Abstract
This study aimed to investigate the association between adolescent overweight and obesity and PTC risk in adulthood. We conducted a case-control study in the Republic of Korea with 1,549 PTC patients and 15,490 controls individually matched for age and sex. We estimated body mass index (BMI) at age 18 years from self-reported weight at this age. Compared with BMI < 23.0 at age 18 years, BMI ≥ 25.0 at age 18 years was associated with higher PTC risk (odds ratio [OR] = 4.31, 95% confidence interval [CI]: 3.57, 5.22). The association between BMI ≥ 25.0 at age 18 years and PTC risk was stronger among men (OR = 6.65, 95% CI: 4.78, 9.27) than among women (OR = 3.49, 95% CI: 2.74, 4.43), and stronger among individuals with current BMI ≥ 25.0 (OR = 8.21, 95% CI: 6.34, 10.62) than among those with current BMI < 25.0 (OR = 2.21, 95% CI: 1.49, 3.27). Among PTC patients, BMI ≥ 25.0 at age 18 years was associated with extra-thyroidal extension and T stage ≥2, but not with N stage ≥1 or BRAFV600E mutation. Adolescent overweight and obesity was associated with higher risk of PTC in adulthood. Our results emphasise the importance of weight management in adolescence to decrease the PTC risk.
Similar content being viewed by others
Introduction
Adolescent overweight and obesity is a major public health issue. The prevalence of adolescent overweight and obesity has increased worldwide, including in the United States1 and the Republic of Korea2,3. Approximately one-fifth of children and adolescents in the United States have obesity4. Adolescent overweight and obesity has been associated with higher risk of premature mortality, cardiovascular disease, and cancer in adulthood5,6,7.
Thyroid cancer is most common endocrine cancer in the U.S.8 and the Republic of Korea9. Although there have been concerns regarding an over-diagnosis and over-treatment of thyroid cancer, previous studies have suggested that the reported increase in thyroid cancer incidence cannot be explained solely by enhanced detection of small tumours owing to the increase in use of ultrasonography and fine-needle aspiration biopsy10,11.
Although the reason for the increase in thyroid cancer incidence is still not clear, an increase in the prevalence of adolescent overweight and obesity might be partially responsible. The association between overweight and obesity in adulthood and thyroid cancer risk in later life has been previously reported12,13,14,15,16,17,18. However, there have been a limited number of studies directly investigating the association between overweight and obesity at age 20 years or younger and thyroid cancer risk afterwards19,20,21, and the results of these studies have been inconsistent.
In the present study, we hypothesised that adolescent overweight and obesity, defined as a body mass index (BMI, kg/m2) ≥25.0 at age 18 years, would be associated with higher risk of papillary thyroid cancer (PTC), the most common histologic type of thyroid cancer (>90% of all thyroid cancer cases)22. We evaluated this hypothesis using a large-scale case-control study conducted in the Republic of Korea.
Methods
Case and control selection
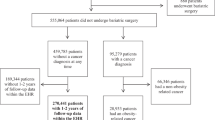
We selected cases from incident thyroid cancer patients admitted at Seoul National University Hospital, Seoul National University Bundang Hospital, and National Medical Centre in the Republic of Korea, between 2010 and 2013. Patients aged ≥20 years who agreed to participate and provided an informed consent were included in the present study. Detailed information on case selection (the Thyroid Cancer Longitudinal Study [T-CALOS] is presented elsewhere23,24.
From a total of 1,576 histologically-confirmed PTC patients (the International Classification of Disease for Oncology, 3rd edition: 8050, 8260, 8340–8344, 8350, and 8450–8460), we excluded 27 patients with no information on weight at age 18 years and at enrolment (current) and current height, resulting in 1,549 cases included in the main analyses. We further excluded individuals with no information on extra-thyroidal extension (n = 24), thyroid tumour size (n = 15), lymph node metastasis (n = 116), and BRAFV600E mutation (n = 248) for analysis of individual associations.
We selected controls from individuals who visited 38 health examination centres for general health examinations, agreed to participate in the study, and provided an informed consent. Detailed information on control selection (the Health Examinees [HEXA] study) is described elsewhere25. The study protocol and information obtained during the survey were similar to those for case selection26.
