Abstract
We conducted a systematic review and meta-analysis to assess the clinical efficacy of high-flow nasal cannula (HFNC) therapy as apneic oxygenation in critically ill patients who require endotracheal intubation in the intensive care unit (ICU). This systematic review and meta-analysis included six randomized controlled trials and a prospective study identified in PubMed, Embase, Cochrane Library, and the Web of Science until August 18, 2019. In this meta-analysis including 956 participants, HFNC was noninferior to standard of care during endotracheal intubation regarding incidence of severe hypoxemia, mean lowest oxygen saturation, and in-hospital mortality. HFNC significantly shortened the ICU stay by a mean of 1.8 days. In linear meta-regression interaction analysis, the risk ratio of severe hypoxemia decreased with increasing baseline partial oxygen pressure (PaO2) to fraction of inspired oxygen (FiO2) ratio. In subgroup analysis, HFNC significantly reduced the incidence of severe hypoxemia during endotracheal intubation in patients with mild hypoxemia (PaO2/FiO2> 200 mmHg; risk difference, −0.06; 95% confidence interval, −0.12 to −0.01; number needed to treat = 16.7). In conclusion, HFNC was noninferior to standard of care for oxygen delivery during endotracheal intubation and was associated with a significantly shorter ICU stay. The beneficial effect of HFNC in reducing the incidence of severe hypoxemia was observed in patients with mild hypoxemia.
Similar content being viewed by others
Introduction
Each year in the United States, approximately 1.5 million patients are estimated to receive endotracheal intubation and the rate of intubation is increasing in the hospital1. Hypoxemia, a frequently reported complication of intubation, is considered a predisposing factor for cardiac arrest and death2,3,4,5. Therefore, oxygenation during endotracheal intubation plays an important role in prolonging the maintenance of acceptable oxygen saturation levels.
In 1959, Frumin et al.6 were the first to develop apneic oxygenation, which delivered supplemental oxygen via nasal cannulation during surgery and anesthesia to allow for sustained levels of sufficient oxygen in alveoli and blood. A recent clinical trial convincingly demonstrated that apneic oxygenation during endotracheal intubation reduced the incidence of hypoxemia while increasing first-pass success rate and peri-intubation oxygen saturation7. Nevertheless, conventional oxygen therapy, which utilizes nasal cannulas, simple face masks, or Venturi masks for oxygenation, is sometimes ineffective in critically ill patients, especially in those with hypoxemia8.
High-flow nasal cannula (HFNC) is a novel respiratory management strategy that delivers humidified and warm supplemental oxygen at flow rates of up to 60 L/min in adults. Compared to standard of care oxygen therapy, HFNC has several physiological advantages, including increased positive end-expiratory pressure (PEEP), and constant fraction of inspired oxygen (FiO2)9. Due to the ease of setting, tolerance, and effectiveness, HFNC is a widely used in patients in the intensive care unit (ICU) for hypoxemic respiratory failure10.
Consequently, HFNC as apneic oxygenation during endotracheal intubation is proposed to be beneficial in preventing hypoxemia. Miguel-Montanes et al.11 reported that HFNC led to a significant reduction in the incidence of severe hypoxemia (peripheral capillary oxygen saturation [SpO2] < 80%) compared with the bag-valve mask during intubation (2% vs 14%). Similarly, Vourc’h et al.12 found that the lowest oxygen saturation level was higher with HFNC. Despite these encouraging benefits of HFNC, the latest randomized control trial (RCT)13 by Frat et al. reported that HFNC did not significantly alter the risk of severe hypoxemia during intubation.
RCTs reported contradictory outcomes with HFNC as apneic oxygenation, and there is currently no consensus regarding whether the rate of hypoxemia is lower with HFNC during endotracheal intubation than with standard of care oxygen treatment. Therefore, there is an urgent need for evidence synthesis based on the comparison of HFNC with standard of care during intubation in critically ill patients.
