Abstract
This study investigated the effect of ethylenediamine-N,N′-disuccinic acid (EDDS), oxalic acid (OA), and citric acid (CA) on phytoextraction of U- and Cd-contaminated soil by Z. pendula. In this study, the biomass of tested plant inhibited significantly following treatment with the high concentration (7.5 mmol·kg−1) EDDS treatment. Maximum U and Cd concentration in the single plant was observed with the 5 mmol·kg−1 CA and 7.5 mmol·kg−1 EDDS treatment, respectively, whereas OA treatments had the lowest U and Cd uptake. The translocation factors of U and Cd reached the maximum in the 5 mmol·kg−1 EDDS. The maximum bioaccumulation of U and Cd in the single plants was 1032.14 µg and 816.87 µg following treatment with 5 mmol·kg−1 CA treatment, which was 6.60- and 1.72-fold of the control groups, respectively. Furthermore, the resultant rank order for available U and Cd content in the soil was CA > EDDS > OA (U) and EDDS > CA > OA (Cd). These results suggested that CA could greater improve the capacity of phytoextraction using Z. pendula in U- and Cd- contaminated soils.
Similar content being viewed by others
Introduction
Uranium (U) is an important radioactive element and widely used in irradiation breeding, insect disease prevention, radiotherapy, nuclear reactor, and other industrial sectors in the form of U compounds and metallic U1. The development of nuclear industry as well as U mineral activities have been affecting the quality of soils in natural environments2. According to a survey, the average content of U in topsoil (0–20 cm) was 3.03 mg/kg in China3; however, the U content in the contaminated soil around U tailings is 3.21–62.37 mg·kg−1 in Hunan Province, China4. Multiple heavy metals (U, Cd, Cr, Fe, Pb, and Cu) have been found in U tailings area5. Meanwhile, Cd has become one of the most severe concerns in U tailings area due to its high mobility and toxicity in the soil environment6. Therefore, U tailing contaminated soils need be paid more attention in certain areas of China because of extensive industry activities of mining7. To date, the remediation technologies of metals contaminated soils, such as adsorption8, electrokinetic remediation9, soil washing10, and phytoextraction11 have been widely applied for reducing the total and/or available metals concentration in soils. Among these, phytoextraction is regarded as one of the most effective treatments because of its simple operation in practical applications. Meanwhile, it is also cost-effective and eco-friendly. However, low shoot uptake and translocation results in a low accumulation capability in plant shoot. Therefore, many methods, including chelators, have been widely applied to accumulate higher quantities of metals in plant shoot, through improving the bioavailability of the metals in soil and stimulating metal uptake in tested plant12,13.
In principle, chelators can be divided into two types: natural and artificial chelators. Natural chelators are mainly low-molecular-weight organic acids (LMWOA) such as, oxalic acid (OA) and citric acid (CA), which can increase the solubility and potential bioavailability of metals in soil, and OA and CA have been widely used in phytoremediation enhancement of metal-polluted soils14,15,16,17. Artificial chelators include diethylenetriaminepentaacetic acid (DTPA), ethyl diglycol acetate (EDGA) and ethylenediaminetetraacetic acid (EDTA), which can chelate insoluble metals to soluble species in soil18. Meanwhile, they usually have stronger capacity to chelate metals in soil compared with low-molecular-weight organic acids19,20. However, DTPA, EDGA, and EDTA have negative impacts that include low biodegradability in soil and increasing the risk of leaching of metals into groundwater21,22. Considering these negative effects, EDDS and CA have been widely used to increase the capacity of metals translocation from contaminated soil to harvestable parts of the tested plant because of strong chelate capacity and good biodegradability23,24. Lan et al. showed that 2 mmol·kg−1 EDDS treatment increased significantly shoot Cd concentrations in Sigesbeckia orientalis L25. The results of Yang et al. indicated that 5 mmol·kg−1 CA had the best effect on U phytoremediation by rye grass26.
