Abstract
Well-adapted pathogens must evade clearance by the host immune system and the study of how they do this has revealed myriad complex strategies and mechanisms. Classical bordetellae are very closely related subspecies that are known to modulate adaptive immunity in a variety of ways, permitting them to either persist for life or repeatedly infect the same host. Exploring the hypothesis that exposure to immune cells would cause bordetellae to induce expression of important immunomodulatory mechanisms, we identified a putative regulator of an immunomodulatory pathway. The deletion of btrS in B. bronchiseptica did not affect colonization or initial growth in the respiratory tract of mice, its natural host, but did increase activation of the inflammasome pathway, and recruitment of inflammatory cells. The mutant lacking btrS recruited many more B and T cells into the lungs, where they rapidly formed highly organized and distinctive Bronchial Associated Lymphoid Tissue (BALT) not induced by any wild type Bordetella species, and a much more rapid and strong antibody response than observed with any of these species. Immunity induced by the mutant was measurably more robust in all respiratory organs, providing completely sterilizing immunity that protected against challenge infections for many months. Moreover, the mutant induced sterilizing immunity against infection with other classical bordetellae, including B. pertussis and B. parapertussis, something the current vaccines do not provide. These findings reveal profound immunomodulation by bordetellae and demonstrate that by disrupting it much more robust protective immunity can be generated, providing a pathway to greatly improve vaccines and preventive treatments against these important pathogens.
Similar content being viewed by others
Introduction
Pathogens are under natural selection for the ability to colonize and persist in their host, and have evolved complex mechanisms of immune modulation that to date we only poorly recognize, appreciate or understand. Natural host experimental systems have allowed some of these mechanisms to be studied in detail in classical bordetellae, which include Bordetella species that are very successful respiratory pathogens of humans and other animals including mice, in which there are sophisticated molecular tools for experimental manipulation of host immunity. In this system these tools have been used to define the contribution of each immune function to the control and clearance of infection. In the context of such studies, we and others have observed that various Bordetella species manipulate immunity in a variety of ways that would be undetectable in other experimental systems1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. This immunomodulation allows each Bordetella species to achieve remarkable success by either persisting for life with limited pathology (B. bronchiseptica) or causing acute coughing disease that facilitates extraordinarily rapid transmission to new hosts (B. pertussis)26,27. Despite their divergent ecological strategies and niches, these species share a large set of genes whose products mediate interactions with the host, modulating host immunity in various ways to optimize their success. The importance of their many immunomodulatory mechanisms is only beginning to be appreciated10.
We recently hypothesized that an aspect of the success of several pathogens including Bordetella species involve their in vivo differential regulation of an array of host-manipulating factors28, each of which are poorly understood. In exploring host signals that might alert the bacteria of both anti-bacterial challenges and immune-modulating opportunities, we and others examined how bordetellae respond to blood components, a signal of inflammation and/or access to deeper body tissues28,29,30,31. Blood contains a constellation of antimicrobials, immunological signals and immune cells. We have previously reported that Bordetella spp. are able to respond to blood and serum by differentially regulating several sets of genes28, including a SigE-type sigma factor annotated as brpL32,33 that was up-regulated 6-fold in blood and 3-fold in serum. This gene, located in the same operon as Type 3 Secretion System (T3SS) regulators such as btrV/btrW34,35, was previously shown to work in conjunction with BtrA (anti-sigma factor) to regulate the expression of the T3SS and was therefore renamed btrS35,36. However, our transcriptomic analysis revealed that there is a broader set of genes whose expression is potentially affected by BtrS. These include autotransporters and ABC transport systems, regulatory proteins, and proteins involved in various metabolic functions. Interestingly, genes required for pertussis toxin production (ptxA to -E) and secretion (ptlA to -L), previously thought to be quiescent in B. bronchiseptica, were also found regulated by BtrS. Altogether, these data suggest that BtrS regulates the T3SS as well as a set of other more poorly characterized genes implicated in immunomodulation.
Here we describe a STRING analysis that associated btrS28,36 with various genes including some known immunomodulatory factors, implicating BtrS as a potential regulator of immunomodulatory functions. Deleting btrS altered in vitro expression of T3SS genes as well as genes encoding motility, outer membrane proteins, transporters, and other genes with undefined functions, many of which have the potential to affect the host immune response in a variety of ways. Deleting btrS did not affect the ability of the bacterium to colonize and grow in the respiratory tract, but did profoundly prevent the inhibition of inflammasome activation, and resulted in greater inflammation and local immune responses. The mutant was not defective in colonizing or persisting in mice lacking B and T cells, indicating that btrS is not required for early steps of the infection process, but is primarily involved in immunomodulation that inhibits effective protective anamnestic immunity. The mutant induced the rapid recruitment of larger numbers of B and T cells that efficiently and rapidly assembled into pronounced Bronchus-Associated Lymphoid Tissue (BALT). The resulting rapid immune response produced much higher antibody titers and allowed mice to completely clear infections with the deletion mutant, while the wild type strain persisted indefinitely. Furthermore, mice convalescent from infection with this mutant were protected from re-infection with all three wild-type classical Bordetella species, being much better protected against both B. pertussis and B. parapertussis than mice given the current vaccine.
Results
btrS contributes to immunomodulation
We recently showed that bordetellae can detect signals in blood/serum and respond by altering expression of various genes, upregulating expression of the gene btrS35,36 (brpL32,33), among others28. BtrS has been shown to be a sigma factor that interacts with at least one anti-sigma factor to regulate the T3SS34,35,36,37,38. The particular ability of sigma/anti-sigma factor combinations to integrate input from multiple and branched signaling pathways led us to consider BtrS as a potentially pivotal regulator of the complex response to dynamic signals from diverse and rapidly changing host environments as Bordetella infection and the immune response it induces develop over time in different locations within the host. To further examine its potential status as an integrator of diverse input signals, we evaluated its association with other known and putative immunomodulators by STRING (Fig. 1A) which mines databases for data on gene neighborhood, gene fusion, co-occurrence, co-expression, protein homology, and text mining. This analysis revealed a network with connections to many other factors, some of which are known to be involved in complex interactions with the host. In addition to T3SS, this analysis indicated that btrS is connected with other genes encoding known virulence regulators such as hfq39,40 and sigE (RpoE)41,42, as well as iron and heme sensors30,43,44, transporters, and other membrane proteins. Roles for some of these factors in immunomodulation and/or pathogenesis of several organisms has been reported45,46,47,48,49,50,51,52,53,54,55,56,57,58,59. We also observed that btrS was up-regulated 2.5-fold when B. bronchiseptica was internalized in macrophages60, revealing responsiveness to interactions with immune cells. Altogether, these data led us to speculate that btrS is not only a regulator of the T3SS, but potentially integrates multiple, diverse signals to coordinate the regulation of numerous immunomodulatory factors that must be carefully controlled in a complex, choreographed manner to optimize bacterial success in the challenging environment of the host immune response.
Transcriptional regulation role of btrS in medium and serum. STRING analysis of the btrS sigma factor. (A) Connections are coded as follow: pink and blue show the known connections, where pink shows gene fusions and blue gene co-occurrence; green indicates gene neighborhood, yellow shows textmining, black shows co-expression, and finally purple shows protein homology. Heat map of the genes differentially regulated in response to blood vs. medium in RB50 measured by RNA sequencing (B), blue is associated with low expression. Selected statistically significantly up- (red) or down-regulated (blue) Gene Ontology (GO) terms of the genes shown in B. (C) The table below reflects whether these terms are positively (+) or negatively (−) regulated by BtrS in blood or medium +/−. Overlap between the genes significantly up-regulated (red) and down-regulated (blue) in RB50ΔbtrS compared to RB50 in blood (D).
btrS is required for the regulation of multiple virulence and immunomodulatory factors
Previous studies assessed the role of BtrA/S in the regulation of nearby T3SS genes34,35,36; however, the role of btrS as a regulator of other genes has not yet been studied. To identify btrS-regulated genes, we generated a clean, in-frame deletion mutant (Fig. S1) and performed an RNAseq analysis of wild-type (RB50) and the mutant (RB50ΔbtrS) after 1 hour incubation in LB media or in blood at 37 °C. Incubation in blood for 1 hour had a profound effect on the transcriptome, altering expression of approximately 7% of the genes (Fig. 1B–D and Supplementary Dataset 1). Genes associated with T3SS products, iron acquisition, as well as protein transport and localization were significantly induced upon blood exposure in the wild-type strain, but not the mutant (Fig. 1C), revealing a role for BtrS in their induction. On the other hand, flagellum biosynthesis and metabolism (nucleic acids, carbohydrates, organic compounds, phosphate-containing compounds, and glycosylated compounds) genes were significantly down-regulated in response to blood in the wild type bacteria, but not the mutant, implicating BtrS in their regulation (Fig. 1D). To test whether these changes in transcript levels translate into differences in phenotype we examined flagellin-dependent motility. The btrS null-mutant was significantly more motile than its wild-type parent (Fig. S3B), supporting the RNAseq data and highlighting the differences in flagella expression that might affect interactions with the host, as examined further below. These data indicate that in addition to T3SS, BtrS is involved, directly or indirectly, in the regulation of multiple genes involved in motility, membrane proteins, and toxin production, as well as multiple genes of unknown function.