Methods
Data sources and searches
We performed a comprehensive search without language restrictions using PubMed, Embase, Cochrane Library, and the Web of Science to identify studies that assessed the outcomes of HFNC as apneic oxygenation during endotracheal intubation in critically ill patients, including those with hypoxemic respiratory failure, those in a comatose stage, and those with hemodynamic dysfunction, in the setting of ICU.
Two independent investigators (P.H.C. and H.J.J.) conducted a systematic search using the terms “high-flow nasal cannula”, “apneic oxygenation”, and “intubation” and utilized medical subject headings or their equivalents and normal text keywords as search terms (Supplementary Information 1) until 18 August, 2019. Manual screening for references from original articles, previous systematic reviews, and conference abstracts was performed to identify eligible studies.
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) (Supplementary Information 2) and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (Supplementary Information 3) guidelines for performing systematic reviews and meta analyses of RCTs and observational studies14,15. The protocol for this systematic review had registered with PROSPERO (CRD42019139408). The first or corresponding authors of the studies were contacted to provide additional information if required.
Eligibility criteria and exclusion criteria
To be included in the analysis, the studies had to meet the following criteria: (1) study cohort comprising only adult patients requiring endotracheal intubation who were admitted to the ICU; (2) administration of HFNC cannula therapy, also known as trans-nasal humidified rapid-insufflation ventilatory exchange16 (oxygen delivery system comprising 100% humidified and heated oxygen at a flow rate > 15 L/min and up to 70 L/min) during the apneic period of endotracheal intubation, compared to standard of care (i.e., no management or oxygen administration by nasal cannulas, simple face masks, or Venturi masks during endotracheal intubation); (3) RCT or prospective non-randomized study.
The studies meeting the following criteria were excluded from the analysis: (1) endotracheal intubation performed in an out-of-hospital setting or in the operating room, (2) lack of reporting on outcomes of interest such as lowest oxygen saturation and number of desaturation events during endotracheal intubation.
Two reviewers (H.J.J. and P.H.C.) appraised all eligible citations independently and extracted various data into an electronic database from original manuscripts of eligible studies. In case of disagreement, the same authors consulted with another author (C.H.L.), the decisions were obtained after group discussion.
Outcome measurement
The following outcomes were extracted: (1) major outcomes: incidence of severe hypoxemia (SpO2 < 80%) during the endotracheal intubation, mean lowest oxygen saturation during endotracheal intubation, ICU length of stay, and in-hospital mortality; (2) minor outcomes: incidence rates of mild hypoxemia (SpO2 < 90%) and life-threatening hypoxemia (SpO2 < 70%) during the endotracheal intubation, first-pass success (success on the first laryngoscopy attempt), duration of endotracheal intubation procedure period, shock (defined by systolic blood pressure <80 mmHg or that requiring vasopressor introduction or increasing vasopressor dose by more than 30%)17, cardiovascular complications (defined as shock, arrhythmia, and cardiac arrest)18, ventilator-associated pneumonia (pneumonia that occurs within 48–72 hours following mechanical ventilation)19, and duration of ventilation.
For dichotomous outcomes, we extracted the proportions in both the experimental and the comparator arms. For continuous outcomes, we extracted the number of participants as well as the mean values with the standard deviation for the outcome measurement per arm.
The quality of the RCTs (Supplementary Information 4) was appraised by H.J.J. and P.H.C using the Cochrane Handbook for Systematic Reviews of Interventions20. Furthermore, we assessed the quality of prospective non-randomized studies using the Newcastle-Ottawa Scale21. Any disagreement was resolved via group discussions22. Risk of bias graphs were generated using Review Manager 5.3 software23.
Data synthesis and analysis
We conducted data analysis as recommended in the Cochrane Handbook for Systematic Reviews of Interventions20. We calculated dichotomous outcomes by conducting random-effects meta-analysis proposed by DerSimonian and Laird24 and the Mantel-Haenszel fixed-effects model25 using risk difference (RD) with 95% confidence interval (CI). To measure continuous outcomes, we employed the generic inverse variance method fixed-effect model and DerSimonian and Laird random-effects model24 meta-analysis using the mean difference (MD) approach with 95% CIs.