Zebrina pendula Schnizl is a fast-growing evergreen herbage found at a uranium tailing site in Hengyang, Hunan province, China. The results of our preliminary experiment have shown that Z. pendula has the higher potential to absorb and accumulate U compared with other tested plants (Table S1). Meanwhile, the species also have advantages of strong tolerance to U, high biomass, and easy management.
In this study, we investigated whether the biodegradable chelators could increase the phytoextraction efficiency of U and Cd from the soil. The specific objectives of this study were to: (1) investigate the influence of three chelators on the biomass production of Z. pendula; (2) analyze the potential of different chelators to improve U and Cd phytoextraction; (3) assess the suitable dosage of chelators for enhancing the effect of U and Cd phytoextraction.
Materials and Methods
Materials
The seeds of Z. pendula were purchased from farm product market of Fucheng district, Mianyang City, Sichuan Province, China. All soil samples were collected from the topsoil (0–20 cm) in the Longshan vegetable garden (ferralosols), and were not contaminated by heavy metals. Soil pH was measured using a pH electrode in a 1:2.5 soil/water ratio27. The organic matter (OM) content was determined according to the method of Nelson and Sommers28 using the samples sieved through a 100 mesh (150 μm) sieve. The available nitrogen (N), phosphorus (P), and potassium (K) contents were measured according to Shen et al.29 using the samples sieved through a 100 mesh (150 μm) sieve. The basic physicochemical properties of the soil are shown in Table 1.
Experimental design
The present study simulated U- and Cd-contaminated soil in a greenhouse. First, the tested soil (ferralosols) was naturally air-dried. The weeds and gravel in the soil were removed, and the soil was crushed and mixed. Subsequently, each plastic pot with a hole at the bottom was filled with 3.0 kg of grounded soil. Uranium (15 mg·kg−1) and Cd (15 mg·kg−1) were spiked into the air-dried soils by uniformly spraying an aqueous solution of UO2(CH3CO2)2·2H2O and CdCl2·2.5H2O onto the soil, and base fertilizer was applied in a single dose that contained (NH4)2SO4, KH2PO4, and K2SO4 powders (nitrogen content: 225 mg·kg−1, phosphorus content: 65 mg·kg−1, and potassium content: 227 mg·kg−1)5. Soil ageing was conducted in the pots containing the artificially contaminated soil in the greenhouse for another 35 days. The pots were arranged in a completely randomized design. The seeds of the Z. pendula were sowed at pollution-free farmland near the greenhouse. After 20 days of growing seedlings, three uniform healthy seedlings of the Z. pendula with 2–3 fronds were transplanted from the pollution-free farmland into each pot. All treatments (36 pots in total) were arranged in the greenhouse (15–25 °C) in a completely randomized design. The moisture content of soil in the pots was regularly adjusted according to Chen et al.5.
On the 90th day after the transplanting of Z. pendula, EDDS, OA, and CA were applied to the soils at rates of 0 (control group, CK), 2.5 (low concentration), 5 (moderate concentration) and 7.5 mmol·kg−1 (high concentration) in solutions, respectively. An equal amount of aqueous solution (distilled water) was used as the control group. The 100 mL solution of chelator were irrigated slowly to the soil around the roots in three batches (each batch was 33 mL in a pot, and the third batch was 34 ml) at 3-day intervals. After the chelators were applied, the soil was irrigated regularly. The tested plants were harvested after 1 week in the third batch, and measurements were taken.
Biomass production
The plant samples were carefully removed, washed with tap water followed by distilled water. The samples were divided into shoots and roots. The samples were dried at 75 °C after 30 min at 105 °C for 48 h. The dried plant samples were weighed using an electronic balance.
U and Cd concentration
The U and Cd concentrations of dried plant samples (shoots and roots) were measured by Chen et al.5 Concentrations of U and Cd in plant samples were determined using an inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x; PerkinElmer, Waltham, Massachusetts, USA).