btrS plays an important role in internalization and survival within RAW macrophages
Based on the analyses above, we hypothesized that the RB50ΔbtrS mutant will fail to coordinate factors involved in complex interactions with its host. To investigate potential roles in interactions with immune cells, we inoculated RAW 264.7 macrophages with wild type or ΔbtrS mutant at Multiplicity of Infection (MOI) of 100 (Fig. S2). 15 minutes later, less than 2.5% of the wild type bacteria were internalized, while 8.3% of the ΔbtrS mutant bacteria were intracellular (Fig. 2A). 24 hours post-inoculation (p.i.), the numbers of wild-type bacteria were reduced by >95%, while the RB50ΔbtrS mutant not only survived but grew in numbers within macrophages (Fig. 2B). This apparent gain of function, albeit in an artificial in vitro assay, was unexpected as we hypothesized that the deletion mutant strain would be defective in survival due to the loss of expression of several virulence factors.
btrS mediates intracellular survival in macrophages. Internalization and survival of RB50 (blue) and RB50DbtrS (red) in RAW 264.7 was measured at 15 minutes (n = 4) (A), and up to 24 hours (n = 6) (B), respectively. Caspase-1 activity, as measured via luminescence with the Caspase-Glo 1 Inflammasome Assay, induced by RB50 and RB50DbtrS in THP-1 human-derived macrophages (C) and mouse bone marrow-derived macrophages (D) within 1 hour (n = 6). Statistical significance was calculated using Two-Way ANOVA. *p < 0.05, **p < 0.005, ***p < 0.0005, and ****p < 0.0001. Error bars indicate SEM.
Transmission Electron Microscopy (TEM) revealed that macrophages challenged with the mutant strain in the assay above contained as many as 22 bacteria in the cytoplasm 4 hours later, while those challenged with the wild-type strain contained no more than 8 bacteria within them (Fig. S2B,C). However, the deletion of btrS significantly reduced bacterial cytotoxicity against macrophages, with the mutant killing less than 5% of macrophages while the wild-type killed nearly 70% (Fig. S2C), as we and others have previously shown5,31,61. Since dead cells do not effectively harbor and protect intracellular bacteria, the decrease in cytotoxicity can partially explain the greater recovery of the mutant within macrophages at 4 hours, but does not explain the noted increase in CFUs recovered in intracellular bacteria over 24 hours. Together, these results reveal that btrS contributes significantly to macrophage cytotoxicity, and affects internalization and growth within macrophages, suggesting that the two effects may be potentially related (Fig. S2D). These effects implicate btrS in the regulation of interactions of B. bronchiseptica with macrophages, which play multiple important roles in generating and shaping the protective immune response to infection.
Deleting btrS alters induction of macrophage immune signaling pathways
To investigate the role of btrS in the transcriptional activation of macrophages, we performed an RNAseq analysis of RAW 264.7 macrophages at 15 minutes and 4 hours post-challenge with either wild type or ΔbtrS mutant. IPA analysis of canonical pathways (Qiagen)62 revealed multiple macrophage-signaling pathways that are differentially expressed following challenge with the two strains. While wild-type increased the expression of genes associated with the inflammasome and ephrin pathways, the ΔbtrS mutant increased expression of pathways involved in macropinocytosis, interferons, innate immunity, and T cell activation/differentiation (Supplementary Dataset 2). Differential induction of these by the mutant indicates that in wild-type B. bronchiseptica, btrS mediates the suppression of this important set of immune-related genes.
To more directly examine the effects on inflammation, we challenged macrophages with wild-type or mutant bacteria for 15 minutes and assessed expression of IL-1β (Fig. S2E and Supplementary Dataset 2) and several other genes involved in apoptosis and cell death. All these were strongly up-regulated by wild type bacteria, indicating activation of the inflammasome pathway and an oxidative stress response, as well as positive regulation of TNF-α production (Supplementary Dataset 2). Interestingly, macrophages challenged with the ΔbtrS mutant increased expression of cytokines and chemokines associated with an increase in immune cell recruitment, NF-|B signaling (pro-inflammatory response), and other factors that positively regulate the activation of an adaptive immune response (T cell activation, leukocyte differentiation, leukocyte activation, and others). The ΔbtrS mutant also activated genes associated other pathways such as hematopoiesis (Supplementary Dataset 2).
To further investigate the effects of exposure to wild-type or ΔbtrS mutant bacteria, we performed an RNAseq analysis of macrophages after 4 hours post-inoculation. RB50-challenged macrophages mostly activated genes involved in T cell migration. Macrophages inoculated with RB50ΔbtrS increased the expression of genes involved in innate immunity such as antigen presentation or positive regulation of the acute inflammatory response, as well as genes involved in adaptive immunity, including lymphocyte proliferation, differentiation, and activation. Together, these assays revealed that the ΔbtrS mutant, relative to the wild type strain, induces a more profound increase in the expression of genes involved in stimulating various aspects of immunity. Altogether, these results suggest that in the wild-type bacteria, BtrS plays a role in suppressing various aspects of inflammation and immune signaling pathways, potentially modulating the development of effective adaptive immunity.
btrS induces inflammasome activation
The up-regulation of genes related to the inflammasome pathway led us to investigate the role of btrS in its activation, a critical aspect of both inflammation and the generation of adaptive responses63,64,65. To do so, we measured caspase-1 levels in the human-derived macrophage-like cell line THP-1 and primary murine bone marrow-derived macrophages. 30 minutes post-inoculation, THP-1 cells challenged with RB50 (Fig. 2C) contained approximately 8-fold more caspase-1 compared with those infected with the deletion mutant. 2 hours post-challenge, murine bone marrow-derived macrophages inoculated with wild type bacteria expressed 2-fold more caspase-1 than those challenged with the mutant (Fig. 2D). These results in both human- and mouse-derived macrophages demonstrate that the inflammasome pathway is substantially induced by RB50 but not by the mutant strain, indicating that BtrS mediates robust activation of the inflammasome pathway.
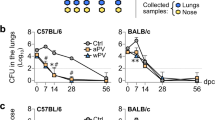
btrS is required for respiratory tract persistence
The effect of btrS deletion on various in vitro measures of aspects of interactions with host cells paints a complex picture difficult to extrapolate to understanding its true in vivo role(s). The B. bronchiseptica natural host infection model in mice allowed us to evaluate this more directly. We therefore followed the course of infection of wild-type and mutant strains in wild type C57BL/6J mice (Fig. 3). Both strains efficiently colonized and grew in the lower respiratory tract during the first few days of infection, indicating that btrS is not required for colonization or growth in the host (Fig. 3B,C). However, after one week, when adaptive immunity begins to be detectable66,67, the mutant began to demonstrate profound defects in persistence throughout the respiratory tract. Wild-type bacteria persisted in the lungs up to 56 days, whereas the mutant was nearly cleared by day 14 and was undetectable by day 21 (Fig. 3C). Even more striking was the phenotype of the mutant in the nasal cavity (Fig. 3A). As expected, wild-type B. bronchiseptica persisted in the nasal cavity of mice for at least 56 days in this experiment, consistent with observations from many previous experiments demonstrating that this wild-type strain always persists indefinitely in the nares of mice41,68. In contrast, the btrS mutant was completely cleared from the nasal cavity by day 56. This is the first B. bronchiseptica mutant described that efficiently colonizes and grows within mice, but is completely cleared from the upper and lower respiratory tract23,36,69,70. To rigorously verify that this profound defect of the ΔbtrS mutant is not due to any other mutation, we compared wild-type and ΔbtrS mutant, to the ΔbtrS complemented with btrS contained on the plasmid pBBR1MCS (ΔbtrS::btrS complemented strain). After 28 days complemented strain persisted in the respiratory tract of mice, confirming that this profound defect in persistence is due to the ΔbtrS deletion (Fig. S3A). This phenotype is much more profound than that reported for a mutant lacking a functional T3SS, indicating that BtrS mediates persistence via effects involving other genes.
btrS promoted persistence in the respiratory tract by suppressing TLR5 mediated clearance. Recovery of B. bronchiseptica RB50 (blue) or RB50ΔbtrS (red) from the respiratory tract of C57Bl/6 J mice, nasal cavity (A), trachea (B), and lungs (C), over time (4–6 mice per experiment, n = 5). Number of neutrophils (D) and macrophages (E) was evaluated in the lungs (n = 5). Recovery of RB50 or RB50ΔbtrS bacteria from the nasal cavity (F) and lungs (G) of TLR5 deficient mice over time (4 mice per group). X-axis indicates days post-inoculation. Statistical significance was calculated using Two-Way ANOVA. *p < 0.05, **p < 0.005, ***p < 0.0005, and ****p < 0.0001. Error bars indicate SEM, ND stands for Not Detected. The dotted line indicates the limit of detection.
btrS mediates decreases in neutrophil and macrophage recruitment to lungs
The remarkable early clearance of the mutant suggests that btrS mediates the modulation of the functions of immune effector cells that can otherwise clear infection. This is consistent with our observation that macrophages infected with the btrS mutant have increased expression of chemokines such as PPBP (neutrophil recruitment) and CCL7 (macrophage recruitment) (Supplementary Dataset 2). To evaluate the effect of deleting btrS on innate immune cell recruitment during infection, we enumerated neutrophils and macrophages recruited to the lungs of mice challenged with the mutant and wild-type strains71,72. By three days post-inoculation, lungs of wild-type infected mice showed a modest increase in numbers of neutrophils compared with naïve mice (Fig. 3D). However, mice challenged with RB50ΔbtrS had almost three times as many neutrophils in their lungs. These numbers decreased by day 7, but still remained significantly higher than RB50-infected mice. These results indicate that BtrS is required to suppress neutrophil recruitment to the lungs.
