Abstract
P75 neurotrophic receptor (p75NTR) is an important receptor for the role of neurotrophins in modulating brain plasticity and apoptosis. The current understanding of the role of p75NTR in cellular adaptation following pathological insults remains blurred, which makes p75NTR’s related signaling networks an interesting and challenging initial point of investigation. We identified p75NTR and related genes through extensive data mining of a PubMed literature search including published works related to p75NTR from the past 20 years. Bioinformatic network and pathway analyses of identified genes (n = 235) were performed using ReactomeFIViz in Cytoscape based on the highly reliable Reactome functional interaction network algorithm. This approach merges interactions extracted from human curated pathways with predicted interactions from machine learning. Genome-wide pathway analysis showed total of 16 enriched hierarchical clusters. A total of 278 enriched single pathways were also identified (p < 0.05, false discovery rate corrected). Gene network analyses showed multiple known and new targets in the p75NTR gene network. This study provides a comprehensive analysis and investigation into the current knowledge of p75NTR signaling networks and pathways. These results also identify several genes and their respective protein products as involved in the p75NTR network, which have not previously been clearly studied in this pathway. These results can be used to generate novel hypotheses to gain a greater understanding of p75NTR in acute brain injuries, neurodegenerative diseases and general response to cellular damage.
Similar content being viewed by others
Introduction
Neurotrophins are secreted dimeric proteins, including BDNF, the most well-known, as well as nerve growth factor (NGF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4)1,2. These neurotrophins and their receptors function in an extensively well-regulated mechanism resulting in a delicate equilibrium that is highly responsive to nervous system injury and change3. Neurotrophins bind to two distinct types of receptors including p75 neurotrophin receptor (p75NTR) with a lower affinity, as well as to the tropomyosin receptor kinases (Trk) receptors, which function as tyrosine kinase receptors with high affinity and selectivity4. Precursor forms of neurotrophins acts as potent ligands for p75NTR and can induce apoptosis, while lacking affinity to Trk receptors5. The Trk receptors activate numerous downstream pathways including the MEK/MAPK pathway, extracellular signal-regulated kinase (Erk), the PI3K-Akt pathway, and the phospholipase C gamma pathway6. In contrast the p75NTR does not have intrinsic tyrosine kinase activity, but rather associates with other transmembrane proteins including the Trks, Nogo and myelin associated glycoprotein (MAG) for its downstream effects5.
This modulating role of p75NTR have been shown in several animal model studies. Inhibition of p75NTR and its associated Nogo co-receptor exerts a neuroprotective effect in models of middle cerebral artery occlusion7. Similarly, a down-regulation of both Nogo and p75NTR promoted improved stroke recovery in another pre-clinical model of middle cerebral artery occlusion8. Nogo associates with p75NTR by MAG, oligodendrocyte myelin glycoprotein or Nogo that activates RhoA leading to inhibition of neurite outgrowth5. In-line with downregulation exhibiting possible neuroprotective and stimulatory effects, activation of p75NTR by proneurotrophins in culture induces a pro-apoptotic effect on neurons9. This is particularly interesting since cleaved neurotrophins promoted recovery through Trks9. In another model of central nervous system (CNS) injury, a small-molecule modulator of the p75NTR receptor demonstrated that modulation of this receptor and its associated pathways results in improved outcomes following traumatic brain injuries10. All of these studies point to the strong role of neurotrophins and p75NTR in the response to CNS injury.
Viewing the role of neurotrophins in exhibiting neuroprotective effects from a slightly different viewpoint, it has previously been suggested that Alzheimer’s disease (AD) amyloid β peptide also acts as ligand to p75NTR, but not Trk receptors while inducing neuronal apoptosis11. This mechanism of p75NTR mediated apoptosis without Trk receptor activation likely plays a significant role in AD pathogenesis11. Interestingly, further findings by Yao et al. (2015) demonstrated physiological neuroprotective effects of p75NTR ectodomain against amyloid β peptide toxicity in the brain of AD patients12. In addition, AD patients have increased levels of p75NTR and proBDNF in hippocampal tissue samples and a greater proBDNF/BDNF ratio in cerebrospinal fluid that may result in imbalance of death and survival counter-regulation mechanisms13. Of note, in AD an increased level of proNGF leads to p75NTR activation and apoptosis in the absence of TrkA14. P75NTR has been shown to have similarities in the biology of Amyloid precursor protein (APP) which plays a significant role in AD patophysiology15. Also, patients with amyotrophic lateral sclerosis (ALS), another neurodegenerative disease, revealed highly increased p75NTR ectodomain concentrations in urine when compared to healthy controls. In addition, high concentrations of p75NTR ectodomain correlated to progression of ALS disease16.