Available U and Cd content
The soil samples around the roots were collected, air-dried at room temperature, sifted (0.1 mm), and weighed (1.0000 g). In this study, we employed the single extraction method, which was used for available U and Cd extraction30,31. Mehlich III extractant (50 mL) (0.2 N CH3COOH, 0.25 N NH4NO3, 0.015 N NH4F, 0.013 N HNO3, and 0.001 N EDTA adjusted to pH 2.5) was mixed with each soil sample, oscillated for 30 min, and then filtered. The available U and Cd content in the soil was measured using the ICP-MS by Elrashidi et al.31.
Computational method
where the Cshoot and Croot is the concentration of heavy metals (mg·kg−1) in the shoot and root, respectively. The Mshoot and Mroot is the mass of shoots and roots, respectively.
Statistical analysis
All statistical tests were performed using Excel 2013, SPSS 23.0, and Origin 9.0 for Windows. Values are expressed as mean ± standard error (SE) (triplicate). Least significant difference (LSD) was applied to test for significant differences (p < 0.05).
Results
Growth parameter
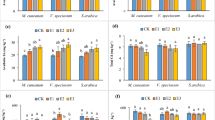
Figure 1 displays the variation in dry weight of shoots, roots, and single plants of Z. pendula in different treatments. The dry mass of shoots showed average increases of 12.7% and 16.77% in the 2.5 mmol·kg−1 OA and CA treatments, respectively, and with increasing concentrations, the shoot dry mass decreased constantly. Compared with the control, the plant growth was severely inhibited, and the shoot dry mass decreased 30.04% following treated with 7.5 mmol·kg−1 EDDS (Fig. 1(a)). The variation trends in the root dry mass under the different concentrations of chelators were the same as that of the shoot dry mass. The root dry mass increased 15.7% and 10.58% in 2.5 mmol·kg−1 OA and CA treatments compared with the control, respectively (Fig. 1b). However, the root dry mass was 4.32 g in 7.5 mmol·kg−1 EDDS treatment, which was 26.28% lower than that of the control (5.86 g) (Fig. 1(b)). The effect of the chelators on the single plant mass was different (Fig. 1(c); CA > OA > EDDS). Furthermore, 2.5 mmol·kg−1 OA and 2.5 mmol·kg−1 CA treatments promoted the growth of Z. pendula, but the biomass production decreased with increasing the concentration. This could be attributed to the fact that low concentrations of small molecular organic acids alleviate the toxicity of heavy metals to plants, whereas high concentrations cause two-fold increase in toxicity of the chelators and heavy metals to plant physiological growth32,33.
Influence of chelators application on shoot dry mass (a), root dry mass (b), and single plant dry mass (c) of Z. pendula under U and Cd stress. EDDS, OA, and CA represents different chelator application, respectively. CK indicates control. Values are reported as means ± SE. Means with different letter are significantly different (p < 0.05) using LSD test.
U uptake and translocation
The potential capacity of EDDS, OA, and CA to remove U from contaminated soil is illustrated in Table 2. Compared with the control, the U absorbtion of different treatments increased by 12.41% to 444.25%. The order of U absorbtion was as follows: CA > EDDS > OA. Meanwhile, CA significantly promoted U absorption by Z. pendula. with the single plant U concentration increasing by 444.25% in the 5 mmol·kg−1 treatment, reaching a maximum of 30.03 mg·kg−1. Meanwhile, the U concentration in the shoots and roots also reached a maximum in the 5 mmol·kg−1 CA treatment (9.04 mg·kg−1 and 127.62 mg·kg−1, respectively), which was 7.35-fold and 6.1-fold higher than that of the control group (1.23 mg·kg−1 for U and 20.91 mg·kg−1 for Cd), respectively (Table 2). The TF of U of Z. pendula increased in each treatment. Compared with the control, the TF of U increased by 15.25% to 77.97%, and the promotion rank order was: EDDS > CA > OA (Table 2). Meanwhile, the maximum TF of U was observed following treated with 5 mmol·kg−1 EDDS, which was higher than that of the other treatments.
