Abstract
In this works, a simple, efficient and repeatable protocol was developed for in vitro regeneration via callus-mediated organogenesis of Neolamarkia Cadamba using cotyledonary petioles and hypocotyls. Effects of basal medium, plant growth regulators, the types and age of explant on the formation of adventitious buds/shoots were studied. Meanwhile, histological analysis for early ontogenic stages and genetic stability assessment by flow cytometry were investigated. Our investigation demonstrated that, compared with 6-benzyladenine (BA), N6-(2-isopentenyl) adenine (2-ip), Thidiazuron (TDZ) was the optimal cytokinin for buds/shoots induction on cotyledon and hypocotyl explants. Douglas-fir and sugar pine medium (DCR) supplemented with 22.7 μM TDZ and 0.27 μM α-naphthalene acetic acid (NAA) was most effective on bud induction, with the highest bud-induction rate and numbers of buds on cotyledon and hypocotyl explants. The available shoot per explant hit 35.2 when the induced callus sub-cultured to a medium without TDZ. It was found that TDZ could promote induction of the callus and the buds, however, continuous exposure beyond 4 weeks of supplemented high concentration (exceed 11.35 μM), TDZ was harmful to the proliferation and growth of buds/shoots. DCR appeared more efficiency than Murashige and Skoog medium (MS), Woody Plant medium (WPM), anther culture of cereal crops medium (N6) on bud induction. Age of cotyledon and hypocotyl explants in 20-day to 25-day was most beneficial to adventitious buds/shoots formation. Histological investigation confirmed that the buds originated from the wounded incisions of cotyledonary petiole and hypocotyl fragments, with callus formation. The regeneration plantlets were successfully acclimatized in greenhouse, yielded above 95% survival rate in field, exhibited normal morphology and growth characteristics. The analysis of flow cytometry on N. cadamba indicated no variation in the ploidy levels between the regenerated plantlets and the donor trees. The developed procedure can be used for mass production, germplasm exchange and transgenic studies to improve the resistance of the species via Agrobacterium-mediated.
Similar content being viewed by others
Introduction
Neolamarckia cadamba (commonly known as burflower tree, kadamba tree or kadamb) is a tropical tree of great economic importance. First, N. cadamba trees grow fast, and their wood is good for pulp production, furniture, and other construction purposes1. Second, N. cadamba has been traditionally used as medicine to treat diseases such as dysentery, fever and snake bites 2,3. Now, from its bark, leaves, and flowers people extract phytochemical compounds, including monoterpenoid, triterpenoid saponin, and ethylene glycol4,5,6,7,8,9, alkaloid10, cadambine7,11, and use them to treat hyperglycemia12, hyperlipidemia13,14, hypertension15, and wound and skin diseases16. Third, N. cadamba is a desirable landscape tree, and is suitable for reforestation programs. Because it grows extremely fast and all parts of the tree can bring profits to the growers, N. cadamba is called “miracle tree” and “gems tree”.
N. cadamba, however, is susceptible to many biotic and abiotic stresses, and needs improvement. For example, because they are juicy and nutritious, buds and young leaves are often attacked by Lepidoptera and Coleoptera insects such as Dianhania glauculelis and Acalolepta cervina17. Because they are especially sensitive to frost, currently N. cadamba trees are broadly planted only in India, Nepal, Thailand, Malaysia, Papua New Guinea and warm areas of China18,19. In order to make it more profitable to grow N. cadamba trees, breeding to improve important agronomic traits becomes increasingly important.
Since N. cadamba trees produce seeds through cross-pollination and seeds take above 5 years to grow into mature trees that start to flower, breeding N. cadamba using conventional methods will be too slow. Therefore, to shorten its breeding process, we must develop and adopt new techniques. One such technique is micropropagation, which can rapidly produce clones of selected individuals that carry the desired traits and thus save years on segregation and selection. Protocols for micropropagating N. cadamba is now available20,21,22,23,24, although further refinement is needed for the protocols to be effective on broader genotypes and explant sources. Another way to speed up N. cadamba breeding is genetic engineering. By using genetic engineering techniques, we can deliver trait-determining genes into somatic cells and from transformed cells regenerate trees with the desired traits, a procedure that may be completed in months instead of the many years that a single sexual reproduction cycle takes. Genetic engineering includes multiple steps. For its successful use in the improvement of N. cadamba, a key step is genetic transformation, which requires an efficient regeneration protocol25.