Wild-type bacteria did not substantially increase numbers of macrophages in the lungs over control mice. However, challenge with RB50ΔbtrS increased macrophage numbers in the lungs at day 14 post-challenge by over 60% (Fig. 3E), suggesting that BtrS is involved in the suppression of macrophage recruitment. Together, these data demonstrate that btrS mediates suppression of recruitment and retention of macrophages and neutrophils, potentially modulating their important roles in the generation of adaptive immunity.
Rapid clearance of the btrS mutant requires TLR5
The increased neutrophil and macrophage recruitment in response to similar or lower numbers of mutant bacteria led us to consider whether BtrS modulates expression of bacterial molecules that contribute to inflammation. The increased expression of flagella genes and motility in the mutant strain and its rapid clearance suggests an immunological mechanism potentially linking these observations21,73,74,75,76,77. Flagella are known to be agonists of TLR5, raising the possibility that BtrS might mediate suppression of flagellar expression to prevent a flagellin-TLR5 response. To examine the role of TLR5 activation in the robust response to and clearance of the ΔbtrS strain, we compared the ability of wild-type and mutant bacteria to colonize, infect and persist in TLR5-deficient mice. Our results revealed that TLR5-deficient mice did fail to rapidly clear the ΔbtrS mutant (Fig. 3F,G), indicating that the rapid clearance of this mutant by wild-type mice is TLR5-mediated. These data provide additional evidence that the phenotype of this mutant is not simply due to the effects of BtrS on T3SS expression, but rather suggest that BtrS-mediated suppression of flagellin expression prevents a TLR5 response that would otherwise substantially augment protective immunity.
btrS modulates T cell recruitment to lungs
The rapid clearance of the ΔbtrS mutant strain and the increased recruitment of innate cells in the lungs prompted a histological examination of immune cell recruitment to the lungs, which revealed both substantial lymphocyte recruitment by the mutant as well as unique distribution of those cells. Histological sections of lungs revealed the formation by day 14 p.i. of pronounced Broncho-alveolar Associated Lymphoid Tissue (BALT) in the lungs of mice challenged with the ΔbtrS mutant but not wild type (Fig. S3C). Analysis of T cell numbers in the lungs 14 days post-inoculation via flow cytometry (Fig. S3)71,72 revealed that infection with wild-type RB50 did not significantly increase CD4+ T cell numbers in the lungs (Fig. 4A), and only modestly increased CD8+ T cells (Fig. 4B) compared to the non-infected control, while challenge with the ΔbtrS mutant resulted in more than twice as many CD4+ and CD8+ T cells in the lungs. It is important to highlight that at this time point (day 14), infection with RB50ΔbtrS induced a much greater T cell response even though the mutant was present at roughly 1/1000 the numbers of the wild-type strain (Fig. 3C). This suggests that wild-type bacteria, via mechanisms that require BtrS, can substantially block the recruitment of T cells and the formation of organized local lymphoid organoids (BALT), allowing it to grow and persist at high numbers in the lungs.
btrS dampens the host adaptive response. Number of CD4+ T cells (A), CD8+ T cells (B), and B cells (C) in the lungs of mice at day 14 post-infection with B. bronchiseptica RB50 (blue), RB50ΔbtrS (red) or naïve control (grey). Anti-B. bronchiseptica IgG antibody titers (D) at day 10 post-infection (4–6 mice per group, n = 5). Statistical significance was calculated using Two-Way ANOVA. *p < 0.05, **p < 0.005, ***p < 0.0005, and ****p < 0.0001. Error bars indicate SEM, ND stands for Not Detected.
btrS suppresses B cell recruitment
RB50ΔbtrS induced macrophages to produce significantly higher levels of CCL18 and IL-6 than did wild-type bacteria (Supplementary Dataset 2 and Fig. S2F), suggesting the mutant might increase recruitment of B cells to the site of infection. Inoculation with wild-type RB50 only slightly increased B cell numbers in lungs compared with the non-infected control, whereas RB50ΔbtrS more than doubled B cells numbers in lungs (Fig. 4C). The anti-B. bronchiseptica serum IgG antibody titers induced by the mutant were measurably higher as early as day 7 post-inoculation (Fig. 4D). This is a striking finding, since antibodies are generally not detectable this early after initial colonization, indicating that BtrS mediates robust B cell suppressive mechanism(s) that delay and/or reduce anti-B. bronchiseptica antibody titers.
Clearance requires adaptive immunity
To investigate if rapid clearance of RB50ΔbtrS is mediated by the T and B cells it rapidly recruits, wild-type and ΔbtrS mutant were compared in Rag−/− mice, which lack T and B cells. 21 days post inoculation both wild-type and mutant bacteria were recovered in similar numbers from all organs of the respiratory tract, indicating that the profound defects of the ΔbtrS mutant are B and T cell dependent. To ensure that these results were not affected by the relatively large inoculum potentially overcoming the remnant immune functions in these immunodeficient animals, we performed a second challenge in which mice were intranasally inoculated with 5 μl of PBS containing 150 bacteria. 24 days later, RB50 and RB50ΔbtrS were both recovered in similarly high numbers (Fig. S4) indicating that the mutant is not defective in any of the functions necessary for colonization, growth and spread to the lower respiratory tract, although slightly lower numbers were recovered from the nasal cavity. The observations that btrS is required for persistence in wild-type mice but does not affect infection and persistence in trachea and lungs of T and B cell-deficient (Rag−/−) mice indicates that BtrS is required for the disruption of a robust host adaptive immune response that is otherwise able to completely clear this notoriously persistent bacterium.
The ΔbtrS mutant induces sterilizing protective immunity to B. bronchiseptica
The substantially increased recruitment of B and T cells leading to rapid and complete clearance of the ΔbtrS mutant suggests that it induces robust immunity that could protect against subsequent infection. To test this, protective immunity was evaluated in three groups of mice that were 1) challenged with RB50ΔbtrS, 2) vaccinated and boosted with the commercial acellular pertussis vaccine Adacel, or 3) administered PBS as a control. Two months later, mice were intranasally challenged with 5 × 105 wild-type B. bronchiseptica (schematic in Fig. 5A). PBS-treated mice (control group) showed high levels of colonization across the entire respiratory tract 7 days post-challenge (Fig. 5B). Mice previously vaccinated with the acellular vaccine eliminated bacteria from the trachea and lungs, demonstrating protection against disease that is conferred by this vaccination (Fig. 5B). However, Adacel vaccination provided no significant reduction in the number of bacteria isolated from the nasal cavity. Thus, despite conferring protection of the lower respiratory tract, the acellular vaccine does not prevent colonization of the nasal cavity, allowing for transmission of the disease, consistent with clinical observations and previous results in mice and baboons78,79. Importantly, mice convalescent from challenge with the ΔbtrS mutant were completely free of B. bronchiseptica in the lungs, trachea, and nasal cavity, indicating that this mutant confers completely protective sterilizing immunity throughout the respiratory tract (Fig. 5B). This is both qualitatively and quantitatively greater protective immunity than has been previously reported for any vaccine against B. bronchiseptica.
Vaccination with RB50ΔbtrS induces sterilizing immunity to classical bordetellae. Workflow of the vaccination experiment; PBS control (black), mice vaccinated with Adacel (violet), and mice vaccinated with the RB50ΔbtrS mutant (red). (A) Number of CFU recovered from the nose, trachea, and lungs of mice at day 7 post re-challenge with B. bronchiseptica RB50. (B) Number of CFU in the nasal cavity 7 days after re-challenge with B. bronchiseptica (BB), B. pertussis (BP), or B. parapertussis (BPP). (C) CFU recovered from the respiratory tract of mice vaccinated with 5 ml of PBS containing 5 CFU of RB50ΔbtrS and re-infected with B. bronchiseptica (D), B. pertussis (E), or B. parapertussis. (F) 4–5 mice per group, n = 3. Statistical significance was calculated using Two-Way ANOVA. *p > 0.05, **p > 0.005, ***p > 0.0005, and ****p > 0.0001. The dotted line indicates the limit of detection.