Understanding the role that p75NTR and associated molecules may play in AD also supports the notion that these molecular mechanisms should not be underestimated in the pathophysiological response to acute brain injury. Especially when considering the significant role that plasticity and associated adaptive mechanisms may play in these insults, including chronic traumatic encephalopathy15,17,18. With p75NTR being studied in various different diseases and fields suggests that it may play an important central role in cellular response to injury. The body of evidence surrounding p75NTR is diffusely spread out over numerous fields suggesting that a review of these separate bodies of work in one study may identify new connections, pathways, and mechanisms that were previously identified as isolated characteristics.
Herein, we investigate the published literature through machine-learning approaches to study the role of p75NTR and its related gene networks in an attempt to elucidate new factors that may be important to future development of therapeutic targets. We hypothesized that by using already published results of p75NTR signaling, we would be able to investigate p75NTR interaction networks and pathways to understand how these genes form networks, connections, and signaling pathways across the broad literature that involves p75NTR. By using machine learning educated linkage gene analysis, we aimed to identify new gene and protein candidates that may be involved in a network with p75NTR, but have not been widely identified or established as possible targets in the literature regarding acute and chronic response to cellular injury.
Materials and methods
Literature search and identification of target genes
In order to identify p75NTR and its related genes, we performed an extensive literature search using PubMed on published articles from the past 20 years (1998/11/13–2018/11/13) related to p75NTR. The final query term was: (p75[All Fields] AND ("neurons"[MeSH Terms] OR "neurons"[All Fields] OR "neuron"[All Fields])) OR (p75[All Fields] AND ("brain"[MeSH Terms] OR "brain"[All Fields])) OR (p75[All Fields] AND neural[All Fields]) AND ("1998/11/13"[PDAT] : "2018/11/13"[PDAT]). The query resulted in a total of 2041 publications. We used R statistical software with “PubMed.mineR” package to mine all gene names appearing in the 2041 publication abstracts19 (Core-team R 2015). Appropriate gene names were used in accordance with HUGO Gene Nomenclature Committee (HGNC) approved symbols (genenames.org). The results of data mining were adjudicated by the authors JK and AS.
Bioinformatic analyses
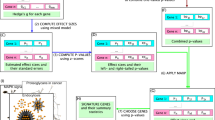
A flow of the study is presented in Fig. 1. We used two approaches to study the p75NTR gene and its role in gene networks and pathways. Network analyses were performed with ReactomeFIViz in Cytoscape (https://www.cytoscape.org/) based on a highly reliable Reactome functional interaction (FI) network. The entire FI network was constructed by merging interactions extracted from human curated pathways with interactions predicted using a machine learning approach20,21. Network analyses were followed with Reactome pathway analyses, and false discovery rate (FDR) corrected p-value < 0.05 was considered to be significant in order to avoid false positive results22. Visualization of genome-wide pathway enrichment analysis and generation of hierarchical cluster figure was performed with Reactome analysis tool provided in reactome.org20.
Work-flow diagram. 1 PubMed database queried with inclusive specified search terms. 2 Acquired data mined resulting in target genes. 3 Network analyses performed using a highly reliable algorithm extracted from multiple human-curated pathways. Network analyses followed with pathway enrichment analysis with hypergeometric testing.
Ethics approval
Institutional ethics board evaluation was not applicable due to the nature of this study. Good scientific practice was used throughout this study.