Previously, we reported a procedure for adventitious buds/shoots induction from the cotyledons of N. cadamba21. The directly regeneration protocol is ideal for micropropagation to obtain a large number of elite seedlings, because the buds/shoots originate directly from epidermal cells, bypassing a prominent callus stage and thus reducing the chance of epigenetic variation26. The protocol, however, is not suitable for genetic transformation (based on this directly regeneration protocol, we attempted to do many genetic transformation experiments, but all failed), which usually includes a step of selection for transformations at the callus-induction stage. Therefore, we set about developing a new protocol that would be easily adapted for genetic transformation. We first tested and identified plant growth regulators that could efficiently induce adventitious buds/shoots through callus-mediated organogenesis, and then optimized the protocol by testing different basal media, subculture media and types of explants. Last, we tested procedures for root induction, acclimation, ploidy of regenerated plants.
Materials and Methods
Explant sources
We collected mature seeds from a N. cadamba tree of over 10 years old in Guangxi Botanical Garden of Medicinal Plants (Nanning, China). To promote germination, we first soaked seeds in 40 °C water for 24 h, by placing the container on a thermostat shaker set to 40 °C and 120 rpm. Then we sterilized the seeds with 20% bleach (5.0% sodium hypochlorite) for 15 min, rinsed them with water for three times, and planted them on MS agar medium supplemented with 3.0% (w/v) sucrose and 0.6% (w/v) plant agar. Seeds germinated, and seedlings grew under this condition: 25 ± 2 °C; 14-h photoperiod of 90 μmol m−2 s−1 irradiance; and relative humidity of 70%. From 20-day to 45-day seedlings we collected cotyledons (with or without cotyledonary petiole) and hypocotyl fragments (3–4 mm in length), and used them as explants.
Adventitious buds/shoots induction
To induce adventitious buds/shoots, we cultured cotyledon explants (abaxial side touching medium surface) and hypocotyl fragments (flat on medium surface) on different induction media under this condition: 25 ± 2 °C; 14-h photoperiod of 90 μmol m−2 s−1 irradiance; and relative humidity of 70%. All induction media were supplemented with 3.0% (w/v) sucrose and 0.6% (w/v) plant agar, and their pH was adjusted to 5.8. Media were autoclaved at 121 °C for 20 min, cooled to ~60 °C before adding plant growth regulators (PGRs), and aliquoted into sterile 250-ml flasks (~25 ml each). The PGRs used in this study (2-ip, TDZ, BA and NAA) were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd (Beijing, China). The basal media (MS, DCR, N6, WPM) were prepared according to original publications27,28,29,30.
Root induction and plantlet acclimatization
Procedures follow Huang et al.21. Briefly, elongated adventitious shoots (3–4 cm) were transferred to MS agar medium supplemented with 0.25 μM IBA and 0.27 μM NAA, and cultured at 25 ± 2 °C, first in dark for 2 days and then under a 12-h photoperiod (90 μmol m−2 s−1) for 8 days. For acclimatization, flasks containing plantlets were moved from culture room to greenhouse and kept there for 3–4 days. The plantlets were then removed from flask and transferred to soil.