Heterogeneity was evaluated using the I square (I2) statistic and Cochran’s Q test. Statistically significant heterogeneity was defined as I2 > 50% and Cochran’s Q test P < 0.126. We used a mixed-effects linear meta-regression model27,28 to evaluate the cause of heterogeneity for main outcomes, with variables including publication year, mean age, sex, and procedural variables including Body Mass Index (BMI), Simplified Acute Physiology Score (SAPS II), PaO2/FiO2 ratio, duration of intubation, and proceduralist expertise.
All statistical analyses were performed using the “metafor” and “meta”29,30 packages of R software version 3.3.131. A P value < 0.05 with a two-tailed test indicated statistical significance without multiplicity correction in all analyses.
Subgroup analysis and sensitivity analysis
Subgroup analysis was performed to detect clinical and statistical heterogeneity in cases of observable heterogeneity via the meta-regression test. We evaluated whether treatment effects on primary outcomes were robust by sensitivity analysis, which were performed based on the specific features of study design to explore the impact of excluding the prospective non-randomized study in meta-analysis.
Results
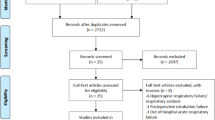
A total of 243 articles were identified, and 212 articles remained after the removal of duplicates. After we screened the titles and abstracts, there were a total of 61 potentially associated articles. Ultimately, seven articles met the inclusion criteria for meta-analysis after the full-text review (Fig. 1) (Supplementary Information 5). Table 1 summarized the basic characteristics of the seven studies, including six RCTs and one prospective non-randomized study, which were published between 2015 and 201911,12,13,32,33,34,35. There were a total of 956 participants, including 501 patients receiving HFNC during the apneic period and 455 receiving standard of care oxygen therapy.
Major outcomes (Summary of major findings were described at Supplementary Information 6)
The incidence of severe hypoxemia during endotracheal intubation was examined in all seven studies11,12,13,32,33,34,35. Our analysis revealed that the incidence of severe hypoxemia did not differ between the HFNC and the standard of care groups during endotracheal intubation with the random-effects model; however, there was moderate heterogeneity among the studies (RD = −0.05, 95% CI −0.11 to 0.01; I2 = 38%, P = 0.14; Fig. 2A).
Meta-analysis of major outcomes high-flow nasal cannula as apneic oxygenation during endotracheal intubation in critically ill patients in the intensive care unit: Incidence of (A) severe hypoxemia (peripheral capillary oxygen saturation [SpO2] < 80%), (B) lowest oxygen saturation during intubation, (C) intensive care unit length of stay, (D) in-hospital mortality.
In the meta-analysis of all seven studies that evaluated lowest oxygen saturation (501 and 455 patients in the HFNC and the standard of care groups, respectively)11,12,13,32,33,34,35, the mean lowest oxygen saturation during endotracheal intubation did not differ significantly between the HFNC and the standard of care groups with the random-effects model and exhibited high heterogeneity among the studies (MD = 2.04, 95% CI = −0.17 to 4.26; I2 = 68%, P < 0.01; Fig. 2B).
In the meta-analysis of the five studies examining the length of ICU stay (430 and 385 patients in the HFNC and the standard of care groups, respectively)12,13,32,33,35, the patients in the HFNC group had significantly shorter ICU stays than those in the standard of care group with the random-effects model (MD = −1.80, 95% CI = −2.98 to −0.63; I2 = 17%, P = 0.31; Fig. 2C).
In the meta-analysis of the six studies examining in-hospital mortality (481 and 435 patients in the HFNC and the standard of care groups, respectively)11,12,13,32,33,35, there was no significant difference in the in-hospital mortality between the HFNC and the standard of care groups with the random-effects model (RD = −0.04, 95% CI = −0.10 to 0.01; I2 = 0%, P = 0.81; Fig. 2D).
HFNC, high-flow nasal cannula; RD, risk difference; MD, mean difference; CI, confidence interval; I2, I square statistic; X2, Cochran’s Q test.