The ΔbtrS mutant induces sterilizing protective immunity to B. pertussis and B. parapertussis
Since the protective immunity generated by the ΔbtrS mutant against B. bronchiseptica is substantially improved over that conferred by the acellular pertussis Adacel vaccine, and we have previously demonstrated variable cross-immunity between classical Bordetella spp.80,81,82,83, we tested whether immunity induced by the ΔbtrS mutant could also protect against the closely related and antigenically similar human pathogens, B. pertussis and B. parapertussis. PBS-treated control mice challenged with B. pertussis or B. parapertussis contained high bacterial numbers in all respiratory organs at day 7 post-challenge (Figs. 5C, S5A,B). Vaccination with Adacel prior to infection resulted in reduced numbers of bacteria in the lower respiratory tract, but all three Bordetella species were still able to persist in the nasal cavity at day 7 post-challenge (Figs. 5C, S5A–C). These results are consistent with clinical and laboratory findings that acellular vaccination confers protection against disease, but is not completely successful at preventing colonization and transmission79. In contrast, mice convalescent from prior exposure to the ΔbtrS strain were completely protected against B. pertussis and B. parapertussis colonization of the nasal cavity, trachea, and lungs at day 7 post-challenge coinciding with the peak of colonization in naïve animals (Figs. S5A–C, and 5C), demonstrating fully sterilizing immunity against all three species.
To determine if a very low dose of the ΔbtrS mutant is sufficient to provide protection against the classical Bordetella species, mice were challenged with 5 CFU of the mutant and 60 days later challenged with B. bronchiseptica, B. pertussis, or B. parapertussis. While naive control animals were colonized to high levels, prior exposure to 5 CFU of the ΔbtrS mutant resulted in nearly complete protection against colonization by B. bronchiseptica (Fig. 5D) and B. parapertussis (Fig. 5F). Prior exposure to 5CFU of of the ΔbtrS mutant resulted in complete protection against B. pertussis colonization of the lower respiratory tract and greater than 99% reduction in numbers in the nasal cavity.
In all cases, exposure to even very low numbers of the ΔbtrS mutant effectively protected against all three classical Bordetella species.
Discussion
During the course of infection, bacteria are confronted with an ever-changing assortment of antimicrobials in a variety of challenging host micro-environments with varying levels of inflammation and adaptive immune functions10,28,29,84,85,86,87,88,89,90,91,92,93. The host immune response involves a complex series of signals that recruit and activate a succession of immune cells to confront the bacterial threat. Yet successful pathogens, such as Bordetella spp., rapidly respond to, suppress and evade host immunity to persist and mediate their own transmission to new hosts, and can even lay the groundwork for future reinfection of the same host94,95,96. Our results indicate that by deleting a single bacterial sigma factor, btrS, we were able to interrupt the ability of B. bronchiseptica to suppress effective host adaptive immunity, indicating that this sigma factor is a key regulator of immunomodulatory functions including T3SS, flagella, and membrane proteins. BtrS appears to mediate an increase in inflammasome activation and macrophage cell death, conditions that would be expected to cause a deficient adaptive immune response, as observed here and as previously reported for other bacterial species63,64,65. The BtrS-mediated decrease in chemokine production by macrophages and immune cell recruitment may explain the reduction in B and T cell recruitment that delays clearance from the lower respiratory tract and allows long-term persistence in the upper respiratory tract. The robust immune response elicited by challenge with this mutant led to protection against further challenge with any of the three classical bordetellae. Importantly, this protection was even better than that conferred by convalescent immunity with the wild-type strain, highlighting that these immunomodulatory abilities can also suppress protective memory97. Even 5 CFU of the mutant is enough to robustly protect against further challenge with a half-million CFU of B. bronchiseptica, B. pertussis, or B. parapertussis. Together, these data demonstrate that the sigma factor BtrS is involved in regulation of numerous genes and that these collectively mediate profound immunomodulation.
Although we previously showed that TLR4-deficient mice are highly susceptible to B. bronchiseptica98,99,100,101, TLR5-deficient mice were not highly susceptible to B. bronchiseptica, indicating that its detection of Microbe-Associated Molecular Patterns (MAMPs) such as flagellin are not crucial to the normal immune response to the wild-type strain. Combined with transcriptional data demonstrating that btrS is required to suppress flagellin expression, these data lead to a compelling model: BtrS mediates the suppression of the TLR5 agonist flagella to prevent the generation of more effective protective immune response. In 1995, Akerley et al. demonstrated that Bvg is important not only for activation of virulence factors, but also for the suppression of other factors21. In that study, ectopically expressing flagella during infection led to a severe defect in persistence in rats and rabbits. At the time, it was known that flagella can be highly immunogenic, but its effects on TLR5 were yet to be discovered. Our results revealed that the mutant lacking BtrS fails to suppress flagella expression and induces robust protective immunity that is TLR5-dependent, suggesting that the careful regulation of potential immunostimulants is critical for successful infection and persistence. Our results suggest that TLR5 contributes to the generation of much more robust protection that can be cross-protective against other Bordetella species an area of investigation that could lead to the development of improved vaccines against these and other pathogens75,76,77. These results are consistent with the observations that addition of TLR5 agonists have increased the performance of vaccines against malaria, bubonic plague, flu, Enterococcus spp., and Francisella tularensis102,103,104,105,106,107,108,109. Our results suggest that TLR5 agonists could significantly increase the performance of the current acellular pertussis vaccine.
Overall, the evidence that BtrS is a regulator of immunomodulatory functions provides a novel perspective on Bordetella-host interactions. The SigE family of sigma factors is conserved among a wide range of bacterial species, but despite intriguing evidence that implicates them in host-pathogen interactions41,42,52,110,111, their in vitro roles in stress response are better understood than their roles in vivo. Efficient natural-host infection and mouse immunological tools available in Bordetella spp. experimental infection systems allow such interactions to be probed in detail. Our results reveal that regulators such as sigma factors can respond to host signals and play important roles in manipulation of host immune responses. Moreover, their roles appear to be significantly more sophisticated than the linear control of one or two virulence factors and may not be accurately simulated in vitro. Exquisite in vivo regulation of immunomodulatory mechanisms, such as secretion systems, flagella, toxins, modulins, adjuvants and antigens, is likely to be an important facet of efficient infection and pathogenesis of other well-adapted pathogens.
Immunity observed in convalescent animals is commonly believed to be the “gold standard” against which vaccines are compared. However, this concept is in conflict with substantial evidence that many well-adapted pathogens modulate immunity and, as in the case of B. pertussis, do not provide lifelong immunity. The results of this work suggests that Bordetella spp. harbor mechanisms that are finely tuned to modulate the host immune response, enabling them to increase persistence while modulating inflammation and immunity to enhance their opportunity to transmit to another host. The rapid and robust innate and adaptive immunity that resulted in early clearance of the btrS mutant is measurably increased over that induced by the wild-type strain, raising questions about whether natural immunity to infection with this and other Bordetella species should be considered the standard against which vaccines are judged. The ability of the btrS mutant to confer protective immunity against further encounters with this and related Bordetella species demonstrates that immunity induced by infection is substantially suppressed and should not be considered the “gold standard” for optimal immunity. Our vision of what is possible should set our sites much higher, as we have now demonstrated that significantly better protection is possible.
Materials and Methods
Bacterial strains and culture conditions
Bordetella spp. were grown on plates of Difco Bordet-Gengou agar (BD, cat. 248200) supplemented with 10% sheep defibrinated blood and 20 μg/mL working concentration of streptomycin28,112. Strains used in this study are the same strains of B. bronchiseptica, B. pertussis and B. parapertussis used in our previously published manuscript28. Knock out mutants were generated as previously described5,22,112,113,114,115.
Complemented strain generation
PCR was used to amplify btrS as well as to linearize the plasmid pYS003 (a pBBR1-mcs5 derivative). pBBR-fhaB-GFP was derived from pLC007(pBBR-gfp) by swapping the lac promoter of GFP with Bordetella bronchiseptica fhaB promoter region. The parental vector pLC007 was linearized by PCR with primers YS031 & YS032 to delete the lac promoter regions (YS031_PIPEve_pLC009_F CACATTAATTGCGTTGCGCTCACTGC//YS032_PIPEve_pLC009_R ATGAGTAAAGGAGAAGAACTTTTCACTGGA). The fhaB promoter region was amplified by PCR with YS037 & YS038 (YS037_PIPEin_fhaB_F 5′-3′ GCAACGCAATTAATGTGcggtttcgccgatgacttcgaatc//YS038_PIPEin_fhaB_R 5′-3′ CTTCTCCTTTACTCATattccgaccagcgaagtgaagtaatc). In both cases, PCR was performed without the final extension step in order to leave single-strand DNA fragments on both ends of the products (PIPE Cloning PCR116). The plasmid was digested with Dpn1 (BioLabs, cat. R0176S) and reamplified. The two products were then purified again and ligated. The plasmid was extracted and electroporated into competent knockout mutant bacteria (RB50ΔbtrS) at 2500 V. The presence of the btrS insert in RB50ΔbtrS and in DH5α was verified via PCR.