Paraffin sections
To trace the early ontogenic stages of bud regeneration, samples were periodically taken and fixed in FAA solution30 (37% formalin: glacial acetic acid: 50% ethanol, ratio 5:5:90, in volume) until the adventitious buds were discernible with the naked eye. The samples were progressively dehydrated in a graded ethanol series (70–100%), then embedded in paraplast and mounted on block-holders. Paraffin blocks were sectioned into 8-μm slices with a Reichert 820 H Histostat rotary microtome (Warer-Lambert Tech. Inc., USA). The sections were affixed to slides, stained with fast green, covered with a cover slip in place with help of a thin coating of Neutral Balsam, and then dried at 38 °C for 48 h30. All sections were observed and photographed with a Leica DMLB microscope (Leica, Inc. Germany).
Assessment of the ploidy of regenerated plants
Young leaves from regenerated plants and donor plants were collected, and their DNA contents were analyzed using a flow cytometer (BD Accuri C6 Plus, USA). Sample process and operation followed the instruction manual. Software CFlow Plus was used to analyze the data and generate the figures.
Statistical analysis
Data from all experiments were subjected to ANOVA. The means were compared using software SPSS (version 19.0) to carry out Duncan’s multiple range test.
Result and Discussion
Effects of PGRs on induction of adventitious buds/shoots
In our previous study21, the most efficient bud/shoot induction was achieved by culturing cotyledon explants on DCR medium supplemented with synthetic plant hormones NAA (0.27 μM) and BA (22.22 μM). In this study, we continued to use DCR medium supplemented with a fixed concentration (0.27 μM) of auxin NAA, but tested different concentrations of 3 cytokinins: BA, 2-ip and TDZ. In addition to cotyledons, we also tested hypocotyl fragments as explants. At the end of 4-week culture, we counted and calculated the percentages of explants that produced adventitious buds (originated from callus, about 0.3 cm in length) and the average number of shoots (originated from the adventitious buds above 0.7 cm length) per explant.
Plant hormone cytokinins are divided into two main types: adenine-type cytokinins and phenylurea-type cytokinins. Both BA and 2-ip belong to the first group, and they showed similar effects in our study. First, at concentration of 22.22 μM, BA induced adventitious buds on 52.4% of cotyledon explants, and each explant produced, on average, 4 shoots (Table 1), a result that was comparable with our previous observations, 54.0% and 4.4 shoots21. Second, also at its optimum concentration (12.30 μM), 2-ip induced adventitious buds on 49.8% of cotyledon explants, although explants produced fewer shoots, only 1 shoot per explant (Table 1). Third, similar to BA, 2-ip induced buds/shoots to develop directly from the epidermal cells at the edge of the cuts, without an intermediate stage of callus (data not shown). Based on these observations, we concluded that BA and 2-ip did not suit our purpose.
Different from BA and 2-ip, TDZ is a phenylurea-type and potent cytokinin for plant tissue culture31. The biological action of TDZ has been suggested to be superior or similar to that of the most active adenine-type cytokinins32. It plays a role in modulation of the endogenous PRGs either directly or as a result of induced stress33,34, and has been shown to be the most critical factor for somatic embryogenesis induction and buds/shoots regeneration35,36.
In in vitro regeneration of N. cadamba, we found that TDZ was far more effective in inducing adventitious buds of N. cadamba, and deduced that the bud formation was through callus-mediated organogenesis. On cotyledon explants and for all tested concentrations except the lowest one (2.27 μM), the percentage of bud-induction was 100% (Table 1). On hypocotyl explants and for the two higher concentrations (11.35, 22.70 μM), the percentage of induction also reached 100% (Table 1). At all concentrations tested, TDZ induced shoot formation on both cotyledon and hypocotyl, and the average numbers of shoots per explant ranged from 1.3 to 3.3, with no obvious difference between the two types of explants.
This effect of TDZ is in stark contrast to that of BA and 2-ip, which could effectively induce the formation of buds/shoots on cotyledon explants, but had no or little effect on hypocotyl explants (Table 1). This contrast hinted that TDZ-induced buds did not arise directly from the cells of cotyledon or hypocotyl, two plant organs that differ obviously in shape and conceivably in cell states and the competency for bud formation, instead, they had to arise from calluses–cell masses that originate from different explants but have similar cell states and thus similar rates of buds/shoots formation.