Minor outcomes (Supplementary Information 7)
There was no significantly difference between the HFNC and the standard of care groups with regard to the minor outcomes including the incidence of mild hypoxemia (5 studies, n = 815 patients; RD = −0.03; 95% CI = −0.10 to 0.03)12,13,32,33,35, the incidence of life-threatening hypoxemia (5 studies, n = 815 patients; RD = −0.01, 95% CI −0.05 to 0.03)12,13,32,33,35, first-pass success (5 studies, n = 671 patients; RD = 0.01, 95% CI = −0.05 to 0.06)12,13,32,33,34, duration of mechanical ventilation (5 studies, n = 815 patients; RD = −0.56, 95% CI −1.51 to 0.40)12,13,32,33,35, shock (4 studies, n = 665 patients; RD = −0.04, 95% CI = −0.09 to 0.02)12,13,32,35, cardiovascular complications (5 studies, n =766 patients; RD = −0.03, 95% CI = −0.08 to 0.01)11,12,13,32,35, and ventilator-associated pneumonia (4 studies, n = 665 patients; RD = −0.02; 95% CI = −0.07 to 0.03)12,13,32,35.
Meta-regression, subgroup analyses and sensitivity analysis
The meta-regression analysis examined the relationship of the following nine variables (publication year, study country, sex, mean age, BMI, SAPS II, PaO2/FiO2 ratio, duration of intubation, and proceduralist expertise) and two major outcomes (severe hypoxemia and mean lowest oxygen saturation during endotracheal intubation) (Table 2).
The meta-regression analysis showed no difference in the risk ratio of interactions of the incidence of severe hypoxemia with overall variables except PaO2/FiO2 ratio. We found a borderline significant benefit that the incidence of severe hypoxemia was lower in the patients with high PaO2/FiO2 ratio undergoing oxygen therapy with HFNC during endotracheal intubation compared to those under standard of care (risk ratio for interaction = 0.993, 95% CI = 0.987 to 0.999; I2 = 10.52%; Table 2). Moreover, there was no difference in the interaction of all variables with mean lowest oxygen saturation during endotracheal intubation (Table 2).
Based on the meta-regression analysis showing a significant interaction between severe hypoxemia and PaO2/FiO2 ratio, we performed subgroup analysis with the following clinical cut-off values for the PaO2/FiO2 ratio according to the Berlin definition of acute respiratory distress syndrome36 and the study by Frat et al.13): the patients with mild hypoxemia (PaO2/FiO2 ratio > 200 mmHg) and those with moderate-to-severe hypoxemia (PaO2/FiO2 ratio ≤ 200 mmHg). The analysis revealed that the benefit of HFNC in the incidence of severe hypoxemia was significantly lower among the patients with mild hypoxemia (RD, −0.06; 95% CI, −0.12 to −0.01; I2 = 0%; Fig. 3).
Subgroup analysis of outcomes between high-flow nasal cannula therapy and standard of care in studies investigating severe hypoxemia (SpO2 < 80%): The included patients were categorized by PaO2/FiO2 ratio (mild hypoxemia, PaO2/FiO2 ratio > 200 mmHg; severe-to-moderate hypoxemia, PaO2/FiO2 ratio ≤ 200 mmHg). Outcome analyses were performed using risk difference (RD) with related 95% confidence intervals (95% CI).
In the linear meta-regression interaction analysis, PaO2/FiO2 ratio significantly modified the incidence of severe hypoxemia in a linear trend. Furthermore, the risk ratio of severe hypoxemia decreased accompanied with increasing baseline PaO2/FiO2 ratios. As the cut-off PaO2/FiO2 ratio reached approximately 250, the upper 95% CI of severe hypoxemia incidence risk ratio equaled to 1 and then the incidence of severe hypoxemia decreased (Fig. 4). Overall, these results indicated that the benefit of HFNC in reducing the incidence of severe hypoxemia decreased in a linear fashion with increasing PaO2/FiO2 ratio.