From a total of 173,422 eligible participants, we excluded individuals with a history of cancer or thyroid diseases (n = 16,578) and those without data on weight at age 18 years and current weight and height (n = 32,547); this resulted in 124,297 potential controls. We subsequently performed 1:10 individual matching for age (±5 years) and sex using the greedy matching algorithm of the SAS GMATCH macro27.
We conducted the main analyses in 1,549 cases (300 men and 1,249 women) and 15,490 controls (3,000 men and 12,490 women). All study participants in the present study belonged to the same ethnic group (East Asian). The study protocols were designed and followed according to the Declaration of Helsinki23,25. The Institutional Review Board of the Seoul National University Hospital approved the study protocols (IRB numbers: 0809-097-258, 1001-067-307, and 1202-088-398). We obtained an informed consent from all study participants.
Assessment of anthropometric measures
Information on participants’ weight at age 18 years were obtained from an in-person interview by a trained interviewer using a structured questionnaire. Current weight and height were measured at study enrolment by a trained member of the survey staff. We calculated the BMI at age 18 years as self-reported weight (kg) at age 18 years divided by the square of current height (m2).
Although the World Health Organization (WHO) suggests BMI cut-offs of <18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, or ≥30.0 for Asian populations28, we combined BMI categories and used the cut-off of <23.0, 23.0–24.9, or ≥25.0, which were not conventional, in further analyses, as the number of participants included in the category of BMI ≥ 30.0 (n = 9) was limited and we observed a monotonic association between BMI and PTC risk. We defined adolescent overweight and obesity as a BMI ≥ 25.0 at age 18 years.
In analyses evaluating the associations of height with PTC risk and clinicopathologic features of PTC, we used sex-specific quartiles of height (cm, <165.5, 165.5–169.5, 169.6–173.7, or ≥173.8 for men; <153.1, 153.1–156.9, 157.0–160.2, or ≥160.3 for women) as an independent variable. These cut-offs were arbitrarily chosen considering the characteristics of the research data, because there have been no conventional cut-offs for height.
Clinicopathologic features of PTC
Information on clinicopathologic features of PTC, such as extra-thyroidal extension, tumour size, lymph node metastasis, and BRAFV600E mutation, were obtained from the medical records by a certified medical records officer and confirmed by an endocrine surgeon. The BRAFV600E mutation was analysed through DNA amplification by polymerase chain reaction (PCR), purification of DNA amplification product with QIAquick PCR purification kit (Qiagen, Hilden, Germany), and sequencing of DNA with ABI 3130XL Genetic Analyzer BigDye Terminator (Applied Biosystems, Foster City, CA)23.
In further analyses, we considered the presence of extra-thyroidal extension (no or yes), T stage (1 or ≥2), N stage (0 or ≥1), and BRAFV600E mutation (no or yes).
Covariates
Based on previous studies on the association between adolescent overweight and obesity and PTC19,20,21, we initially collected the data on age (year), sex, educational level (<high school or ≥high school), tobacco smoking (never-smoker or ever-smoker), alcohol consumption (never-drinker or ever-drinker), history of chronic diseases including type 2 diabetes (no or yes), hypertension (no or yes), and dyslipidaemia (no or yes); data on reproductive factors including menopause (no or yes) were also collected.
Since lifestyle factors did not differ appreciably between cases and controls, we included the afore-mentioned variables, other than tobacco smoking and alcohol consumption, as covariates in further analyses.
Statistical analysis
Using individually matched case-control pairs, we performed conditional logistic regressions to explore the association between BMI ≥ 25.0 at age 18 years and PTC risk, compared with BMI < 23.0 at age 18 years.
Because previous studies suggested heterogeneity of the association by sex14,18,21, we conducted sex-stratified analyses using the same conditional logistic regression models.
Current overweight and obesity may be related to both adolescent overweight and obesity and PTC risk and may confound the association between adolescent overweight and obesity and PTC risk. To confirm that the association between BMI ≥ 25.0 at age 18 years and PTC risk would be independent of current weight status, we conducted stratified analyses by current BMI (≥25.0 vs. <25.0), employing unconditional logistic regression models adjusted for the same covariates. Among various methods to control for the effects of potential confounders, such as stratification, adjustment, and restriction, we used a stratification method, because we wanted to investigate further the heterogeneity of the association between BMI at age 18 year and PTC risk by current BMI.