Macrophages assays
Internalization and intracellular survival assay
Intracellular survival in RAW264.7 and THP-1 cell lines was assessed following the traditional gentamicin protection assay method117. In brief, macrophages were challenged at MOI of 100, followed by centrifugation at 300 g for 5 minutes. After 1 hour, after which the media was replaced by media containing 300 μg/ml of gentamicin. Antibiotics were maintained for the whole length of the experiment. For all consecutive experiments, we also followed this approach (adding gentamicin after the first hour of incubation). To measure internalization, cells were washed twice with PBS followed by 5 minutes incubation in ice with 1% Triton (Invitrogen, cat. HFH10). The recovered bacteria were plated on BG/strep plates and enumerated 48 hours later. For the intracellular survival assays, we followed the same procedure at all different time points specified on the graphs. All the experiments were performed in triplicate; see details of the numbers of replicates on each figure.
Transmission electron microscopy
Samples were fixed at different times (15 minutes and 4 hours post-challenge) with 2% glutaraldehyde.
Cytokine production
To asses IL-1β, TNF-α and IL-6 expression, the supernatant of infected cells was utilized to quantify levels of cytokines via commercially available ELISA kits (DuoSet ELISA system, R&D Systems)118. Inflammasome activity was evaluated following the protocol of the Caspase-Glo 1 Inflammasome Assay Kit (Promega, cat. G9951).
Cytotoxicity assay
Briefly, RAW murine macrophages RAW264.7 were cultured in RPMI medium 1640 (Gibco, Life Technologies) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Life Technologies) and 100 U/ml Penicillin-Streptomycin (Gibco, Life Technologies) to 95% confluency. Then macrophages were washed twice with RPMI supplemented with 2% FBS and lacking antibiotics to allow bacterial infection. Multiplicity of infection was 100 (MOI 1:100). After the bacterial addition samples were centrifuged at 300 g for 10 min and incubated for 15 minutes or 4 h at 37 °C in a 5% CO2 atmosphere. The cytotoxicity was measured as the release of LDH using the CytoTox 96 Kit (Promega) following the manufacturer’s protocol. As controls, we performed the same assay on media containing bacteria at the same concentration, lacking macrophages, confirming that no-cytotoxicity is detected.
RNA sequencing
RNA was extracted following manufacturer’s recommendations of the RNAeasy Kit (Qiagen, cat. 74104) as previously detailed28,62.
Bioinformatics
RNA was sequenced on an illumina Hiseq platform by Mr. DNA Lab, Shallowater, TX. For the analysis of the response of B. bronchiseptica to medium and serum, a total of 15 to 18 million 2 × 150 bp reads were obtained for each sample. For the analysis of the macrophage transcriptome in response to B. bronchiseptica infection, approximately 500 million 2 × 150 bp reads were obtained for each sample. Quality control, trimming, and mapping were performed using the CLC Genomics v.11 analysis suite. Samples were mapped either to the transcriptome of B. bronchiseptica or Mus musculus and changes in gene expression analyzed by performing an empirical analysis of DGE119. Changes in gene expression were considered significant if the false discovery rate was <0.05. Additional data analysis included gene set enrichment analysis (GSEA120) and string analysis121. Datasets were deposited at SRA (Bioproject numbers PRJNA559660).
Enzyme-linked immunosorbent assays
96-well microtiter plates (Costar) were coated with heat-killed B. bronchiseptica wild-type or mutant as previously reported67. SureBlue (SeraCare, cat. 5120-0076) was added to start the reaction, which was terminated with HCl after three minutes. The plates were read at an OD of 450 nm. The titer was determined to be the reciprocal of the lowest dilution in which an OD of 0.1 was obtained.
Animal experiments
Wild-type C57BL/6J and Rag−/− (B6.129S7 Rag1tm1Mom/J) mice were obtained from Jackson Laboratories, Bar Harbor, ME or our breeding colony (established from Jackson laboratories mice). Mice were bred and maintained at Paul D. Coverdell Center for Biomedical and Health Sciences, University of Georgia, GA, (AUP: A2016 02-010-Y2-A3)28,122. All experiments were carried out in accordance with all institutional guidelines (Bordetella Host Interactions AUP: A2016 02-010-Y2-A6). Nasal cavity, trachea, lungs, spleen and blood were collected post-mortem in 1 mL of cold PBS. When tissues were used to enumerate colonies, collection was performed in beaded tubes, and when organs were used for immunology, they were collected in 15 mL falcon tubes containing sterile PBS. All results were graphed in GraphPrism v8 and statistical significance was calculated using two-way ANOVA. A power analysis was used (G-Power 3) to compare the immunological response and colonization and we utilized at least 6 mice per group (and experiments were performed in triplicate) at alpha 0,05 and power of 80%.
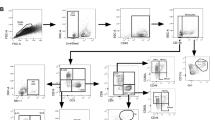
Flow cytometry
Spleen and lungs were processed and stained as previously described71,72. Numbers of live cells were enumerated with Countess II (Thermo Fisher) with trypan blue stain. Two million live cells were seeded in each well for staining. Antibody panels are shown in Supplementary Table S1. The acquisition of the data was performed using BD-LSR II (Becton Dickinson) and analysis was performed with FlowJo 10.0 following standard gating strategy. Statistical significance was calculated using two-way ANOVA in GraphPrism.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens (A2016 02-010-Y2-A3 Bordetella-Host Interactions and A2016 07-006-Y2-A5 Breeding protocol). All animals were anesthetized using 5% isoflurane and euthanized using carbon dioxide inhalation followed by cervical dislocation to minimize animal suffering. Animals were handled following institutional guidelines, in keeping with full accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care International.
References
Hoffman, C. et al. Bordetella adenylate cyclase toxin interacts with filamentous haemagglutinin to inhibit biofilm formation in vitro. Mol. Microbiol. 103, 214–228, https://doi.org/10.1111/mmi.13551 (2017).
Gorgojo, J., Scharrig, E., Gomez, R. M., Harvill, E. T. & Rodriguez, M. E. Bordetella parapertussis Circumvents Neutrophil Extracellular Bactericidal Mechanisms. PLoS One 12, e0169936, https://doi.org/10.1371/journal.pone.0169936 (2017).
Valdez, H. A., Oviedo, J. M., Gorgojo, J. P., Lamberti, Y. & Rodriguez, M. E. Bordetella pertussis modulates human macrophage defense gene expression. Pathog. Dis. 74, https://doi.org/10.1093/femspd/ftw073 (2016).
Gorgojo, J., Lamberti, Y., Valdez, H., Harvill, E. T. & Rodríguez, M. E. Bordetella parapertussis survives the innate interaction with human neutrophils by impairing bactericidal trafficking inside the cell through a lipid raft-dependent mechanism mediated by the lipopolysaccharide O antigen. Infect. Immun. 80, 4309–4316, https://doi.org/10.1128/IAI.00662-12 (2012).
Bendor, L. et al. Type Six Secretion System of Bordetella bronchiseptica and Adaptive Immune Components Limit Intracellular Survival During Infection. PLoS One 10, e0140743, https://doi.org/10.1371/journal.pone.0140743 (2015).
Weyrich, L. S. et al. A Type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo. PLoS One 7, e45892, https://doi.org/10.1371/journal.pone.0045892 (2012).
Zhang, X., Rodríguez, M. E. & Harvill, E. T. O antigen allows B. parapertussis to evade B. pertussis vaccine-induced immunity by blocking binding and functions of cross-reactive antibodies. PLoS One 4, e6989, https://doi.org/10.1371/journal.pone.0006989 (2009).
Goebel, E. M., Wolfe, D. N., Elder, K., Stibitz, S. & Harvill, E. T. O antigen protects Bordetella parapertussis from complement. Infect. Immun. 76, 1774–1780, https://doi.org/10.1128/IAI.01629-07 (2008).
Pilione, M. R. & Harvill, E. T. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect. Immun. 74, 1043–1049, https://doi.org/10.1128/IAI.74.2.1043-1049.2006 (2006).
Gestal, M. C., Whitesides, L. T. & Harvill, E. T. Integrated Signaling Pathways Mediate Bordetella Immunomodulation, Persistence, and Transmission. Trends Microbiol. 27, 118–130, https://doi.org/10.1016/j.tim.2018.09.010 (2019).
Kirimanjeswara, G. S., Agosto, L. M., Kennett, M. J., Bjornstad, O. N. & Harvill, E. T. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J. Clin. Invest. 115, 3594–3601, https://doi.org/10.1172/JCI24609 (2005).