Results
Genes associated to p75NTR
With the data mining approach, we identified a total of 235 genes associated to p75NTR and its role in neuronal signaling (Tables S1 and S2). Using gene frequencies in the mined literature, the top 20 genes that emerged were NGF, BDNF, TNF, MAG, GDNF, GFAP, APP, p75NTR, TRAF6, CNTF, EGF, EGFR, PGP, TRPC6, PTEN, AR, CHL1, SOX10, MBP and NTRK1. A full list of the identified 235 genes is available in the supplemental material.
Hierarchical genome-wide gene set enrichment analysis
We used the 235 p75NTR related genes to investigate the hierarchical genome-wide pathways and which of these pathways were enriched in known pathways. The enrichment analysis initially demonstrated that p75NTR and related genes aggregate in different hierarchical clusters (Fig. 2). Overall, 16 out of 27 hierarchical pathway clusters were enriched with at least one pathway. Hierarchal pathway clusters that included 10 or more enriched pathways were programmed cell death, immune system, signal transduction, developmental biology, gene expression, disease, and extracellular matrix organization.
Genome-wide overview of pathway enrichment analysis Enriched pathways are highlighted in yellow. Out of the 27 shown hierarchical clusters, 16 clusters had enriched pathways. Immune system, signal transduction, programmed cell death, developmental biology, gene expression, disease and extracellular matrix organization pathways cluster were the most enriched with numerous pathways.
Enriched pathways
We further identified enriched pathways related to 235 p75NTR related genes without first modeling through hierarchal pathway clusters. Through this analysis, 278 enriched pathways were identified (p < 0.05, FDR corrected, Table S3). The five most enriched pathways (according to FDR corrected p-value) were pathways in cancer, signaling by interleukins, signaling by NGF, melanoma, and proteoglycans in cancer. A list of the top 20 pathways are presented in Table 1. A full list of enriched pathways and individual genes in the nodes are presented in supplemental material.
Gene network analyses
After pathway analyses, we performed gene network interaction analyses to understand how p75NTR and related genes connect to each other with related functions (Fig. 3). Analysis of 235 genes identified 10 highly interconnected genes with more than 20 connections. These highly connected genes were STAT1, STAT3, SP1, JUN, EGFR, TRAF6, JAK2, NRAS, TP53 and MDM2. In addition, P75NTR, NGF, FGFR3, EGF, TNF, NTRK1, EGR1 and CBL were identified to have 15–19 connections. The rest of the mined genes related to p75NTR had less than 15 connections in the formed in silico network analysis.
We performed further gene analyses with the use of linkage genes to identify genes linked to 235 genes through educated machine learning analysis across the Reactome FIViz database. Linkage genes were those genes that were not identified as constituents of the 235 genes. With this approach we identified a total of 38 linkage genes that were mechanistically connected to the mined 235 genes (Fig. 4). Seven of the genes identified were highly connected with 40 or more connections. These genes were GRB2, SRC, EP300, MAPK1, MAPK3, UBC and CTNNB1. Fifteen linkage genes were identified to have 20–39 connections. The remaining 16 linkage genes had less than 20 connections.
Gene interaction network of mined genes related to p75NTR incorporating linkage genes A total of 38 linkage genes were identified, which were functionally related to mined genes. Seven genes identified were highly connected with 40 or more connections. Fifteen genes were identified to have 20–39 connections. Lastly, 16 genes with less than 20 connections were identified. Circles correlate to mined genes, while diamonds correlate to linkage genes.
Finally, we performed focused functional interaction subnetwork analyses of identified highly connected (≥ 40) genes and linkage genes in relation to the p75NTR and its main ligand NGF. P75NTR and NGF were identified to both activate GRB2. In addition, p75NTR had direct connection to linkage gene UBC and association to MAPK1. Furthermore, p75NTR had activation connection to its main ligand NGF and TP53. In the same network, we identified several additional linkage genes including SRC, EP300, MAPK3 and CTNNB1 (Fig. 5).
Subnetwork analysis of highly connected genes with linkage genes P75NTR and NGF were both directly activating GRB2. In addition, p75NTR had connections to linkage genes UBC and MAPK1, and, as expected, to its main ligand NGF as well as TP53. In the same network, several linkage genes SRC, EP300, MAPK3 and CTNNB1 were also identified.