We assessed the interactions of BMI at age 18 years with sex and current BMI (≥25.0 vs. <25.0) with respect to PTC risk by testing each corresponding product term added to the main logistic regression models.
By using generalised additive models, we further investigated the associations between BMI (continuous) at age 18 years and PTC risk among men with current BMI ≥ 25, men with current BMI < 25, women with current BMI ≥ 25, and women with current BMI < 25 (Fig. 1).
We conducted unconditional logistic regressions adjusted for the same covariates among the PTC patients to evaluate the associations between BMI at age 18 years and clinicopathologic features of PTC.
Because height is used to calculate BMI and has been associated with PTC risk in previous studies29,30, we also repeated all analyses using sex-specific quartiles of height as an independent variable.
We conducted a series of sensitivity analyses after stratifying the study population according to menopausal status in women (premenopausal vs. postmenopausal), birth year (<1950, 1950–1964, or ≥1965), age (<45 years, 45–59 years, or ≥60 years), and history of type 2 diabetes, hypertension, and dyslipidaemia.
We conducted all analyses using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and Stata version 14 (Stata Corp., College Station, TX).
Results
Age (50.7 years vs. 50.8 years), current BMI (23.7 vs. 23.7), tobacco smoking rate (16.8% vs. 17.3%), and alcohol consumption rate (43.8% vs. 45.0%) were similar among the cases and controls. However, the cases had a higher likelihood of having educational levels ≥high school (85.0% vs. 69.5%) and a history of type 2 diabetes (7.0% vs. 5.2%), hypertension (23.8% vs. 15.8%), and dyslipidaemia (16.3% vs. 7.9%) compared to the controls (Table 1).
After adjustment for potential confounders, BMI ≥ 25.0 at age 18 years was found to be associated with higher PTC risk compared with BMI < 23.0 at age 18 years (odds ratio [OR] = 4.31, 95% confidence interval [CI]: 3.57, 5.22). When we categorised BMI according to the WHO criteria for Asians, the results were similar. We also found that greater height was associated with higher PTC risk (p-value for trend <0.01) (Table 2).
When the analyses were stratified by sex, both men and women with BMI ≥ 25.0 at age 18 years have higher PTC risk compared with men and women with BMI < 23.0 at age 18 years (OR = 6.65, 95% CI: 4.76, 9.27 for men; OR = 3.49, 95% CI: 2.74, 4.43 for women). However, the association was found to be stronger among men than women (p-value for interaction = 0.01) (Table 3).
Stratified analyses by current BMI (≥25.0 vs. <25.0) showed that BMI ≥ 25.0 at age 18 years was associated with higher PTC risk compared with BMI < 23.0 at age 18 years, in both the group with current BMI ≥ 25.0 (OR = 8.21, 95% CI: 6.34, 10.62) and with current BMI < 25.0 (OR = 2.21, 95% CI: 1.49, 3.27). However, the association was stronger in the group with current BMI ≥ 25.0 than in that with current BMI < 25.0 (p-value for interaction < 0.01) (Table 3).
In penalised regression spline models, BMI at age 18 years was positively associated with PTC risk among men with current BMI ≥ 25, men with current BMI < 25, women with current BMI ≥ 25, and women with current BMI < 25 (Fig. 1).
Penalised regression splines for the association between body mass index at age 18 years and the risk of papillary thyroid cancer. (a) among men with current body mass index ≥25. (b) among men with current body mass index <25. (c) among women with current body mass index ≥25. (d) among women with current body mass index <25.
We also found that greater height was associated with higher PTC risk among both men and women and among individuals of any current BMI (≥25.0 and <25.0) (all p-values for trend <0.01). However, the association did not differ between sex (p-value for interaction = 0.56) or current BMI (p-value for interaction = 0.79) (Table 3).
Among PTC patients, compared with BMI < 23.0 at age 18 years, BMI ≥ 25.0 at age 18 years was associated with extra-thyroidal extension (OR = 1.50, 95% CI: 1.06, 2.12) and T stage ≥2 (OR = 1.94, 95% CI: 1.03, 3.65), but not with N stage ≥1 (OR = 1.08, 95% CI: 0.64, 1.84) or BRAFV600E mutation (OR = 0.82, 95% CI: 0.55, 1.22). Greater height was only associated with N stage ≥1 (p-value for trend = 0.01) (Table 4).