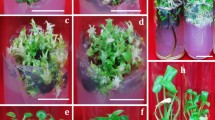
Indeed, we observed that clusters of calluses started to emerge from the incisions of cotyledonary petioles and hypocotyl fragments at day 6, and, after continued culture on the same TDZ-containing medium, on some calluses developed adventitious buds (Fig. 1c,d). We sampled explants at different culture stages and did histological analysis. We confirmed that the calluses were truly unstructured cell masses and adventitious buds arose randomly from calluses, clearly different from the bud formation induced by BA or 2-ip, which was directly from epidermal cells21. Therefore, cytokinin TDZ well suited our purpose, and next we needed to choose a concentration.
Effect of adventitious bud formation from N. cadamba on DCR medium containing 11.35 μM TDZ and 0.27 μM NAA. Callus (arrow) developed from the cotyledons (a) and hypocotyls (b) after 4 weeks of incubation. (c,d) Adventitious buds exhibited abnormal and the leaf became involute after 6 weeks of culture. After 4 weeks of culture, subculture on DCR medium containing 22.70 μM BA and 0.27 μM NAA in 20 d (e), 30 d (f).
Among the 4 TDZ concentrations, as shown in Table 1, 22.7 μM is most effective in inducing adventitious buds/shoots. However, on medium containing 22.7 μM TDZ, many adventitious buds refused to grow after formation and elongated shoots grew involute leaves (Fig. 1c,d). The problem became more severe after extended culture (>6 weeks). On medium containing 11.35 μM TDZ, the problem was less prominent, but the average number of shoots per explant was lower. Liu et al.37 also reported the similar observations on Jatropha curcas that both the concentration and the exposure duration of TDZ on explants influence shoot proliferation and elongation. And, in the in vitro regeneration of Rauvolfa tetraphylla38, Vitex trifolia39, it was found that prolonged exposure of the culture to TDZ had an adverse effect, too.
To solve the problem and to optimize the protocol, we used two strategies: (1) culture for 4 weeks on medium containing 22.7 μM TDZ to induce adventitious buds, and then subculture on medium containing a lower concentration of TDZ or a different cytokinin for buds to elongate; meanwhile, (2) fix TDZ concentration to 11.35 μM and test other factors that may affect efficiency: basal medium and explant type and age.
Effect of cotyledonary petiole and explant age on the formation of adventitious buds
When cotyledon explants were cultured on TDZ-containing medium, calluses consistently emerged from the cut zone of the petiole and, less frequently, they also formed on the lower epidermis (also the side touching medium surface) of cotyledon. On the epidermis-derived calluses, however, we never saw adventitious buds. This phenomenon prompted us to compare cotyledon explants with and without petioles. As shown in Table 2, without petioles, calluses could still form on 100% of the cotyledon explants, but none developed adventitious buds. This result and our previous studies21 suggested that it was key to include with the cotyledon explant a section of the petiole. The cotyledon explants without petioles could not be induced to buds/shoots formation in the induction medium with only plant hormone cytokinins and NAA.
As shown above, calluses derived from cotyledons without petioles did not give rise to adventitious buds, suggesting a strong influence of the type of explants. We wondered whether bud formation was also affected by the age of explants. Therefore, we collected cotyledon and hypocotyl explants from seedlings of different ages, and determined their shoot formation rates on DCR medium containing 11.35 μM TDZ and 0.27 μM NAA. Surprisingly, the rate was not significantly affected by the age in 21-day to 35-day; the induction rate remained above 97.0% when explants reached 35-day old, and the lowest percentage (78.7%) occurred on cotyledon in 45 days (Fig. 2). Moreover, result hinted that buds/shoots formation appeared gradually decrease when the explants got older (after 25-day), perhaps cell dedifferentiation became more difficult on the induction medium. In our research, we continued to use explants from 20-day to 25-day seedlings, because they were not only easier to collect than the smaller explants of about 2 weeks old, but also could produce more buds/shoots than the explants of above 30-day old (data not shown).