Cd uptake and translocation
The chelators promoted the absorption of Cd by Z. pendula in U- and Cd- contaminated soil (Table 3). The order of Cd absorption was EDDS > CA > OA. The Cd concentration in the roots and single plant reached a maximum in the 7.5 mmol·kg−1 EDDS treatment (148.48 mg·kg−1 and 32.09 mg·kg−1, respectively), which was 1.99-fold and 2.32-fold that of the control, respectively. However, the shoot Cd content reached a maximum of 5.34 mg·kg−1 at the 5 mmol·kg−1, which increased 4.32-fold compared to the control. This may be due to the ability that the plant translocated heavy metal from roots to shoots was inhibited in high concentration EDDS treatments. The TF of Cd reached the maximum in 5 mmol·kg−1 EDDS, which increased 109.38% compared to that of the control (Table 3). However, the TF of Cd decreased following treatment with OA and CA. These results indicates that it is more feasible to apply EDDS to improve the efficiency of Cd phytoextraction than OA and CA treatments.
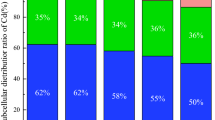
U and Cd accumulation
Heavy metal accumulation in plants is a key index for evaluating remediation efficiency, and the overground accumulation is more important than the underground accumulation. The ability of EDDS, OA, and CA to alter the accumulation of U (a) and Cd (b) are illustrated in Fig. 2. Each treatment promoted the U accumulation by the plants (Fig. 2a). The order of U accumulation was as follows: CA > EDDS > OA. The U accumulation in the shoots, roots, and single plants increased significantly in the CA treatments. The maximum U accumulations in the shoots, roots, and single plants was 130.36, 385.71 and 516.07 µg in the 5 mmol·kg−1 CA treatment, respectively, which was significantly (p < 0.05) higher than that of the control and other treatments (Fig. 2a). Furthermore, CA is an inexpensive, natural, small molecule organic acid that does not produce secondary pollution to the environment; therefore, it is feasible to apply CA to remove the U from U- and Cd-contaminated soil. As shown in Fig. 2(b), the ability of different treatments to assist Z. pendula in accumulating Cd was inconsistent. EDDS has a more significant effect on shoot Cd accumulation compared with the other treatments. The shoot Cd accumulation reached a maximum (165.63 µg) in 7.5 mg·kg−1 EDDS, which increased 3.21-fold compared to the control (Fig. 2b). However, the maximum of root and single plant Cd accumulation was 727.47 µg and 816.87 µg in 5 mg·kg−1 CA treatment, respectively. These results show that EDDS has better capacity to promote Cd accumulation in shoot than OA and CA, whereas CA has a more significant effect on the root Cd accumulation compared to EDDS and OA.
Available U and Cd content in soils
The addition of the EDDS and CA increased the available U and Cd content in the soil (Table 4). The order of U activation by the chelators was CA > EDDS > OA. In the CA treatment, the available U content first increased and then decreased, reaching a maximum (14.58 mg·kg−1) at 5 mmol·kg−1, which was significantly (p < 0.05) higher than that of the control and the other treatments. The available U content in the CA treatment was 1.86-fold and 1.42-fold higher than that of the control and 5 mmol·kg−1 EDDS treatment, respectively (Table 3). However, the ability of EDDS to activate Cd in the soil was better than OA and CA treatments. The maximum available Cd content was 8.93 mg·kg−1 in 5.0 mmol·kg−1 EDDS treatment, which was 43.33% higher than that of the control (6.23 mg·kg−1) (Table 3). In general, CA could more efficiently activate U from the soil, whereas EDDS has greater effect on activating Cd from the soil. The reason was that the ability to activate U and Cd and chelation stability constants were not identical among three chelators. Furthermore, this difference could be attributed to the variation in soil type, soil physicochemical properties, etc.