We conducted several sensitivity analyses. The results did not change appreciably in each stratum on evaluating the association between BMI at age 18 years and PTC risk stratified by menopausal status in women (premenopausal vs. postmenopausal) (data not shown), birth year (<1950, 1950–1964, or ≥1965), age (<45 years, 45–59 years, and ≥60 years) (Supplementary Table 1), history of type 2 diabetes, hypertension, or dyslipidaemia (Supplementary Table 2).
Discussion
In the present study, we found an association between adolescent overweight and obesity and higher PTC risk in adulthood. The association was found to be stronger among men and among individuals with current BMI ≥ 25, compared with women and those with current BMI < 25. Among PTC patients, adolescent overweight and obesity was associated with extra-thyroidal extension and larger tumour size of PTC.
There have been a limited number of studies investigating the association between body weight in early life and thyroid cancer risk in adulthood, and the results of these studies are inconsistent. In a case-control study conducted in Japan, BMI at age 20 years was associated with higher risk of thyroid cancer in later life19. In a cohort study in Denmark, BMI at age 7–13 years was associated with higher thyroid cancer risk in adulthood21. However, in a case-control study in Serbia, BMI in childhood or adolescence was not associated with the risk of PTC among cases and controls younger than age 20 years20. The results of these studies suggest that overweight and obesity in early life might be associated with PTC risk in adulthood but not with risk of early-onset PTC, defined as PTC development in those younger than 20 years of age.
Previous studies examining the association between body weight in early life and thyroid cancer risk in adulthood19,21, as well as those exploring the association between overweight and obesity in adulthood and thyroid cancer risk in later life14,18, have reported a stronger association among men than among women. These results, which are consistent with the results of the present study, suggest that imbalance of sex hormones may contribute to the observed association between overweight and obesity and PTC risk31,32.
We found that adolescent overweight and obesity was associated with higher PTC risk not only among individuals with current BMI ≥ 25.0, but also among those with current BMI < 25.0. These results suggest that adolescent overweight and obesity may increase the PTC risk even among individuals who are no longer overweight or have obesity when they reach adulthood and emphasise the importance of maintaining proper body weight in adolescence with respect to the PTC risk. However, the point estimate for the association between BMI at age 18 years and PTC risk was substantially lower among those with current BMI < 25.0 than those with current BMI ≥ 25.0, implying that PTC risk might be lowered by controlling body weight during adulthood even among individuals who were overweight or had obesity in adolescence.
Adolescent overweight and obesity was found to be associated with extra-thyroidal extension and larger tumour size, suggesting aggressiveness and poor prognosis. Previous studies have reported that BMI in adulthood is associated with extra-thyroidal extension, advanced stage of PTC33, and postoperative locoregional events34 among PTC patients. However, to the best of our knowledge, no previous study has investigated the association between adolescent overweight and clinicopathologic features of PTC. Further studies, including those employing a cohort design, are warranted to confirm these results.
Previous studies13,21,29, although not all12, have reported that greater height is associated with higher risk of thyroid cancer, which is consistent with the results of the present study. The positive association between greater height and PTC risk may be due to, at least in part, shared biological processes of growth hormone and insulin-like growth factor, which could promote both postnatal growth and mutation, proliferation, adhesion, and migration of thyroid cells30,35,36. However, the associations between greater height and clinicopathologic features of PTC have not been investigated in previous studies. Further studies are needed to confirm the results of the present study, such as the association between greater height and N stage ≥1.
Several biological pathways can be responsible for the association between adolescent overweight and obesity and PTC risk (Supplementary Fig. 1). Adolescent overweight and obesity can accelerate growth by increasing growth hormone or insulin-like growth factor 1, and can induce imbalance of hormones such as insulin, leptin, and thyroid stimulating hormone37; this can lead to higher risk of thyroid carcinogenesis35,38. Adolescent overweight and obesity may also induce an imbalance in the estrogen level, which can activate mitogen-activated protein kinase and other growth factors, affecting the development and aggressiveness of thyroid cancer31,32. In addition, chronic subclinical inflammation of the adipose tissue and subsequent local inflammation of the thyroid may also contribute to thyroid cancer development39.