Effect of basal medium on the formation of adventitious buds/shoots
When determining that cytokinin TDZ, in combination with auxin NAA, could induce adventitious buds on 100% of explants, we did all tests on DCR medium. We wondered whether the number of buds per explant could be increased by using different media. For this purpose, we fixed the concentrations of TDZ and NAA to 11.35 μM and 0.27 μM, and compared the effects of 4 commonly used basal media, DCR, MS, N6 and WPM. As shown in Table 3, basal medium indeed had a profound effect on bud regeneration. Medium DCR was most effective, with bud induction rate of 100% and the highest numbers of buds on both cotyledon and hypocotyl explants. As DCR, MS medium also had bud induction rate of 100%, but produced fewer buds than DCR. Basal media N6 and WPM had significantly lower bud induction rates and produced fewer buds. Therefore, basal medium DCR was the best choice, and then followed by MS, N6, WPM. It was similar to the publication of Joshi et al.23, that is, on three basic medium (MS, B5 and White), they found that MS medium was more suitable for the bud break and shoots developed, too. These results showed that different basic medium had a significant effect on buds/shoots formation, which was probably due to the difference of chemical element content in different basic medium.
Effect of subculture medium on TDZ-induced adventitious buds/shoots
We had found that 22.70 μM TDZ, in combination with 0.27 μM of NAA, was most effective in inducing adventitious buds and shoots, but many buds refused to grow after formation and elongated shoots were morphologically abnormal. We solved this problem by using a simple strategy–subculture. To ensure efficient bud induction, explants were first cultured for 4 weeks on 22.70 μM TDZ-containing medium, and then, for the adventitious buds to grow, explants were subcultured for another 3 to 4 weeks on a fresh medium, which contained a lower concentration of TDZ, no hormones, or a different cytokinin. As shown in Table 4, when TDZ concentration was reduced to 9.08 μM, subculture yielded 8.9 shoots per explant, a significant increase from the ~3 shoots produced during initial culture, and the high concentration TDZ-caused bud/shoot abnormalities diminished. When hormones were entirely eliminated, subculture yielded 7.6 shoots per explant, also a significant increase, and furthermore, shoots from the hormone-free subculture medium were perfectly normal. The most striking results, however, were obtained when TDZ was replaced with 22.70 μM BA. On this subculture medium, not only shoots grew normal (Fig. 1e,f)), the average number of available shoots per explant (35.2) also reached unprecedentedly high. The new shoots originated from both the adventitious buds formed during initial culture and the auxiliary buds of adventitious shoots. Similar effect has been reported in several species regeneration including Phalaenopsis40, Diospyros kaki41 and Cicer arietinum42.
Shoot propagation, root formation and acclimatization
In these sections, we carried out in accordance with the protocol of our previous publication21, the result showed that shoot propagation was achieved successively on the MS medium containing 4.44 μM BA and 0.25 μM IBA, the shoots grew robust, well elongate and more adventitious shoots. The 1/2MS medium containing 0.25 μM IBA and 0.27 μM NAA was the most effect for in vitro rooting, root induction rates and numbers of roots were 95.4% and 6.0, respectively. The plantlets yielded above 95% successful acclimatization rate, and grew in the field with normal phenotypes (Fig. 3).
Histological investigations
Histological analysis provided morphological details that help explain the process of organogenesis from the explants43. At different regeneration stages of explants, it was found that the incision of explants was the source of callus and organs.No significant histological changes were observed during the first 5 days. The first distinct change was that the cells dedifferentiated along the incision of cotyledon and hypocotyl. After 3 weeks of culture, the clusters of callus structures were formed, a few developed adventitious buds/shoots, but most callus were to maintain clusters of callus structures, and the origin of the bud was random happened from the callus (Fig. 4). From the histological analyses on this experiment and our previously study21, it was indicated that the regeneration process on DCR medium contained TDZ and NAA was indirect organogenesis pattern.