Discussion
The complex role of p75NTR in brain plasticity and apoptosis has proven a challenging subject of investigation leaving researchers without a full understanding of the role it plays in various pathophysiological aspects. Herein we investigated previously published literature using data mining and machine learning approaches to gain a better understanding of p75NTR’s gene networks and pathways. We identified p75NTR related genes, gene networks, and pathways while identifying new associated p75NTR network genes. While a portion of these results validate previously published mechanistic links, the advantage of reviewing vast amounts of previously unconnected literature through this approach also identified new genes and proteins for future studies. The pathway analyses provide a general overview of the functions of p75NTR in a network with its closely related genes. The data presented here will also serve as a useful resource to the research community in querying potential biomarkers, therapeutic targets, and potential areas of future studies.
Our results obtained from genome-wide cluster and pathway analyses validated the current understanding of p75NTR in that it plays a role in various pathways including programmed cell death, immune system modulation, signal transduction, developmental biology, gene expression regulation, and extracellular matrix organization2,9,23,24,25,26,27. The most enriched pathways identified validated the roles of previous studies, such as the finding that p75NTR has been shown to be highly expressed in human cancers including melanoma26,28,29. The link of p75NTR to cancer pathology is particularly interesting, given its role in immune modulation, matrix remodeling, and cellular adaptation. However, there are currently no studies demonstrating p75NTR’s effects on modulating the immune response in cancer, nor in acute or chronic brain disease suggesting this area may warrant further investigation, noting also the fact that p75NTR belongs to tumor necrosis factor receptor superfamily. The pathway analyses that resulted provide a general overview of the functions of p75NTR in a network with its closely related genes. Overall, 278 enriched pathways and 16 hierarchical pathway clusters were identified generating vast amounts of data that may be useful for validation and future research by other investigators.
The gene network analyses in combination with the focused subnetwork analysis using linkage genes identified functionally related genes that were not part of our original datamined p75NTR identified genes. GRB2 has been shown to be directly downstream of TrkA acting as a signaling adaptor protein30. Associations or links of p75NTR and GRB2 are not well established. Interestingly, in the brain tissue of scrapie infected rodents, BDNF, TrkB, phospho-TrkB, GRB2 and p75NTR were all significantly down-regulated supporting the direct association identified in our results between GRB2 and p75NTR31,32. Viral infection etiology of AD has been a widely known controversial topic. This makes the finding about scrapie viral infection particularly interesting. Results of three independent AD cohorts showed disruption of molecular, genetic, and clinical networks by human herpesvirus33. However, the role of p75NTR in possible viral pathophysiology of AD remain unknown, but these results in conjunction with previous works may shed some light generating new hypotheses and studies.
Another linkage gene identified was UBC, the gene for polyubiquitin precursor protein, which is known to have a role in proteasome degradation34. Conjugation of ubiquitins has been well established as highly important for protein degradation and the associated role that plays in larger cellular process such as DNA repair, cell cycle regulation, kinase modification and endocytosis system34,35. Loss of UBC is associated with the pathophysiological molecular factors of AD via decreased proteasome degradation system, which may be thought of as cells inefficiently removing malfunctioning, damaged, or old proteins36. These connections with p75NTR underline the important role in AD’s pathophysiology17. However, it is important to understand that the role of p75NTR in AD pathophysiology is only a model for which p75NTR likely acts as a central protein signaler in response to various elements of cellular damage.
MAPK1/ERK2 and MAPK3/ERK1 pathways were also identified and their roles have been reported in apoptosis, neuronal repair, and axonal growth37,38. As shown in several studies, MAPKs are downstream targets of Trks and p75NTR38,39. Our results suggested that NGF and p75NTR link with MAPK1, and NGF activates MAPK3, which are in line with previously published studies39,40. An interpretation for these interactions in the broader context of a disease can be seen with inhibition of ERK1/2 following the acute phase of stroke promoting long-term functional outcome and enhanced later-stage recovery processes in rats41. Other experiments using preclinical rat models have shown how MAPK activity also plays a fundamental role during postnatal neurogenesis when hippocampal apoptosis and synaptogenesis are occurring42. The significant connections between MAPK and p75NTR highlight that p75NTR should also be further investigated in relation to these disease processes, as well as finding possible targets for future medical therapy.