Correlation analysis
The correlation coefficients among plant growth, uptake of metals, translocation factor (TF) of metals, available metal content in the soil, and accumulation of metal by the plant are shown in Table 5 (U) and Table 6 (Cd). The shoot U concentration, root concentration, and available U content were extremely significantly positive correlated with U accumulation in the plant (p < 0.01). Meanwhile, there was no obvious correlation between plant growth (shoot and root dry mass) and U accumulation. This result indicates that the U concentration of the shoot and root and available U content in the soil were the main ingredients in the phytoextraction process compared with plant growth. For Cd, the shoot Cd concentration was extremely significantly positive correlated with shoot Cd accumulation (p < 0.01). Dramatically, there was no obvious correlation between shoot Cd concentration and Cd accumulation in single plant, possibly due to that Cd is mainly accumulated in the root of Z. pendula. In addition, the Cd concentration of the shoots (roots) exhibited a significantly negative correlation with the dry mass of the shoots (roots), suggesting that high concentration Cd in the tested plant might engender an adverse effect on the plant growth.
Discussion
The application of chelators in phytoremediation can chelate metals in soil and facilitate absorption by tested plants34,35. However, chelator treatments of high dose also have a toxic effect on tested plant growth, resulting in even death of the plants36. In this study, the results indicate that CA and OA treatments promote the growth of Z. pendula at low concentrations (2.5 mmol·kg−1). It might be due to that the growth of the tested plant was not negatively affected by low dose heavy metal concentrations, whilst the biomass yield was increased following treatment with moderate CA and OA concentrations36,37. Nevertheless, all chelators in the present study inhibited the growth of Z. pendula following treatment with high concentration, and EDDS treatment had stronger inhibition effect on the growth of Z. pendula compared with OA and CA treatments. This is agree with the conclusions of Moslehi et al.38 that treating Helianthus annuus L. with 5 mmol·kg−1 EDDS decreased significantly the dry weight of shoot and root in uncontaminated soils. Chen et al.39 found that the dry mass of the shoot and the root decreased in 7.5 mmol·kg−1 EDDS treatment in the Co contaminated soils. The reason is possible that low concentrations of organic acids can mitigate the toxicity of heavy metals in plants40. However, excessive concentration of chelators lead to a significant increase in metal ions concentration in the soil solution, which in turn causes more severe stress to plants. In addition, excessive concentration of chelators have a certain degree of toxicity to plant41; therefore, the tested plant biomass decreased42. Wei et al. reported that low concentrations (2.5 mmol·kg−1) of L glutamic acid N, N-diacetic acid (GLDA) increased the biomass of Sedum alfredii, whereas high concentrations (10 mmol·kg−1) produced toxic effects on growth, which was consistent with the findings of the present study43.
Chelators can promote absorption, translocation, and accumulation of heavy metals in plants, possibly due to that chelators can enhance desorption of metals from the soil matrix to the soil solution, chelate insoluble metals to water soluble species in soil, change the form of heavy metals in soil, increase the content of available heavy metals in soil, and facilitate metal transport into the xylem and increase metal translocation from roots to shoots19,44. In the present study, the addition of chelators increased the available U and Cd content in the soil. CA and EDDS had the best effect on the absorption of U and Cd by Z. pendula, respectively. These results indicate that the available U and Cd content in the soil was directly correlated to the absorption of U and Cd by Z. pendula. Meanwhile, correlation analysis also showed that the available U and Cd content in the soil was extremely significant positive with the concentration of U and Cd in Z. pendula. Furthermore, chelators have different chelation effects on different heavy metals in soil due to mutual selectivity. For example, Zhang et al.45 indicated that EDTA was the most effective of the three chelators for Pb phytoremediation due to the promotion of the available Pb content in the soil.