The present study has some limitations. First, there is a possibility that adolescents who are overweight or have obesity are more likely to receive medical care (including thyroid screening) and be included in the case group due to differences in detection rate. Second, we obtained the data on weight at age 18 years from self-reports; information bias could have occurred. However, a systematic difference in weight at age 18 years recalled by the PTC patients and that recalled by the control group is unlikely. Third, the results of the present study should be cautiously generalised to other ethnic populations due to the differences in body size and reported heterogeneous associations between weight and other health outcomes, such as mortality, between ethnic groups40.
However, the present study also has some strengths. First, we collected comprehensive data through standardised operating procedures. We collected the epidemiological data through a face-to-face interview using a structured questionnaire and the clinical data through a medical chart review. Second, we obtained data on a sufficient number of male PTC patients (n = 300) to analyse the association among male subgroups; this has not been extensively investigated to date. Although this is a case-control study and the male to female ratio in the present study did not reflect the male to female ratio of papillary thyroid cancer in the population precisely, papillary thyroid cancer is reported to be more common among women than men not only worldwide including Asia, north America, and Europe41. Third, we conducted several analyses on the association between adolescent overweight and the clinicopathologic features of PTC related to tumour aggressiveness, which also had not been previously investigated.
Conclusion
We found that adolescent overweight and obesity was associated with higher risk of PTC. The association was stronger among men than women and stronger among individuals with current BMI ≥ 25 than those with current BMI < 25. Adolescent overweight and obesity was also associated with higher tumour aggressiveness among PTC patients. The results of the present study provide additional evidence that public health concerns and policy intervention are needed for weight management in adolescence to decrease the PTC risk.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ogden, C. L. et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA 315, 2292–2299 (2016).
Moon, J. S. Secular trends of body sizes in Korean children and adolescents: from 1965 to 2010. Korean J. Pediatr 54, 436–442 (2011).
Oh, K. et al. Prevalence and trends in obesity among Korean children and adolescents in 1997 and 2005. Korean Journal of Pediatrics 51, 950 (2008).
US Preventive Services Task Force et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 317, 2417–2426 (2017).
Twig, G. et al. BMI at Age 17 Years and Diabetes Mortality in Midlife: A Nationwide Cohort of 2.3 Million Adolescents. Diabetes Care 39, 1996–2003 (2016).
Keinan-Boker, L., Levine, H., Derazne, E., Molina-Hazan, V. & Kark, J. D. Measured adolescent body mass index and adult breast cancer in a cohort of 951,480 women. Breast Cancer Res. Treat. 158, 157–167 (2016).
Leiba, M. et al. Adolescent weight and height are predictors of specific non-Hodgkin lymphoma subtypes among a cohort of 2,352,988 individuals aged 16 to 19 years. Cancer 122, 1068–1077 (2016).
Jemal, A. et al. Cancer statistics, 2005. CA Cancer J. Clin 55, 10–30 (2005).
Jung, K.-W. et al. Prediction of Cancer Incidence and Mortality in Korea, 2016. Cancer Res Treat 48, 451–457 (2016).
Kitahara, C. M. & Sosa, J. A. The changing incidence of thyroid cancer. Nat Rev Endocrinol 12, 646–653 (2016).
Morris, L. G. T. & Myssiorek, D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am. J. Surg. 200, 454–461 (2010).
Clavel-Chapelon, F., Guillas, G., Tondeur, L., Kernaleguen, C. & Boutron-Ruault, M.-C. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int. J. Cancer 126, 2984–2990 (2010).
Rinaldi, S. et al. Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int. J. Cancer 131, E1004–1014 (2012).
Zhao, Z. G. et al. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J. Int. Med. Res. 40, 2041–2050 (2012).
Peterson, E., De, P. & Nuttall, R. BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One 7, e29177 (2012).
Lauby-Secretan, B. et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N. Engl. J. Med. 375, 794–798 (2016).
Xu, L. et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid 24, 966–974 (2014).
Leitzmann, M. F. et al. Prospective study of body mass index, physical activity and thyroid cancer. Int. J. Cancer 126, 2947–2956 (2010).
Suzuki, T. et al. Anthropometric factors at age 20 years and risk of thyroid cancer. Cancer Causes Control 19, 1233–1242 (2008).