In addition, SRC, CTNNB1, and EP300 were identified in the same linkage gene network with p75NTR. Interestingly, inhibition of SRC family kinases improved cognitive function in rats after intraventricular hemorrhage43. Indeed, these observations further increase the interest of p75NTR and its role following hemorrhagic insult as another form of pathological damage cells suffer acutely. Similarly, CTNNB1 encodes a β-catenin protein that increases proNGF leading to p75NTR activation ultimately promoting neuronal growth44. Further investigation showed that modulation of β-catenin pathway was neuroprotective after intracerebral hemorrhage in rats45. The role of p75NTR, however, was not previously studied in these aspects. Our results suggest an association between CTNNB1 and p75NTR possibly demonstrating a substantial role for p75NTR in the stroke response.
Previously, sortilin (SORT1), lingo (LINGO1) and NRAGE (MAGED1) have been linked to p75NTR functions regulating apoptosis, axonal outgrowth and transporting pro-neurotrophins1,27,39. The genes did not result in the analyses directly. Interestingly however, MAGEL2 was identified in our analysis that belong to the same melanoma-associated antigen (MAGE) family than NRAGE, and shares strong homology to NRAGE and other proteins in MAGE-family. As NRAGE is involved in the p75NTR mediated programmed cell death, MAGEL2 is linked to neurodevelopmental disorders such as Prader-Willi syndrome and Schaaf-Yang syndrome. This suggest significant importance of MAGEL2 in neuronal development in humans46. Previous animal studies have shown that mTOR and autophagy pathways are dysregulated in Magel2 null mice models47. In our analysis, MAGEL2 was directly linked to transcription factor E2F1 that was directly linked to p75NTR. This suggests a role of necdin-related MAGE proteins in p75NTR functions, supported by preclinical observations48.
Two of seven subnetwork linkage genes identified here, namely UBC, and EP300 have not been extensively studied in the context of brain plasticity. These two linkage genes and their encoded proteins could be interesting targets for future studies to explore in patients with acute brain injury or neurodegenerative diseases. This also highlights the benefit of taking large scale approaches through data mining, which allows for an overview of the interactions that may exist between different studies, but have not previously been discovered to exist.
Our study was limited to in silico analyses, and confirmatory in vitro or in vivo analyses were not performed. However, the research approach used herein has the advantage of interrogating a large dataset through a systematical approach which incorporates all currently available knowledge. Analyzing such a search with powerful bioinformatic tools educated with machine learning algorithm from human curated pathways allows for broad investigation of connections that may have previously been missed, or not looked for in the first place. Confirmation of discussed associations are warranted.
Conclusion
We provide the single largest comprehensive gene and functional network library of p75NTR. This study incorporates current knowledge using a large dataset approach that increases the overall understanding of complex p75NTR networks. These results suggest both new possible target genes for further investigation in p75NTR research, while also validating previously conducted research in identifying pathways, genes, and clusters that highlight p75NTR’s biological function. These results can be used to generate novel hypotheses to gain a greater understanding of p75NTR in acute or chronic brain injuries, other neurodegenerative diseases, and the general response to cellular injury.
Data availability
All data generated or analyzed during this study are included in this manuscript and its supplementary information files. Source data is included as supplementary file and also can be retrieved from PubMed.gov with term: (p75[All Fields] AND ("neurons"[MeSH Terms] OR "neurons"[All Fields] OR "neuron"[All Fields])) OR (p75[All Fields] AND ("brain"[MeSH Terms] OR "brain"[All Fields])) OR (p75[All Fields] AND neural[All Fields]) AND ("1998/11/13"[PDAT]: "2018/11/13"[PDAT]).
Code availability
Open source R statistical software were used with “PubMed.mineR” package19. Figure 1 was generated by using biorender tool (biorender.com). Network and pathway analyses were performed with ReactomeFIViz in Cytoscape software platform (https://www.cytoscape.org/), and respective network figures were generated by the same program tool20,21.
References
Dechant, G. & Barde, Y.-A. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat. Neurosci. 5, 1131–1136 (2002).
Park, H. & Poo, M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 (2013).