In the current study, the effects of EDDS, OA, and CA treatments on the uptake and translocation of U and Cd from contaminated soil are different. The chelators significantly promoted the U and Cd uptake, and the order of U and Cd uptake was CA > EDDS > OA (U), EDDS > CA> OA (Cd), respectively. In this study, the concentrations of U and Cd in the shoots and roots of Z. pendula increased highest by 6.35- and 6.1-fold for CA treatment and 4.32- and 0.99-fold for EDDS treatments, respectively, when compared with those of the control groups. The effects on phytoextraction in heavy metals contaminated soil are different using different chelators, which may be because the ability that chelators changed the form of heavy metals in the soil is different due to its differences of physicochemical property. Zhang et al. reported that applying EDDS treatments to Cd and Pb phytoextraction significantly (p < 0.05) increased the Cd concentration of the shoots and roots than the CA treatments; meanwhile, the available Cd content in EDDS treatments is also higher than the CA treatments45. Hu et al. tested CA-assisted U phytoremediation on Macleaya cordata and indicated that the U concentration of the shoots and roots in the 10 mmol·kg−1 CA treatment was significantly higher than (p < 0.05) the EDDS and OA treatments46. In addition, the TF is one of most important indicators that determine whether a plant has the potential to remove metals from the contaminated soils. The TFs of U and Cd of Z. pendula were much lower than 1 in control groups, indicating that U and Cd was mainly absorbed by the roots of Z. pendula. However, the maximum TFs of of U and Cd were 1.78- and 2.11-fold higher than those in the control groups, respectively, which was consistent with the observation by Wan et al.47 and Zhang et al.45.
The accumulation of metals in the tested plant is the key index to evaluate the phytoextraction. The chelator treatments effectively promoted the accumulation of U and Cd by Z. pendula, and the effect of different treatments on accumulation was also different. This study indicates that applying CA caused higher U concentrations and accumulation amount in the shoots and the roots compared with other chelators. This finding is consistent with the result of Jagetiya et al.36 that the chelator strengthened U accumulation in Indian mustard follow the order of CA > EDTA > OA > NTA. The U accumulations of the shoots and single plants following treatment with 5 mmol·kg−1 CA were 6.48-fold and 5.6-fold higher than those in the control groups, respectively. Yang et al. also showed that the application of 5 mmol·kg−1 CA increased the U accumulation coefficient in the shoots and roots of rye grass by 2.31-fold and 1.67-fold that of the control, respectively26. The maximum of shoot Cd accumulation was 165.63 µg in 7.5 mg·kg−1 EDDS treatment. Nevertheless, the maximum of root and single plant Cd accumulation was 727.47 µg and 816.87 µg in 5 mg·kg−1 CA treatment, respectively. The main reason is that biomass production of the tested plant was inhibited significantly in higher dosages of EDDS treatments than the CA treatments.
The application of chelators can increase the bioavailability of metals in soil-plant system through chelation with metals48. The results showed that the available U and Cd content in the soil was influenced by the three chelators, and the order of metals activation was CA > EDDS > OA (U) and EDDS > CA > OA (Cd), respectively. This reason possible is due to the mutual selectivity of the metals and the various chelating agents. This is consistent with the results of Lozano et al., who showed that particular chelator is suitable for particular metal in soil49, such as CA treatment was more efficient to increase the solubility of uranium than EDDS treatment50.
High dosages of chelators result in greater toxicity in plants, as indicated by such signs as leaf etiolation and wilting51. Similar results were observed in the present study. Heavy metals damage the cytoplasmic membrane and normal biochemical mechanisms for controlling transport in plants. Chelators can be used to transfer metals from soil to plants by forming metal chelates. This results in the excessive accumulation of metals in the tested plant tissues, which has a toxic effect on plant growth and can even cause plant dehydration and death36. Due to the high concentration of metals in the current study, further investigation is required to determine whether the toxicity of the chelators is direct or indirect. Based on these results, when conducting chelation-induced phytoextraction, it is important to remember that excessive chelators treatments may engender adverse effects on the growth and development of the plant species.