Meta-regression plot of PaO2/FiO2 ratio: Meta-regression analysis showing a linear relationship between the reduction in the incidence risk ratio of severe hypoxemia and the increase in PaO2/FiO2 ratio. As the cut-off PaO2/FiO2 ratio reached approximately 250, the upper 95% CI of severe hypoxemia incidence risk ratio equaled to 1 and then the incidence of severe hypoxemia decreased. Circles indicate incidence risk ratios of severe hypoxemia in individual studies, and bubble size is proportional to the precision of individual studies.
In the subgroup analysis of flow rate of high-flow nasal cannula as apneic oxygenation equal to 60 L/minutes or lower than 60 L/minutes, the result revealed that the incidence of severe hypoxemia did not differ in both subgroups. (Supplementary Information 8) We also did a sensitivity analysis, altering the choice of studies to remove the non-randomized trial11, and the results did no change substantially. (Supplementary Information 9).
FLORALI-2A = participant stratification in FLORALI-2 study with mild hypoxemia (PaO2/FiO2 ratio > 200 mmHg)
FLORALI-2B = participant stratification in FLORALI-2 study with severe-to-moderate hypoxemia (PaO2/FiO2 ratio ≤ 200 mmHg)
PF, PaO2/FiO2 ratio; HFNC, high-flow nasal cannula; RD, risk difference; CI, confidence interval; I2, I square statistic; X2, Cochran’s Q test.
FLORALI-2A = participant stratification in FLORALI-2 study with mild hypoxemia (PaO2/FiO2 ratio > 200 mmHg)
FLORALI-2B = participant stratification in FLORALI-2 study with severe-to-moderate hypoxemia (PaO2/FiO2 ratio ≤ 200 mmHg)
CI, confidence interval; PF, PaO2/FiO2 ratio.
Discussion
In this systematic review and meta-analysis investigating the efficacy of HFNC therapy as apneic oxygenation during endotracheal intubation in critically ill patients in the ICU setting, the available evidence suggested that HFNC as apneic oxygenation significantly reduced the length of ICU stay and that HFNC as apneic oxygenation was not inferior to standard of care oxygen therapy in the incidence of severe hypoxemia, mean lowest oxygen saturation during intubation, in-hospital mortality, and other minor outcomes. Critically, HFNC as apneic oxygenation was effective in reducing the incidence of severe hypoxemia during intubation in patients with mild hypoxemia defined by a PaO2/FiO2 ratio of >200 mmHg.
HFNC was initially utilized as an alternative breathing support for premature infants to maintain positive airway pressure37,38,39; however, there is a propensity to use HFNC therapy in adults with respiratory distress8,40,41. In the latest meta-analysis conducted by Zhu and colleague40, HFNC had significantly effect on reducing post-extubation respiratory failure rate, respiratory rates, and increasing PaO2, comparing with conventional oxygen therapy in patients after planned extubation. In most of studies42,43,44, HFNC was compared with standard of care to demonstrate that this approach was able to improve oxygenation. Despite several potentially physiological advantages of HFNC, there is no clear evidence of efficacy for HFNC as apneic oxygenation during endotracheal intubation. A number of RCTs provided conflicting results regarding the efficacy of HFNC as apneic oxygenation during intubation12,13,32,33,34,35. The current meta-analysis revealed that the incidence of severe hypoxemia was comparable between HFNC therapy and standard of care.
In the FLORALI-2 study by Frat et al.13, patients with acute respiratory distress syndrome were stratified by the PaO2/FiO2 ratio. The trial revealed that apneic oxygenation with HFNC during endotracheal intubation increased the incidence of severe hypoxemia after adjustment for PaO2 in patients with moderate-to-severe hypoxemia. Similarly, Simon et al.45 uncovered that the oxygen level during bronchoscopy was significantly lower in patients with moderate-to-severe hypoxemia receiving oxygen via HFNC than those receiving oxygen via noninvasive ventilation. In our subgroup analysis, we found a reduction in the incidence of severe hypoxemia in patients with mild hypoxemia among those receiving HFNC (number needed to treat = 16.7). Furthermore, in the linear meta-regression interaction analysis, we observed that the trend of reduction in hypoxemia incidence followed the reduction in baseline PaO2/FiO2 ratio in patients receiving HFNC therapy. Therefore, we propose that optimal oxygen delivery strategy should be based on the patient; by the same token, HFNC might be beneficial for apneic oxygenation in patients with mild hypoxemia.