Thoenen, H. Neurotrophins and neuronal plasticity. Science 270, 593–598 (1995).
Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003).
Kraemer, B. R., Yoon, S. O. & Carter, B. D. The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handb. Exp. Pharmacol. 220, 121–164 (2014).
Patapoutian, A. & Reichardt, L. F. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 11, 272–280 (2001).
Lazarovici, P. et al. Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models. J. Mol. Neurosci. MN 48, 526–540 (2012).
Liu, L. et al. Panax notoginseng saponins promotes stroke recovery by influencing expression of Nogo-A, NgR and p75NGF, in vitro and in vivo. Biol. Pharm. Bull. 37, 560–568 (2014).
Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948 (2001).
Shi, J., Longo, F. M. & Massa, S. M. A small molecule p75NTR ligand protects neurogenesis after traumatic brain injury. Stem Cells 31, 2561–2574 (2013).
Diarra, A., Geetha, T., Potter, P. & Babu, J. R. Signaling of the neurotrophin receptor p75 in relation to Alzheimer’s disease. Biochem. Biophys. Res. Commun. 390, 352–356 (2009).
Yao, X.-Q. et al. p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol. Psychiatry 20, 1301–1310 (2015).
Fleitas, C. et al. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol. Brain 11, 68 (2018).
Fahnestock, M. & Shekari, A. ProNGF and neurodegeneration in Alzheimer’s disease. Front. Neurosci. 13, 129 (2019).
Bai, Y. et al. The in vivo brain interactome of the amyloid precursor protein. Mol. Cell. Proteomics MCP 7, 15–34 (2008).
Shepheard, S. R., Chataway, T., Schultz, D. W., Rush, R. A. & Rogers, M.-L. The extracellular domain of neurotrophin receptor p75 as a candidate biomarker for amyotrophic lateral sclerosis. PLoS ONE 9, e87398 (2014).
Shen, L.-L. et al. Neurotrophin receptor p75 mediates amyloid β-induced tau pathology. Neurobiol. Dis. 132, 104567 (2019).
Smith, D. H., Johnson, V. E., Trojanowski, J. Q. & Stewart, W. Chronic traumatic encephalopathy—confusion and controversies. Nat. Rev. Neurol. 15, 179–183 (2019).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2017).
Wu, G., Feng, X. & Stein, L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 11, R53 (2010).
Wu, G., Dawson, E., Duong, A., Haw, R. & Stein, L. ReactomeFIViz: a Cytoscape app for pathway and network-based data analysis. F1000Res. 3, 146 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995).
Yan, Q. & Johnson, E. M. An immunohistochemical study of the nerve growth factor receptor in developing rats. J. Neurosci. Off. J. Soc. Neurosci. 8, 3481–3498 (1988).
Ernfors, P. et al. The nerve growth factor receptor gene is expressed in both neuronal and non-neuronal tissues in the human fetus. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 9, 57–66 (1991).
Kim, E. Y. & Teh, H. S. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol. Baltim. Md 1950(167), 6812–6820 (2001).
Nalbandian, A. & Djakiew, D. The p75(NTR) metastasis suppressor inhibits urokinase plasminogen activator, matrix metalloproteinase-2 and matrix metalloproteinase-9 in PC-3 prostate cancer cells. Clin. Exp. Metastasis 23, 107–116 (2006).
Schecterson, L. C. & Bothwell, M. Neurotrophin receptors: old friends with new partners. Dev. Neurobiol. 70, 332–338 (2010).
Chesa, P. G., Rettig, W. J., Thomson, T. M., Old, L. J. & Melamed, M. R. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 36, 383–389 (1988).
Boiko, A. D. et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466, 133–137 (2010).
MacDonald, J. I., Gryz, E. A., Kubu, C. J., Verdi, J. M. & Meakin, S. O. Direct binding of the signaling adapter protein Grb2 to the activation loop tyrosines on the nerve growth factor receptor tyrosine kinase, TrkA. J. Biol. Chem. 275, 18225–18233 (2000).
Wang, T.-T. et al. Down-regulation of brain-derived neurotrophic factor and its signaling components in the brain tissues of scrapie experimental animals. Int. J. Biochem. Cell Biol. 79, 318–326 (2016).