Wang, X., Gray, M. C., Hewlett, E. L. & Maynard, J. A. The Bordetella adenylate cyclase repeat-in-toxin (RTX) domain is immunodominant and elicits neutralizing antibodies. J. Biol. Chem. 290, 23025, https://doi.org/10.1074/jbc.A114.585281 (2015).
Henderson, M. W. et al. Contribution of Bordetella filamentous hemagglutinin and adenylate cyclase toxin to suppression and evasion of interleukin-17-mediated inflammation. Infect. Immun. 80, 2061–2075, https://doi.org/10.1128/IAI.00148-12 (2012).
Sindt, K. A. et al. Pertussis toxin activates platelets through an interaction with platelet glycoprotein Ib. Infect. Immun. 62, 3108–3114 (1994).
Kuwae, A., Momose, F., Nagamatsu, K., Suyama, Y. & Abe, A. BteA Secreted from the Bordetella bronchiseptica Type III Secetion System Induces Necrosis through an Actin Cytoskeleton Signaling Pathway and Inhibits Phagocytosis by Macrophages. PLoS One 11, e0148387, https://doi.org/10.1371/journal.pone.0148387 (2016).
Abe, A., Nishimura, R., Tanaka, N., Kurushima, J. & Kuwae, A. The Bordetella Secreted Regulator BspR is Translocated into the Nucleus of Host Cells via Its N-Terminal Moiety: Evaluation of Bacterial Effector Translocation by the Escherichia coli Type III Secretion System. PLoS One 10, e0135140, https://doi.org/10.1371/journal.pone.0135140 (2015).
Nagamatsu, K. et al. Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN. J. Exp. Med. 206, 3073–3088, https://doi.org/10.1084/jem.20090494 (2009).
Andreasen, C. & Carbonetti, N. H. Pertussis toxin inhibits early chemokine production to delay neutrophil recruitment in response to Bordetella pertussis respiratory tract infection in mice. Infect. Immun. 76, 5139–5148, https://doi.org/10.1128/IAI.00895-08 (2008).
Carbonetti, N. H., Artamonova, G. V., Andreasen, C. & Bushar, N. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect. Immun. 73, 2698–2703, https://doi.org/10.1128/IAI.73.5.2698-2703.2005 (2005).
Carbonetti, N. H. et al. Suppression of serum antibody responses by pertussis toxin after respiratory tract colonization by Bordetella pertussis and identification of an immunodominant lipoprotein. Infect. Immun. 72, 3350–3358, https://doi.org/10.1128/IAI.72.6.3350-3358.2004 (2004).
Akerley, B. J., Cotter, P. A. & Miller, J. F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80, 611–620 (1995).
Harvill, E. T., Cotter, P. A., Yuk, M. H. & Miller, J. F. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67, 1493–1500 (1999).
Yuk, M. H., Harvill, E. T., Cotter, P. A. & Miller, J. F. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol. Microbiol. 35, 991–1004 (2000).
Inatsuka, C. S. et al. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect. Immun. 78, 2901–2909, https://doi.org/10.1128/IAI.00188-10 (2010).
Inatsuka, C. S., Julio, S. M. & Cotter, P. A. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc. Natl. Acad. Sci. USA 102, 18578–18583, https://doi.org/10.1073/pnas.0507910102 (2005).
Kilgore, P. E., Salim, A. M., Zervos, M. J. & Schmitt, H. J. Pertussis: Microbiology, Disease, Treatment, and Prevention. Clin. Microbiol. Rev. 29, 449–486, https://doi.org/10.1128/CMR.00083-15 (2016).
Freyberg, Z. & Harvill, E. T. Pathogen manipulation of host metabolism: A common strategy for immune evasion. PLoS Pathog. 13, e1006669, https://doi.org/10.1371/journal.ppat.1006669 (2017).
Gestal, M. C. et al. Blood or Serum Exposure Induce Global Transcriptional Changes, Altered Antigenic Profile, and Increased Cytotoxicity by Classical Bordetellae. Front. Microbiol. 9, 1969, https://doi.org/10.3389/fmicb.2018.01969 (2018).
Gonyar, L. A., Gray, M. C., Christianson, G. J., Mehrad, B. & Hewlett, E. L. Albumin, in the Presence of Calcium, Elicits a Massive Increase in Extracellular Bordetella Adenylate Cyclase Toxin. Infect. Immun. 85, https://doi.org/10.1128/IAI.00198-17 (2017).
Brickman, T. J., Cummings, C. A., Liew, S. Y., Relman, D. A. & Armstrong, S. K. Transcriptional profiling of the iron starvation response in Bordetella pertussis provides new insights into siderophore utilization and virulence gene expression. J. Bacteriol. 193, 4798–4812, https://doi.org/10.1128/JB.05136-11 (2011).
Hester, S. E., Lui, M., Nicholson, T., Nowacki, D. & Harvill, E. T. Identification of a CO2 responsive regulon in Bordetella. PLoS One 7, e47635, https://doi.org/10.1371/journal.pone.0047635 (2012).
Nicholson, T. L. Construction and validation of a first-generation Bordetella bronchiseptica long-oligonucleotide microarray by transcriptional profiling the Bvg regulon. BMC Genomics 8, 220, https://doi.org/10.1186/1471-2164-8-220 (2007).
Moon, K. et al. The BvgAS Regulon of. MBio. 8, https://doi.org/10.1128/mBio.01526-17 (2017).
Kozak, N. A., Mattoo, S., Foreman-Wykert, A. K., Whitelegge, J. P. & Miller, J. F. Interactions between partner switcher orthologs BtrW and BtrV regulate type III secretion in Bordetella. J. Bacteriol. 187, 5665–5676, https://doi.org/10.1128/JB.187.16.5665-5676.2005 (2005).
Mattoo, S., Yuk, M. H., Huang, L. L. & Miller, J. F. Regulation of type III secretion in Bordetella. Mol. Microbiol. 52, 1201–1214, https://doi.org/10.1111/j.1365-2958.2004.04053.x (2004).
Ahuja, U. et al. Differential regulation of type III secretion and virulence genes in Bordetella pertussis and Bordetella bronchiseptica by a secreted anti-σ factor. Proc. Natl. Acad. Sci. USA 113, 2341–2348, https://doi.org/10.1073/pnas.1600320113 (2016).
Guttman, C. et al. BtcA, A class IA type III chaperone, interacts with the BteA N-terminal domain through a globular/non-globular mechanism. PLoS One 8, e81557, https://doi.org/10.1371/journal.pone.0081557 (2013).
Kurushima, J., Kuwae, A. & Abe, A. Btc22 chaperone is required for secretion and stability of the type III secreted protein Bsp22 in Bordetella bronchiseptica. FEMS Microbiol. Lett. 331, 144–151, https://doi.org/10.1111/j.1574-6968.2012.02561.x (2012).
Bibova, I. et al. Transcriptional profiling of Bordetella pertussis reveals requirement of RNA chaperone Hfq for Type III secretion system functionality. RNA Biol. 12, 175–185, https://doi.org/10.1080/15476286.2015.1017237 (2015).
Bibova, I. et al. The RNA chaperone Hfq is required for virulence of Bordetella pertussis. Infect. Immun. 81, 4081–4090, https://doi.org/10.1128/IAI.00345-13 (2013).
Barchinger, S. E. et al. sigE facilitates the adaptation of Bordetella bronchiseptica to stress conditions and lethal infection in immunocompromised mice. BMC Microbiol. 12, 179, https://doi.org/10.1186/1471-2180-12-179 (2012).
Barbier, M. et al. Modulation of Pertussis and Adenylate Cyclase Toxins by Sigma Factor RpoE in Bordetella pertussis. Infect. Immun. 85, https://doi.org/10.1128/IAI.00565-16 (2017).
Brickman, T. J., Suhadolc, R. J. & Armstrong, S. K. Interspecies variations in Bordetella catecholamine receptor gene regulation and function. Infect. Immun. 83, 4639–4652, https://doi.org/10.1128/IAI.00787-15 (2015).
Armstrong, S. K., Brickman, T. J. & Suhadolc, R. J. Involvement of multiple distinct Bordetella receptor proteins in the utilization of iron liberated from transferrin by host catecholamine stress hormones. Mol. Microbiol. 84, 446–462, https://doi.org/10.1111/j.1365-2958.2012.08032.x (2012).
Zhang, Y. F. et al. Probing the sRNA regulatory landscape of P. aeruginosa: post-transcriptional control of determinants of pathogenicity and antibiotic susceptibility. Mol. Microbiol., https://doi.org/10.1111/mmi.13857 (2017).
Mitobe, J. et al. An attenuated Shigella mutant lacking the RNA-binding protein Hfq provides cross-protection against Shigella strains of broad serotype. PLoS Negl. Trop. Dis. 11, e0005728, https://doi.org/10.1371/journal.pntd.0005728 (2017).