In a meta-analysis, e Silva et al.7 found that apneic oxygenation during endotracheal intubation reduced the length of ICU stay. Notably, the inclusion of a variety of ventilation approaches for apneic oxygenation during intubation might lead to a flawed conclusion. Contrariwise, the current meta-analysis focusing on the utility of HFNC was in agreement with previous studies by revealing that providing supplemental oxygen with HFNC during intubation might be associated with a shorter ICU stay. Previous studies11,12,13,32,35 reported conflicting results with respect to cardiovascular complications including shock, arrhythmia, and cardiac death in association with HFNC. Our data showed that the incidence rates of peri-intubation shock and arrhythmia were lower during apneic oxygenation with HFNC, albeit without statistical significance.
Several ongoing RCTs are currently evaluating the efficacy of HFNC as apneic oxygenation during invasive procedures in various fields including diagnostic, emergency, and critical care medicine46,47,48. Pre- and Apneic High-Flow Oxygenation for RApid Sequence Intubation in The Emergency Department (Pre-AeRATE)47 is a multi-center RCT, aiming to elucidate whether HFNC improves oxygen levels during endotracheal intubation, thereby reducing the risk of hypoxemia during rapid sequence intubation. The findings of the study might provide more concrete evidence regarding the benefits and adverse events associated with HFNC.
The current meta-analysis has several limitations. First, although we focused on one approach, HFNC, the included studies employed protocols that differed in certain aspects such as different oxygen flow rates, which might have contributed to the substantial heterogeneity observed in our analyses. Second, the clinical heterogeneity of the included individuals was a critical confounding factor that should be considered. Third, the effect of HFNC as apneic oxygenation might not be isolated due to the different interventions of pre-oxygenation. Last, there was a paucity of large clinical trial to evaluate the true association between PaO2/FiO2 ratio and severe hypoxemia. As a result, a cautious approach in interpreting the results of this meta-analysis is warranted.
Conclusion
As an oxygen delivery strategy, HFNC was noninferior to standard of care during apneic oxygenation when initiated at the time of endotracheal intubation. The meta-analysis suggested that HFNC oxygen therapy as apneic oxygenation might be beneficial by lowering the incidence of severe hypoxemia in patients with mild hypoxemia (PaO2/FiO2 ratio > 200 mmHg). Furthermore, utilizing HFNC during endotracheal intubation might be associated with a shorter ICU stay. Despite these striking findings, there is a need for further research focusing on distinguishing populations that might reap the most benefits from this approach.
References
Casey, J. D. et al. Bag-Mask Ventilation during Tracheal Intubation of Critically Ill Adults. The New England journal of medicine 380, 811–821, https://doi.org/10.1056/NEJMoa1812405 (2019).
Griesdale, D. E., Bosma, T. L., Kurth, T., Isac, G. & Chittock, D. R. Complications of endotracheal intubation in the critically ill. Intensive care medicine 34, 1835–1842, https://doi.org/10.1007/s00134-008-1205-6 (2008).
Jaber, S. et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Critical care medicine 34, 2355–2361, https://doi.org/10.1097/01.Ccm.0000233879.58720.87 (2006).
De Jong, A. et al. Cardiac Arrest and Mortality Related to Intubation Procedure in Critically Ill Adult Patients: A Multicenter Cohort Study. Critical care medicine 46, 532–539, https://doi.org/10.1097/ccm.0000000000002925 (2018).
Simpson, G. D., Ross, M. J., McKeown, D. W. & Ray, D. C. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. British journal of anaesthesia 108, 792–799, https://doi.org/10.1093/bja/aer504 (2012).
FRUMIN, M. J., M.D., EPSTEIN, ROBERT, M., M.D. & COHEN, G., PH.D. APNEIC OXYGENATION IN MAN. Anesthesiology: The Journal of the American Society of Anesthesiologists 20, 789-798 (1959).