The regenerated plants ploidy assessment by flow cytometry
In the regeneration approach of this study, we used sterile cotyledon and hypocotyl as explants to induce adventitious buds/shoots. To verify the effectiveness of this method, Flow cytometry were used to detect the similarities in the DNA ploidy levels between the donor tree and the regenerated plant. The result showed that the peak of fluorescence intensity was approximately equal, the fluorescence intensity of donor tree and induction plantlet were 126964 and 130314, respectively (Figs. 5 and 6). The values indicated, under the condition of in vitro culture, there was good genetic stability between the donor tree and the regenerated plant without any somaclonal variation. Furthermore, it was found that these plantlet phenotypic characteristics had no different from the donor plant after they grew on field in 1.5a.
Conclusion
The study demonstrates, for the first time, an improved, reproducible and highly efficient buds/shoots regeneration protocol of N. cadamba was developed in both cotyledonary petiole and hypocotyl explants, used the cytokinin TDZ via an intermediate callus formation. Obviously, in terms of proliferation multiple and potential genetic transformation ability, this in vitro regeneration strategy is superior to our previous research20. In addition, this is the first report of genetically sustainable proliferation via cotyledonary petiole and hypocotyl explants in N. cadamba. it would facilitate successful approach for the mass production of genetically consistent plants, ex situ conservation of elite germplasm of this sensitive, multipurpose tree in the future. Furthermore, this simple, efficient and reproducible regeneration strategy of regeneration can be applied to transgenic studies to improve the resistance of the species via Agrobacterium-mediated.
References
Lal, M., Dutt, D., Tyagi, C. H., Upadhyay, J. S. & Upadhyay, S. Characterization of Anthocephalus cadamba and its delignification by kraft pulping. Tappi J. 9(3), 30–37 (2010).
Banerji, N. & Dutta, N. L. Structure of a new saponin from stem bark of Anthocephalus cadamba Mig. Indian J. Chem B. 14, 614 (1976).
Buchke, V. A., Bachhav, R. S. & Saudagar, R. B. Analgesic and anti-inflammatory of Anthocephalus cadamba leaves. Indian J. Pharmacol. 40, 90 (2008).
Ahmed, F. et al. Evaluation of Neolamarkia cadamba(Roxb.) Bosser leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr. J. Tradit Complem. 8(1), 79–81 (2011).
Alam, M. A. et al. Antidiarrhoeal property of the hydroethanolic extract of the flowering tops of Anthocephalus cadamba. Rev. Bras. Farmacogn. 18(2), 155–159 (2008).
Banerji, N. Structure of two new saponins from stem bark of Anthocephalus cadamba Miq. J. Indian Chem. Soc. 55(3), 275–278 (1978).
Handa, S. S., Borris, R. P. & Cordel, G. A. Nmr spectral analysis of cadambine from Anthocephalus chinensis. J. Nat. Prod. 46(3), 325–330 (1983).
Sahu, N. P. et al. Structures of two novel isomeric triterpenoid saponins from Anthocephalus cadamba. Magn. Reson. Chem. 37, 837–842 (1999).
Zhou, H., He, H., Kong, N., Wang, T. & Hao, X. Indole alkaloids from the leaves of Anthocephalus chinensis. Helv. Chim. Acta. 91(11), 2148–2152 (2008).
Liu, L. L. et al. c A and B, two novel indole alkaloids from Neolamarckia cadamba. Tetrahedron Lett. 51(43), 5670–5673 (2010).
Takayama, H. et al. Gluco-indole alkaloids from Nauclea cadamba in thailand and transformation of 3 alpha-dihydrocadambine into the indolopyridine alkaloid, 16-carbomethoxynaufoline. Chem. Pharm. Bull. 51(2), 232–233 (2003).
Alam, M. A. et al. Anthocephalus cadamba extract shows hypoglycemic effect and eases oxidative stress in alloxan-induced diabetic rats. Braz J. Pharmacogn. 21(1), 155–164 (2011).