Zivaljevic, V. et al. A case-control study of papillary thyroid cancer in children and adolescents. Eur. J. Cancer Prev. 22, 561–565 (2013).
Kitahara, C. M., Gamborg, M., Berrington de González, A., Sørensen, T. I. A. & Baker, J. L. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 74, 235–242 (2014).
Cho, B. Y. et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid 23, 797–804 (2013).
Lee, K. E. et al. Protocol of a thyroid cancer longitudinal study (T-CALOS): a prospective, clinical and epidemiological study in Korea. BMJ Open 5, e007234 (2015).
Hwang, Y. et al. Annual Average Changes in Adult Obesity as a Risk Factor for Papillary Thyroid Cancer: A Large-Scale Case-Control Study. Medicine (Baltimore) 95, e2893 (2016).
Health Examinees Study Group. The Health Examinees (HEXA) study: rationale, study design and baseline characteristics. Asian Pac. J. Cancer Prev. 16, 1591–1597 (2015).
Yoo, K.-Y. et al. Genomic epidemiology cohorts in Korea: present and the future. Asian Pac. J. Cancer Prev. 6, 238–243 (2005).
Bergstralh, E. J., Kosanke, J. L. & Jacobsen, S. J. Software for optimal matching in observational studies. Epidemiology 7, 331–332 (1996).
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004).
Sado, J. et al. Risk of thyroid cancer in relation to height, weight, and body mass index in Japanese individuals: a population-based cohort study. Cancer Med 7, 2200–2210 (2018).
Jing, Z. et al. Association between height and thyroid cancer risk: a meta-analysis of prospective cohort studies. Int. J. Cancer 137, 1484–1490 (2015).
Manole, D., Schildknecht, B., Gosnell, B., Adams, E. & Derwahl, M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J. Clin. Endocrinol. Metab. 86, 1072–1077 (2001).
Vannucchi, G. et al. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur. J. Endocrinol. 173, 29–36 (2015).
Kim, H. J. et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin. Endocrinol. (Oxf) 78, 134–140 (2013).
Trésallet, C. et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery 156, 1145–1152 (2014).
Schmidt, J. A. et al. Insulin-like growth factor-i and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 23, 976–985 (2014).
Ben-Shlomo, Y. et al. Prenatal and postnatal milk supplementation and adult insulin-like growth factor I: long-term follow-up of a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev. 14, 1336–1339 (2005).
Speiser, P. W. et al. Childhood obesity. J. Clin. Endocrinol. Metab. 90, 1871–1887 (2005).
Kimura, T. et al. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr. Rev. 22, 631–656 (2001).
Marcello, M. A., Cunha, L. L., Batista, F. A. & Ward, L. S. Obesity and thyroid cancer. Endocr. Relat. Cancer 21, T255–271 (2014).
Zheng, W. et al. Association between body-mass index and risk of death in more than 1 million Asians. N. Engl. J. Med. 364, 719–729 (2011).
Pellegriti, G., Frasca, F., Regalbuto, C., Squatrito, S. & Vigneri, R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J. Cancer Epidemiol 2013, 965212 (2013).
Acknowledgements
This study was conducted with research grants as follows: (1) The Korean Foundation for Cancer Research (grant number CB-2011-03-01); (2) The Basic Research Laboratory program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (no. 2011-0001564); (3) The Education and Research Encouragement Fund of Seoul National University Hospital; (4) Radiation Health Institute (grant no. A15IP12) from KHNP (Korea Hydro & Nuclear Power CO., LTD.); and (5) The Brain Korea 21 PLUS Program.
Author information
Authors and Affiliations
Contributions
K.-N.K., Y.H. and K.H.K. conducted analysis and wrote the manuscript. K.E.L., Y.J.P., S.-J.K., H.K., D.J.P., B.C., H.-C.C. and D.K. designed the study, acquired the data, and revised the manuscript. S.K.P. conceptualised the study design, interpreted the results, and supervised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, KN., Hwang, Y., Kim, K.H. et al. Adolescent overweight and obesity and the risk of papillary thyroid cancer in adulthood: a large-scale case-control study. Sci Rep 10, 5000 (2020). https://doi.org/10.1038/s41598-020-59245-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59245-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.