E.Silva, L. O. J. et al. Effectiveness of Apneic Oxygenation During Intubation: A Systematic Review and Meta-Analysis. Annals of emergency medicine 70, 483–494.e411, https://doi.org/10.1016/j.annemergmed.2017.05.001 (2017).
Zhu, Y., Yin, H., Zhang, R. & Wei, J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients with acute respiratory failure: a systematic review and meta-analysis of randomized controlled trials. BMC pulmonary medicine 17, 201, https://doi.org/10.1186/s12890-017-0525-0 (2017).
Nishimura, M. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respiratory care 61, 529–541, https://doi.org/10.4187/respcare.04577 (2016).
Sztrymf, B. et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive care medicine 37, 1780–1786, https://doi.org/10.1007/s00134-011-2354-6 (2011).
Miguel-Montanes, R. et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Critical care medicine 43, 574–583, https://doi.org/10.1097/ccm.0000000000000743 (2015).
Vourc’h, M. et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive care medicine 41, 1538–1548, https://doi.org/10.1007/s00134-015-3796-z (2015).
Frat, J. P. et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. The Lancet. Respiratory medicine 7, 303–312, https://doi.org/10.1016/s2213-2600(19)30048-7 (2019).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of internal medicine 162, 777–784, https://doi.org/10.7326/m14-2385 (2015).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000).
Patel, A. & Nouraei, S. A. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 70, 323–329, https://doi.org/10.1111/anae.12923 (2015).
Reynolds Harmony, R. & Hochman Judith, S. Cardiogenic Shock. Circulation 117, 686–697, https://doi.org/10.1161/CIRCULATIONAHA.106.613596 (2008).
Mort, T. C. Complications of Emergency Tracheal Intubation: Hemodynamic Alterations - Part I. Journal of Intensive Care Medicine 22, 157–165, https://doi.org/10.1177/0885066607299525 (2007).
Kalanuria, A. A., Ziai, W. & Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 18, 208–208, https://doi.org/10.1186/cc13775 (2014).
Higgins JPT, G. S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Journal of clinical epidemiology 66, 408–414, https://doi.org/10.1016/j.jclinepi.2012.09.016 (2013).
Roberts, C. & Torgerson, D. J. Understanding controlled trials: baseline imbalance in randomised controlled trials. BMJ (Clinical research ed.) 319, 185, https://doi.org/10.1136/bmj.319.7203.185 (1999).
Review Manager (RevMan) v. 5.3 Version (Copenhagen. (The Nordic Cochrane Centre, The Cochrane Collaboration) (2014).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Controlled clinical trials 7, 177–188 (1986).
Mantel, N. & Haenszel, W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. JNCI: Journal of the National Cancer Institute 22, 719–748, https://doi.org/10.1093/jnci/22.4.719 (1959).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 327, 557–560, https://doi.org/10.1136/bmj.327.7414.557 (2003).
Berkey, C. S., Hoaglin, D. C., Mosteller, F. & Colditz, G. A. A random-effects regression model for meta-analysis. Statistics in medicine 14, 395–411 (1995).
van Houwelingen, H. C., Arends, L. R. & Stijnen, T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Statistics in medicine 21, 589–624 (2002).
Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software; Vol 1, Issue 3 (2010) (2010).
Wallace, B. C. et al. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. Journal of Statistical Software; Vol 1, Issue 5 (2012) (2012).
The R Project for Statistical Computing (The R Foundation).
Jaber, S. et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive care medicine 42, 1877–1887, https://doi.org/10.1007/s00134-016-4588-9 (2016).
Semler, M. W. et al. Randomized Trial of Apneic Oxygenation during Endotracheal Intubation of the Critically Ill. American journal of respiratory and critical care medicine 193, 273–280, https://doi.org/10.1164/rccm.201507-1294OC (2016).
Simon, M. et al. High-Flow Nasal Cannula Versus Bag-Valve-Mask for Preoxygenation Before Intubation in Subjects With Hypoxemic Respiratory Failure. Respiratory care 61, 1160, https://doi.org/10.4187/respcare.04413 (2016).