Kumar, V. et al. Lipid lowering activity of Anthocephalus Indicus root in hyperlipidemic rats. Evid.-Based Compl. Alt. 7(3), 317–322 (2010).
Kumar, V. et al. Hypolipidemic activity of anthocephalus indicus (Kadam) in hyperlipidemic rats. Med. chem. res. 17(2–7), 152–158 (2008).
Zhong, J. Y., Wang, W. D., Zhang, Z. X. & Jiang, Z. D. Chemical constituents from the bark of Anthocephalus chinensis. Acta Bot. yunnan. 12(4), 453–456 (1990).
Umachigi, S. P. et al. Antimicrobial, wound healing and antioxidant activities of Anthocephalus cadamba. Afr. J. Tradit Complem. 4(4), 481–487 (2007).
Zhao, L., Chen, P. & Li, Q. Investigation on major insect pests damaging six valuable broadleaved tree species in tropical areas of yunnan province. Journal of Southwest Forestry College. 28(3), 30–35 (in Chinese). (2008).
Flora Of China Editorial Committee, Flora of China, 71(1), 261 (in Chinese). (Beijing & St. Louis, 1999).
Dwevedi, A., Sharma, K. & Sharma, Y. Cadamba: a miraculous tree having enormous pharmacological implications. Phcog Rev. 9(18), 107–113 (2015).
Li, J., Zhang, D., Ouyang, K. & Chen, X. High frequency plant regeneration from leaf culture of Neolamarckia cadamba. Plant Biotechnol.-Nar. 36(1), 13–19 (2019).
Huang, H. et al. Direct adventitious shoot organogenesis and plant regeneration from cotyledon explants in Neolamarckia cadamba. Plant Biotechnol.-Nar. 31(2), 115–121 (2014).
Zhan, Y. L. A study on tissue culture and rapid propagation of Neolamarckia cadamba (Roxb.): South China Agricultural University, 2010.((Mater dissertation, in Chinese)
Joshi, A. & Mathur, N. In Vitro propagation and conservation of Anthocephalus cadamba through apical bud and nodal explants a valuable medicinal plant. Plant. J of Biotechnology. 4(3), 8–18 (2015).
Pei-Kieng, M. & Wei-Seng, H. Rapid in vitro propagation and efficient acclimatisation protocols of Neolamarckia cadamba. Asian J. Plant Sci. 18(4), 153–163 (2019).
Sanyal, I., Singh, A. K., Kaushik, M. & Amla, D. V. D. R. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with bacillus thuringiensis Cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Science (Oxford). 168(4), 1135–1146 (2005).
Stroud, H. et al. Plants regenerated from tissue culture contain stable epigenome changes in rice. Elife. 2, e00354, https://doi.org/10.7554/eLife.00354 (2013).
Gupta, P. K. & Durzan, D. J. Shoot multiplication from mature trees of douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 4(4), 177–179 (1985).
Lloyd, G. & McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proceedings of the International Plant Propagators’ Society. 30, 421–427 (1981).
C., C. & W., C. Establishment of an efficient medium for anther culture in rice through comparative experiments on the nitrogen sources. Sci. China Math. 5 (18), 659–668 (1975).
Jensen, W. A. Botanical Histochemistry: Principles and Practice, 408, (Freeman, 1962).
Huetteman, C. A. & Preece, J. E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss. Org. Cult. 33(2), 105–119 (1993).
Mok, M. C., Martin, R. C. & Mok, D. Cytokinins: biosynthesis, metabolism and perception. In Vitro Cell. Dev.-Pl. 36(2), 102–107 (2000).
Murthy, B. N. S., Murch, S. J. & Praveen, K. S. Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regulator levels and significance of cotyledons. Physiol. Plantarum. 94(2), 268–276 (1995).
Xiao-Ying, C., Qing-Sheng, Y. & Wei, L. A review of recent advances in TDZ. Subtropical Plant Sci. 32(03), 59–63(in Chinese) (2003).
