants, were prescribed after PCI. The study protocol was approved by the Institutional Review Board at each participating center including the Korea University Anam Hospital, Seoul, South Korea. Written informed consent was obtained from all patients at enrollment. This study complied with the Declaration of Helsinki and was registered with ClinicalTrials.gov (number NCT02707445).
Genotype and platelet reactivity
Measured single nucleotide polymorphisms (SNPs) included CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), CYP2C19*17 (rs12248560), ABCB1 (rs1045642), PON1 (rs662), and P2Y12 (rs6809699). The genotype of each SNP was determined by pyrosequencing using a PSQ 96MA Pyrosequencer (Pyrosequencing AB, Uppsala, Sweden), as previously reported27. To measure the inhibitory effect of clopidogrel on platelet reactivity, the VeriyfyNow P2Y12 assay (Accumetrics, San Diego, California, USA) was used. Physicians and patients were blinded to residual platelet reactivity and genotype results.
Endpoint
The primary endpoints were major bleeding, defined as Bleeding Academic Research Consortium (BARC) 3, 4, and 5. The secondary endpoints were any cause mortality, cardiac death, myocardial infarction, stent thrombosis, target lesion revascularization, and stroke.
Statistical analysis
Comparisons between groups were performed using independent Student’s t-test or analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables. Post hoc subgroup analysis was performed based on the baseline characteristics. To estimate the effect of the clinical outcomes, including major bleeding, any cause mortality, cardiac death, myocardial infarction, stent thrombosis, target lesion revascularization, and stroke according to genetic variation, the hazard ratio (HR) was calculated using the Cox proportional hazard model. In the multivariate Cox regression analysis, the HR was adjusted for sex, age, hypertension, diabetes mellitus, previous history of myocardial infarction, previous history of PCIs, congestive heart failure, chronic kidney disease, current smoking status, anemia, clinical presentation to acute coronary syndrome (unstable angina, non-ST elevation myocardial infarction, and ST-elevation myocardial infarction), genetic variants (P2Y12 G52T, CYP2C19, PON1, and ABCB1), duration of DAPT, multivessel involvement, minimal stent size, and total stent length. Two-tailed p-values were used, and p-values of < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS version 25.0 software (SPSS Inc., Chicago, IL, USA).
References
Capodanno, D., Alfonso, F., Levine, G. N., Valgimigli, M. & Angiolillo, D. J. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J. Am. Coll. Cardiol. 72, 2915–2931 (2018).
Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165 (2019).
Levine, G. N. et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 68, 1082–1115 (2016).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260 (2018).
Urban, P. et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 40, 2632–2653 (2019).
Urban, P. et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 140, 240–261 (2019).
Wallentin, L. et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: A genetic substudy of the PLATO trial. Lancet 376, 1320–1328 (2010).
Simon, T. et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009).
Bouman, H. J. et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 17, 110–116 (2011).
Staritz, P. et al. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int. J. Cardiol. 133, 341–345 (2009).
Claassens, D. M. F. et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N. Engl. J. Med. 381, 1621–1631 (2019).
Mega, J. L. et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA 304, 1821–1830 (2010).
Galeazzi, R. et al. Clustering of ABCB1 and CYP2C19 genetic variants predicts risk of major bleeding and thrombotic events in elderly patients with acute coronary syndrome receiving dual antiplatelet therapy with aspirin and clopidogrel. Drugs Aging 35, 649–656 (2018).
Sugimoto, K., Uno, T., Yamazaki, H. & Tateishi, T. Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br. J. Clin. Pharmacol. 65, 437–439 (2008).
Sibbing, D. et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 121, 512–518 (2010).
Park, M. W. et al. Impact of the CYP2C19*17 polymorphism on the clinical outcome of clopidogrel therapy in Asian patients undergoing percutaneous coronary intervention. Pharmacogenet. Genom. 23, 558–562 (2013).
Mega, J. L. et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362 (2009).
Fontana, P. et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108, 989–995 (2003).
Collet, J. P. et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet 373, 309–317 (2009).
Zhang, J. H. et al. Effect of platelet receptor gene polymorphisms on outcomes in ST-elevation myocardial infarction patients after percutaneous coronary intervention. Platelets 27, 75–79 (2016).
Kim, K. A., Song, W. G., Lee, H. M., Joo, H. J. & Park, J. Y. Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population. Thromb. Res. 132, 221–226 (2013).
Ou, W., He, Y., Li, A., Liu, B. & Jin, L. Genotype frequencies of CYP2C19, P2Y12 and GPIIIa polymorphisms in coronary heart disease patients of han ethnicity, and their impact on clopidogrel responsiveness. Int. Heart J. 57, 586–592 (2016).
Li, C. et al. Gene variants in responsiveness to clopidogrel have no impact on clinical outcomes in Chinese patients undergoing percutaneous coronary intervention—A multicenter study. Int. J. Cardiol. 240, 360–366 (2017).
Calderón-Cruz, B. et al. C3435T polymorphism of the ABCB1 gene is associated with poor clopidogrel responsiveness in a Mexican population undergoing percutaneous coronary intervention. Thromb. Res. 136, 894–898 (2015).
Vargas-Alarcón, G. et al. Distribution of ABCB1, CYP3A5, CYP2C19, and P2RY12 gene polymorphisms in a Mexican Mestizos population. Mol. Biol. Rep. 41, 7023–7029 (2014).
Scavone, M., Femia, E. A. & Cattaneo, M. P2Y12 receptor gene mutations associated with bleeding. Platelets 28, 421–423 (2017).
Kim, K. A., Song, W. G., Lee, H. M., Joo, H. J. & Park, J. Y. Multiplex pyrosequencing method to determine CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously: Its application to a Korean population and comparisons with other ethnic groups. Mol. Biol. Rep. 41, 7305–7312 (2014).
Acknowledgements
This research was supported by Dong-A ST CO., LTD. All authors declare that the funders had no role in study design, data collection and analysis, preparation of the manuscript or decision to publish.
Author information
Authors and Affiliations
Contributions
D.-S.L. designed the study. J.-J.C. and H.J.J. wrote the first draft. J.-J.C. and H.J.J. planned and performed statistical analyses. J.H.P., S.J.H., T.H.A., B.-K.K., W.S., S.G.A., J.Y., Y.H.K., Y.-H.C., W.C.K., W.K., Y.-H.L., H.G., W.C., and D.-S.L. contributed to the collection of data, discussions, and interpretation of the data. The decision to submit this manuscript for publication was made by all the authors and study principal investigators.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cha, JJ., Joo, H.J., Park, J.H. et al. Impact of genetic variants on major bleeding after percutaneous coronary intervention based on a prospective multicenter registry. Sci Rep 11, 1790 (2021). https://doi.org/10.1038/s41598-020-80319-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80319-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.