Guitton, C. et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive care medicine 45, 447–458, https://doi.org/10.1007/s00134-019-05529-w (2019).
Ferguson, N. D. et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive care medicine 38, 1573–1582, https://doi.org/10.1007/s00134-012-2682-1 (2012).
Manley, B. J. et al. High-Flow Nasal Cannulae in Very Preterm Infants after Extubation. New England Journal of Medicine 369, 1425–1433, https://doi.org/10.1056/NEJMoa1300071 (2013).
Sreenan, C., Lemke, R. P., Hudson-Mason, A. & Osiovich, H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 107, 1081–1083, https://doi.org/10.1542/peds.107.5.1081 (2001).
Lee, J. H., Rehder, K. J., Williford, L., Cheifetz, I. M. & Turner, D. A. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive care medicine 39, 247–257, https://doi.org/10.1007/s00134-012-2743-5 (2013).
Zhu, Y., Yin, H., Zhang, R., Ye, X. & Wei, J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit. Care 23, 180, https://doi.org/10.1186/s13054-019-2465-y (2019).
Zhu, Y., Yin, H., Zhang, R. & Wei, J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: A meta-analysis. Journal of critical care 38, 123–128, https://doi.org/10.1016/j.jcrc.2016.10.027 (2017).
Frat, J.-P. et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. New England Journal of Medicine 372, 2185–2196, https://doi.org/10.1056/NEJMoa1503326 (2015).
Lucangelo, U. et al. High-Flow Nasal Interface Improves Oxygenation in Patients Undergoing Bronchoscopy. Critical Care Research and Practice 2012, 6, https://doi.org/10.1155/2012/506382 (2012).
Tiruvoipati, R., Lewis, D., Haji, K. & Botha, J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. Journal of critical care 25, 463–468, https://doi.org/10.1016/j.jcrc.2009.06.050 (2010).
Simon, M. et al. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy–a prospective randomised trial. Crit. Care 18, 712, https://doi.org/10.1186/s13054-014-0712-9 (2014).
Ostrovsky, I. Apneic Oxygenation Including Precipitous Intubations During RSI in the ED (RAPID), https://clinicaltrials.gov/ct2/show/NCT03694379 (2019)
Chua, M. T. et al. Pre- and Apnoeic high flow oxygenation for RApid sequence intubation in The Emergency department (Pre-AeRATE): study protocol for a multicentre, randomised controlled trial. Trials 20, 195, https://doi.org/10.1186/s13063-019-3305-8 (2019).
Vourc’h, M. et al. Preoxygenation in difficult airway management: high-flow oxygenation by nasal cannula versus face mask (the PREOPTIDAM study). Protocol for a single-centre randomised study. BMJ open 9, e025909, https://doi.org/10.1136/bmjopen-2018-025909 (2019).
Author information
Authors and Affiliations
Contributions
Conceptualization: H.-J.J., and P.-H.C.; methodology: H.-J.J., P.-H.C., C.L., and C.-H.L.; formal analysis: H.-J.J., P.-H.C., and C.-H.L.; investigation: H.-J.J., P.-H.C., and L.-Y.Y.; writing—original draft preparation: H.-J.J., P.-H.C., L.-Y.Y., and C.-K.P.; writing—review and editing: C.-H.L., and C.-K.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jhou, HJ., Chen, PH., Lin, C. et al. High-flow nasal cannula therapy as apneic oxygenation during endotracheal intubation in critically ill patients in the intensive care unit: a systematic review and meta-analysis. Sci Rep 10, 3541 (2020). https://doi.org/10.1038/s41598-020-60636-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60636-9
This article is cited by
-
The Physiologically Difficult Airway and Management Considerations
Current Anesthesiology Reports (2024)
-
Managing the Physiologically Difficult Airway in Critically Ill Adults
Critical Care (2023)
-
Effectiveness of high-flow nasal cannula for tracheal intubation in the emergency department
BMC Emergency Medicine (2022)
-
Practical strategies to reduce nosocomial transmission to healthcare professionals providing respiratory care to patients with COVID-19
Critical Care (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.