Yang, X., Lü, J., Silva, J. A. T. D. & Ma, G. Somatic embryogenesis and shoot organogenesis from leaf explants of Primulina tabacum. Plant Cell Tiss. Org. Cult. 109(2), 213–221 (2012).
Panda, B. M. M. G. & Hazra, S. Micropropagation of Semecarpus anacardium L.: a medicinally important tree species. Plant Biosystems. 146(Suppl. 1), 61–68 (2012).
Liu, Y. et al. Efficient culture protocol for plant regeneration from cotyledonary petiole explants of Jatropha curcas L. Biotechnol. Biotec. Eq. 30(5), 907–914 (2016).
Hussain, S. A., Ahmad, N. & Anis, M. Synergetic effect of TDZ and BA on minimizing the post-exposure effects on axillary shoot proliferation and assessment of genetic fidelity in Rauvolfia tetraphylla (L.). Rend. Lincei-sci Fis. 29(1), 109–115 (2018).
Ahmed, M. R. & Anis, M. Role of TDZ in the quick regeneration of multiple shoots from nodal explant of Vitex Trifolia L.–an important medicinal plant. Appl. Biochem. Biotech. 168(5), 957–966 (2012).
Cheng, Q. et al. The high-frequency induction of adventitious shoots and plant regeneration from leaf explants of Phalaenopsis with added thidiazuron. J. Plant Sci. 29(4), 524–530 (in Chinese) (2011).
Yokoyama, T., Moriyasu, Y. M. M. S. & Sugawara, Y. Adventitious bud formation through nodule induction by thidiazuron in cultured leaf segments of the Japanese persimmon (Diospyros kaki Thunb.). Plant Biotechnol.-Nar. 28(3), 339–344 (2011).
Murthy, B. N. S., Victor, J., Singh, R. P., Fletcher, R. A. & Saxena, P. K. In vitro regeneration of chickpea (Cicer Arietinum L.): stimulation of direct organogenesis and somatic embryogenesis by thidiazuron. Plant Growth Regul. 19(3), 233–240 (1996).
Ghimire, B. K. et al. High-frequency direct shoot regeneration from drymaria cordata willd. Leaves. Plant Cell Tiss. Org. 100(2), 209–217 (2010).
Acknowledgements
This research was jointly supported by Guangxi Natural Science Foundation [2018GXNSFAA138094], Science and Technology Program of Guangdong[2017B020201008], the Forestry Science and Technology Innovation Program of Guangdong Province [2018KJCX001], Guangzhou Science and Technology Innovation Commission [201707010462] and Science and Technology Innovation Team Program of Guangxi Botanical Garden of Medicinal Plant [2019003,2019007]. We are highly grateful to Dr. Changqing Zhang (Plants for Human Health Institute &Department of Plant and Microbial Biology, North Carolina State University) for his helpful modifications and suggestions on the structure and language of the manuscript.
Author information
Authors and Affiliations
Contributions
H.H. conception and design, acquisition of data, analysis and interpretation of data, drafting or revising the manuscript; L.B. and X.C. conception and design. Y.W., Y.Z. and K.O. conducted the experiments, analyzed the results. All authors have read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, H., Wei, Y., Zhai, Y. et al. High frequency regeneration of plants via callus-mediated organogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia Cadamba). Sci Rep 10, 4558 (2020). https://doi.org/10.1038/s41598-020-61612-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61612-z
This article is cited by
-
Indirect organogenesis and in vitro bulb formation of Pancratium maritimum
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Direct organogenesis from cortical cells of hypocotyl segments in soybean
In Vitro Cellular & Developmental Biology - Plant (2023)
-
High frequency adventitious shoot regeneration from hypocotyl-derived callus of Glyptostrobus pensilis, a critically endangered plant
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Shoot organogenesis and somatic embryogenesis from leaf and petiole explants of endangered Euryodendron excelsum
Scientific Reports (2022)
-
Tissue culture mediated biotechnological interventions in medicinal trees: recent progress
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.