Bhatt, S. et al. Hfq and three Hfq-dependent small regulatory RNAs-MgrR, RyhB and McaS-coregulate the locus of enterocyte effacement in enteropathogenic Escherichia coli. Pathog. Dis. 75, https://doi.org/10.1093/femspd/ftw113 (2017).
Fernández, L. et al. Interconnection of post-transcriptional regulation: The RNA-binding protein Hfq is a novel target of the Lon protease in Pseudomonas aeruginosa. Sci. Rep. 6, 26811, https://doi.org/10.1038/srep26811 (2016).
Shiratsuchi, A. et al. Inhibition of Phagocytic Killing of Escherichia coli in Drosophila Hemocytes by RNA Chaperone Hfq. J. Immunol. 197, 1298–1307, https://doi.org/10.4049/jimmunol.1501953 (2016).
Pisu, D. et al. The Alternative Sigma Factors SigE and SigB Are Involved in Tolerance and Persistence to Antitubercular Drugs. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.01596-17 (2017).
Roblin, P., Dewitte, F., Villeret, V., Biondi, E. G. & Bompard, C. A Salmonella type three secretion effector/chaperone complex adopts a hexameric ring-like structure. J. Bacteriol. 197, 688–698, https://doi.org/10.1128/JB.02294-14 (2015).
Casonato, S., Provvedi, R., Dainese, E., Palù, G. & Manganelli, R. Mycobacterium tuberculosis requires the ECF sigma factor SigE to arrest phagosome maturation. PLoS One 9, e108893, https://doi.org/10.1371/journal.pone.0108893 (2014).
Popat, R. et al. Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. Proc. Biol. Sci. 284, https://doi.org/10.1098/rspb.2017.0200 (2017).
Ayllón, N. et al. Comparative Proteomics Reveals Differences in Host-Pathogen Interaction between Infectious and Commensal Relationship with Campylobacter jejuni. Front. Cell Infect. Microbiol. 7, 145, https://doi.org/10.3389/fcimb.2017.00145 (2017).
Reinhart, A. A. et al. The Pseudomonas aeruginosa PrrF Small RNAs Regulate Iron Homeostasis during Acute Murine Lung Infection. Infect. Immun. 85, https://doi.org/10.1128/IAI.00764-16 (2017).
Gasperini, G., Arato, V., Pizza, M., Aricò, B. & Leuzzi, R. Physiopathological roles of spontaneously released outer membrane vesicles of Bordetella pertussis. Future Microbiol. 12, 1247–1259, https://doi.org/10.2217/fmb-2017-0064 (2017).
Behnsen, J. & Raffatellu, M. Siderophores: More than Stealing Iron. MBio. 7, https://doi.org/10.1128/mBio.01906-16 (2016).
Holden, V. I., Breen, P., Houle, S., Dozois, C. M. & Bachman, M. A. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1α Stabilization during Pneumonia. MBio. 7, https://doi.org/10.1128/mBio.01397-16 (2016).
Yılmaz, Ç., Apak, A., Özcengiz, E. & Özcengiz, G. Immunogenicity and protective efficacy of recombinant iron superoxide dismutase protein from Bordetella pertussis in mice models. Microbiol. Immunol. 60, 717–724, https://doi.org/10.1111/1348-0421.12445 (2016).
Rivera, I. et al. Vol. 10 (Frontiers, Frontiers in Microbiology, 2019).
Kurushima, J., Kuwae, A. & Abe, A. The type III secreted protein BspR regulates the virulence genes in Bordetella bronchiseptica. PLoS One 7, e38925, https://doi.org/10.1371/journal.pone.0038925 (2012).
Damron, F. H., Oglesby-Sherrouse, A. G., Wilks, A. & Barbier, M. Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Sci. Rep. 6, 39172, https://doi.org/10.1038/srep39172 (2016).
Pereira, M., Tourlomousis, P., Wright, J., P Monie, T. & Bryant, C. E. CARD9 negatively regulates NLRP3-induced IL-1β production on Salmonella infection of macrophages. Nat. Commun. 7, 12874, https://doi.org/10.1038/ncomms12874 (2016).
Man, S. M. et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl. Acad. Sci. USA 111, 7403–7408, https://doi.org/10.1073/pnas.1402911111 (2014).
Man, S. M. et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol. 191, 5239–5246, https://doi.org/10.4049/jimmunol.1301581 (2013).
Higgs, R., Higgins, S. C., Ross, P. J. & Mills, K. H. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal. Immunol. 5, 485–500, https://doi.org/10.1038/mi.2012.54 (2012).
Kirimanjeswara, G. S., Mann, P. B., Pilione, M., Kennett, M. J. & Harvill, E. T. The complex mechanism of antibody-mediated clearance of Bordetella from the lungs requires TLR4. J. Immunol. 175, 7504–7511 (2005).
Pilione, M. R., Pishko, E. J., Preston, A., Maskell, D. J. & Harvill, E. T. pagP is required for resistance to antibody-mediated complement lysis during Bordetella bronchiseptica respiratory infection. Infect. Immun. 72, 2837–2842 (2004).
Buboltz, A. M., Nicholson, T. L., Weyrich, L. S. & Harvill, E. T. Role of the type III secretion system in a hypervirulent lineage of Bordetella bronchiseptica. Infect. Immun. 77, 3969–3977, https://doi.org/10.1128/IAI.01362-08 (2009).
Fennelly, N. K. et al. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect. Immun. 76, 1257–1266, https://doi.org/10.1128/IAI.00836-07 (2008).
Halim, T. Y. F. & Takei, F. Current Protocols in Immunology. Vol. 3.25 1-13 (Wiley online library, 2014).
Cossarizza, A. et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 47, 1584–1797, https://doi.org/10.1002/eji.201646632 (2017).
Floyd, M. et al. Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa. PLoS Pathog. 12, e1005987, https://doi.org/10.1371/journal.ppat.1005987 (2016).
Asgarian-Omran, H. et al. Interaction of Bordetella pertussis filamentous hemagglutinin with human TLR2: identification of the TLR2-binding domain. APMIS 123, 156–162, https://doi.org/10.1111/apm.12332 (2015).
Tremblay, M. M., Bilal, M. Y. & Houtman, J. C. Prior TLR5 induction in human T cells results in a transient potentiation of subsequent TCR-induced cytokine production. Mol. Immunol. 57, 161–170, https://doi.org/10.1016/j.molimm.2013.09.002 (2014).
Chan, P. L. et al. TLR5 signaling enhances the proliferation of human allogeneic CD40-activated B cell induced CD4hiCD25+ regulatory T cells. PLoS One 8, e67969, https://doi.org/10.1371/journal.pone.0067969 (2013).
Letran, S. E. et al. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur. J. Immunol. 41, 29–38, https://doi.org/10.1002/eji.201040717 (2011).
Smallridge, W. E., Rolin, O. Y., Jacobs, N. T. & Harvill, E. T. Different effects of whole-cell and acellular vaccines on Bordetella transmission. J. Infect. Dis. 209, 1981–1988, https://doi.org/10.1093/infdis/jiu030 (2014).
Warfel, J. M., Zimmerman, L. I. & Merkel, T. J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 111, 787–792, https://doi.org/10.1073/pnas.1314688110 (2014).
Hester, S. E. et al. Horizontally acquired divergent O-antigen contributes to escape from cross-immunity in the classical bordetellae. BMC Evol. Biol. 13, 209, https://doi.org/10.1186/1471-2148-13-209 (2013).
Buboltz, A. M., Nicholson, T. L., Karanikas, A. T., Preston, A. & Harvill, E. T. Evidence for horizontal gene transfer of two antigenically distinct O antigens in Bordetella bronchiseptica. Infect. Immun. 77, 3249–3257, https://doi.org/10.1128/IAI.01448-08 (2009).
Restif, O., Wolfe, D. N., Goebel, E. M., Bjornstad, O. N. & Harvill, E. T. Of mice and men: asymmetric interactions between Bordetella pathogen species. Parasitology 135, 1517–1529, https://doi.org/10.1017/S0031182008000279 (2008).
Wolfe, D. N., Goebel, E. M., Bjornstad, O. N., Restif, O. & Harvill, E. T. The O antigen enables Bordetella parapertussis to avoid Bordetella pertussis-induced immunity. Infect. Immun. 75, 4972–4979, https://doi.org/10.1128/IAI.00763-07 (2007).
Carlson-Banning, K. M. & Sperandio, V. Enterohemorrhagic Escherichia coli outwits hosts through sensing small molecules. Curr. Opin. Microbiol. 41, 83–88, https://doi.org/10.1016/j.mib.2017.12.002 (2018).
Sperandio, V. Pathogens’ adaptation to the human host. Proc. Natl. Acad. Sci. USA 115, 9342–9343, https://doi.org/10.1073/pnas.1813379115 (2018).
Lustri, B. C., Sperandio, V. & Moreira, C. G. Bacterial Chat: Intestinal Metabolites and Signals in Host-Microbiota-Pathogen Interactions. Infect. Immun 85, https://doi.org/10.1128/IAI.00476-17 (2017).
Bäumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93, https://doi.org/10.1038/nature18849 (2016).
Kendall, M. M. & Sperandio, V. What a Dinner Party! Mechanisms and Functions of Interkingdom Signaling in Host-Pathogen Associations. MBio. 7, e01748, https://doi.org/10.1128/mBio.01748-15 (2016).
Curtis, M. M. & Sperandio, V. A complex relationship: the interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol. 4, 133–138, https://doi.org/10.1038/mi.2010.89 (2011).
Kendall, M. M., Gruber, C. C., Rasko, D. A., Hughes, D. T. & Sperandio, V. Hfq virulence regulation in enterohemorrhagic Escherichia coli O157:H7 strain 86-24. J. Bacteriol. 193, 6843–6851, https://doi.org/10.1128/JB.06141-11 (2011).
Pacheco, A. R. & Sperandio, V. Inter-kingdom signaling: chemical language between bacteria and host. Curr. Opin. Microbiol. 12, 192–198, https://doi.org/10.1016/j.mib.2009.01.006 (2009).
Parker, C. T. & Sperandio, V. Cell-to-cell signalling during pathogenesis. Cell Microbiol. 11, 363–369, https://doi.org/10.1111/j.1462-5822.2008.01272.x (2009).
Sperandio, V., Torres, A. G., Jarvis, B., Nataro, J. P. & Kaper, J. B. Bacteria-host communication: the language of hormones. Proc. Natl Acad. Sci. USA 100, 8951–8956, https://doi.org/10.1073/pnas.1537100100 (2003).
Hotson, A. N. et al. Coordinate actions of innate immune responses oppose those of the adaptive immune system during Salmonella infection of mice. Sci. Signal. 9, ra4, https://doi.org/10.1126/scisignal.aaa9303 (2016).
Baxt, L. A., Garza-Mayers, A. C. & Goldberg, M. B. Bacterial subversion of host innate immune pathways. Science 340, 697–701, https://doi.org/10.1126/science.1235771 (2013).
Liu, Y., Liu, W. & Russell, M. W. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 7, 165–176, https://doi.org/10.1038/mi.2013.36 (2014).
Goebel, E. M., Zhang, X. & Harvill, E. T. Bordetella pertussis infection or vaccination substantially protects mice against B. bronchiseptica infection. PLoS One 4, e6778, https://doi.org/10.1371/journal.pone.0006778 (2009).
Wolfe, D. N., Buboltz, A. M. & Harvill, E. T. Inefficient Toll-like receptor-4 stimulation enables Bordetella parapertussis to avoid host immunity. PLoS One 4, e4280, https://doi.org/10.1371/journal.pone.0004280 (2009).
Mann, P. B. et al. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect. Immun. 73, 8144–8152, https://doi.org/10.1128/IAI.73.12.8144-8152.2005 (2005).
Mann, P. B., Elder, K. D., Kennett, M. J. & Harvill, E. T. Toll-like receptor 4-dependent early elicited tumor necrosis factor alpha expression is critical for innate host defense against Bordetella bronchiseptica. Infect. Immun. 72, 6650–6658, https://doi.org/10.1128/IAI.72.11.6650-6658.2004 (2004).
Mann, P. B., Kennett, M. J. & Harvill, E. T. Toll-like receptor 4 is critical to innate host defense in a murine model of bordetellosis. J. Infect. Dis. 189, 833–836, https://doi.org/10.1086/381898 (2004).
Wang, C., Zhu, W., Luo, Y. & Wang, B. Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine 14, 1349–1360, https://doi.org/10.1016/j.nano.2018.03.007 (2018).
Rostami, H., Ebtekar, M., Ardestani, M. S., Yazdi, M. H. & Mahdavi, M. Co-utilization of a TLR5 agonist and nano-formulation of HIV-1 vaccine candidate leads to increased vaccine immunogenicity and decreased immunogenic dose: A preliminary study. Immunol. Lett. 187, 19–26, https://doi.org/10.1016/j.imlet.2017.05.002 (2017).
Fougeron, D. et al. Indirect Toll-like receptor 5-mediated activation of conventional dendritic cells promotes the mucosal adjuvant activity of flagellin in the respiratory tract. Vaccine 33, 3331–3341, https://doi.org/10.1016/j.vaccine.2015.05.022 (2015).
Kim, J. R. et al. Inclusion of Flagellin during Vaccination against Influenza Enhances Recall Responses in Nonhuman Primate Neonates. J. Virol. 89, 7291–7303, https://doi.org/10.1128/JVI.00549-15 (2015).
Jarchum, I., Liu, M., Lipuma, L. & Pamer, E. G. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect. Immun. 79, 1498–1503, https://doi.org/10.1128/IAI.01196-10 (2011).
Kinnebrew, M. A. et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 201, 534–543, https://doi.org/10.1086/650203 (2010).
Bargieri, D. Y. et al. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine 26, 6132–6142, https://doi.org/10.1016/j.vaccine.2008.08.070 (2008).
Honko, A. N., Sriranganathan, N., Lees, C. J. & Mizel, S. B. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74, 1113–1120, https://doi.org/10.1128/IAI.74.2.1113-1120.2006 (2006).
Manganelli, R. et al. The extra cytoplasmic function sigma factor sigma(E) is essential for Mycobacterium tuberculosis virulence in mice. Infect. Immun. 72, 3038–3041 (2004).
Manganelli, R., Voskuil, M. I., Schoolnik, G. K. & Smith, I. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41, 423–437 (2001).
Dewan, K. K. et al. An Extracellular Polysaccharide Locus Required for Transmission of Bordetella bronchiseptica. J. Infect. Dis. 216, 899–906, https://doi.org/10.1093/infdis/jix251 (2017).
Yuk, M. H., Harvill, E. T. & Miller, J. F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28, 945–959 (1998).
Harvill, E. T. et al. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68, 6720–6728 (2000).
Elder, K. D. & Harvill, E. T. Strain-dependent role of BrkA during Bordetella pertussis infection of the murine respiratory tract. Infect. Immun. 72, 5919–5924, https://doi.org/10.1128/IAI.72.10.5919-5924.2004 (2004).
Klock, H. E. & Lesley, S. A. The Polymerase Incomplete Primer Extension (PIPE) method applied to high-throughput cloning and site-directed mutagenesis. Methods Mol. Biol. 498, 91–103, https://doi.org/10.1007/978-1-59745-196-3_6 (2009).
VanCleave, T. T., Pulsifer, A. R., Connor, M. G., Warawa, J. M. & Lawrenz, M. B. Impact of Gentamicin Concentration and Exposure Time on Intracellular. Front. Cell Infect. Microbiol. 7, 505, https://doi.org/10.3389/fcimb.2017.00505 (2017).
Dewan, K. K. et al. Development of macrolide resistance in Bordetella bronchiseptica is associated with the loss of virulence. J. Antimicrob. Chemother. https://doi.org/10.1093/jac/dky264 (2018).
Robinson, M. D. & Smyth, G. K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9, 321–332, https://doi.org/10.1093/biostatistics/kxm030 (2008).
Tian, L. et al. Discovering statistically significant pathways in expression profiling studies. Proc. Natl Acad. Sci. USA 102, 13544–13549, https://doi.org/10.1073/pnas.0506577102 (2005).
Franceschini, A. et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–815, https://doi.org/10.1093/nar/gks1094 (2013).
Dewan, K. K. et al. A model of chronic, transmissible Otitis Media in mice. PLoS Pathog. 15, e1007696, https://doi.org/10.1371/journal.ppat.1007696 (2019).
Acknowledgements
This work was supported by the NIH (Grant Number AI122753), NIH (Grant Number AI116186 Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. The authors would like to thank the Karen Norris Laboratory, particularly Dr. Whitney Rabacal, who helped with immunological experiments. We would also like to thank the UGA undergrads, especially Julie Evans and Margaret Dedloff for their dedication and passion.
Author information
Authors and Affiliations
Contributions
M.C.G., conceptualization, develop and perform experiments, analyze the data, writing. L.K.H., perform experiment and editing of the figures. K.K.D., edit the manuscript. H.M.J. performed experiments and editing of the manuscript. M.B., analyze data, writing. C.B., provide key ideas for experiments and edit the manuscript. I.H.S., mouse experiments. I.R., data analysis. B.L., editing manuscript. U.B.M., performed all the pathological studies included on the manuscript. E.T.H., conceptualization, mentoring, funding, editing of the manuscript, and brainstorming. Competing interests: The authors do not have conflict of interest. Data and materials availability: All data is fully available and RAW data is available under request to authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gestal, M.C., Howard, L.K., Dewan, K. et al. Enhancement of immune response against Bordetella spp. by disrupting immunomodulation. Sci Rep 9, 20261 (2019). https://doi.org/10.1038/s41598-019-56652-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56652-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.