Ma, Y. et al. Reduction of NF-κB (p65) in scrapie-infected cultured cells and in the brains of scrapie-infected rodents. ACS Chem. Neurosci. 8, 2535–2548 (2017).
Readhead, B. et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99, 64-82.e7 (2018).
Caldeira, M. V., Salazar, I. L., Curcio, M., Canzoniero, L. M. T. & Duarte, C. B. Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?. Prog. Neurobiol. 112, 50–69 (2014).
Skaar, J. R. & Pagano, M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr. Opin. Cell Biol. 21, 816–824 (2009).
Latina, V. et al. NGF-dependent changes in ubiquitin homeostasis trigger early cholinergic degeneration in cellular and animal AD-model. Front. Cell. Neurosci. 12, 487 (2018).
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000).
Lad, S. P. & Neet, K. E. Activation of the mitogen-activated protein kinase pathway through p75NTR: a common mechanism for the neurotrophin family. J. Neurosci. Res. 73, 614–626 (2003).
Haddad, Y., Adam, V. & Heger, Z. Trk receptors and neurotrophin cross-interactions: new perspectives toward manipulating therapeutic side-effects. Front. Mol. Neurosci. 10, 130 (2017).
da Silva Meirelles, L., Simon, D. & Regner, A. Neurotrauma: the crosstalk between neurotrophins and inflammation in the acutely injured brain. Int. J. Mol. Sci. 18, 1082 (2017).
Mostajeran, M., Edvinsson, L., Warfvinge, K., Singh, R. & Ansar, S. Inhibition of mitogen-activated protein kinase 1/2 in the acute phase of stroke improves long-term neurological outcome and promotes recovery processes in rats. Acta Physiol. Oxf. Engl. 219, 814–824 (2017).
Costa, A. P. et al. Differential activation of mitogen-activated protein kinases, ERK 1/2, p38(MAPK) and JNK p54/p46 during postnatal development of rat hippocampus. Neurochem. Res. 41, 1160–1169 (2016).
Liu, D. Z. et al. Inhibition of Src family kinases improves cognitive function after intraventricular hemorrhage or intraventricular thrombin. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 37, 2359–2367 (2017).
Schiel, K. A. A new etiologic model for Alzheimers disease. Med. Hypotheses 111, 27–35 (2018).
Zhao, D. et al. PTEN inhibition protects against experimental intracerebral hemorrhage-induced brain injury through PTEN/E2F1/β-catenin pathway. Front. Mol. Neurosci. 12, 281 (2019).
Patak, J. et al. MAGEL2-related disorders: a study and case series. Clin. Genet. 96, 493–505 (2019).
Crutcher, E. et al. mTOR and autophagy pathways are dysregulated in murine and human models of Schaaf–Yang syndrome. Sci. Rep. 9, 15935 (2019).
Kuwako, K., Taniura, H. & Yoshikawa, K. Necdin-related MAGE proteins differentially interact with the E2F1 transcription factor and the p75 neurotrophin receptor. J. Biol. Chem. 279, 1703–1712 (2004).
Funding
This work was supported by grants from the Maire Taponen foundation, Sigrid Juselius Foundation, Maud Kuistila Foundation, Finnish Medical Association and Emil Aaltonen Foundation to JK, and by the Maire Taponen foundation to AS, by the Academy of Finland (grant #17,379) and Maire Taponen Foundation to JPP.
Author information
Authors and Affiliations
Contributions
Studies were designed by J.K. Bioinformatic and statistical analyses were performed by J.K., A.S., Y.C. and Y.L. Results of the study were interpreted and the first draft was written by A.S., S.L, R.G. and J.K. The paper was edited and critically reviewed by J.F., T.R. I.H., C.D., J.U., R.T., J.P.P., S.R., F.K., M.R., J.R., and E.C. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sajanti, A., Lyne, S.B., Girard, R. et al. A comprehensive p75 neurotrophin receptor gene network and pathway analyses identifying new target genes. Sci Rep 10, 14984 (2020). https://doi.org/10.1038/s41598-020-72061-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72061-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.