Conclusion
This study showed that applications of the chelators significantly enhanced phytoextraction of U and Cd by Z. pendula. in the contaminated soils. High concentration (7.5 mmol·kg−1) of EDDS treatment significantly (p < 0.05) inhibited the plant growth, but low (2.5 mmol·kg−1) concentrations of CA and OA were significantly (p < 0.05) conducive to the growth of the tested plant. Chelators enhanced the uptake, translocation, and accumulation of Cd and U by Z. pendula. The maximum U and Cd accumulation amount were observed with the 5 mmol·kg−1 CA treatment, and were significantly higher (p < 0.05) than that of the control. Meanwhile, the available U and Cd concentrations in the soil were influenced by all tested chelator treatments, and the order of available metal contents in the soil was as follow: CA > EDDS > OA (U) and EDDS > CA > OA (Cd), respectively. Available U content in the soil is one of the main parameters controlling accumulation of U in the Z. pendula through correlation analysis. In U- and Cd-contaminated soil, CA was effective in improving absorption and accumulation amounts of U and Cd by Z. pendula. These findings would be beneficial to increase removal efficiency of U and Cd from contaminated soils. Furthermore, the potential impacts on soil properties after soil remediation will be further considered in future studies.
References
Miao, T. & Pan, T. A. Multiphysics Model for Evaluating Electrokinetic Remediation of Nuclear Waste-Contaminated Soils. Water, Air, & Soil Pollution 226(3), 77 (2015).
Selvakumara, R. et al. Challenges and complexities in remediation of uranium contaminated soils: A review. Journal of environmental radioactivity 18(2), 1–10 (2018).
China National Environmental Monitoring Centre. Background Values of Elements in Soils of China.China Environmental Science Press, 87–90. China (1990).
Ma, P. J., Wang, Z., Yi, F. C., Niu, S. J. & Zhou, H. T. Spatial Distribution and Pollution Assessment of Uranium in Soil around Uranium Tailings. Atomic Energy Science and Technology 51(5), 956–960 (2017).
Chen, L., Long, C., Wang, D. & Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 242, 125112 (2020).
Navarro-León, E., Oviedo-Silva, J., Ruiz, J. M. & Blasco, B. Possible role of HMA4a TILLING mutants of Brassica rapa in cadmium phytoremediation programs. Ecotoxicology and Environmental Safety 180, 88–94 (2019).
Kim, I. G., Kim, S. S., Kim, G. N., Han, G. S. & Choi, G. W. Reduction of Radioactive Waste from Remediation of Uranium-Contaminated Soil. Nuclear Engineering and Technology 48, 840–846 (2017).
Yu, Y., Liu, M. & Yang, J. Characteristics of vanadium adsorption on and desorption from humic acid. Chemistry and Ecology 34(6), 548–564 (2018).
Sun, Z., Wu, B., Guo, P., Wang, S. & Guo, S. Enhanced electrokinetic remediation and simulation of cadmium-contaminated soil by superimposed electric field. Chemosphere 233, 17–24 (2019).
Xia, Z. et al. Remediation of cadmium, lead and zinc in contaminated soil with CETSA and MA/AA. Journal of Hazardous Materials 366, 177–183 (2019).
Usman, K., Al-Ghouti, M. A. & Abu-Dieyeh, M. H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Scientific Reports 9(1), 5658 (2019).
Almaroai, Y. A. et al. Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil. Environmental Technology 34(6), 747–755 (2013).
Zhang, T. et al. Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. Journal of Hazardous Materials 262, 464–471 (2013).
Jones, D. L. Organic acids in the rhizosphere - a critical review. Plant and Soil 205(1), 25–44 (1998).
Cieśliński, G., Van Rees, K. C., Szmigielska, A. M., Krishnamurti, G. S. & Huang, P. M. Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant and Soil 203(1), 109–117 (1998).
Hinsinger, P., Plassard, C., Tang, C. & Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant and Soil 248(1/2), 43–59 (2003).
Wu, L. H., Luo, Y. M., Christie, P. & Wong, M. H. Effects of EDTA and low molecular weight organic acids on soil solution properties of a heavy metal polluted soil. Chemosphere 50(6), 819–822 (2003).
Leštan, D., Luo, C. & Li, X. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environmental Pollution 153(1), 3–13 (2008).
Bian, X. G., Cui, J., Tang, B. P. & Yang, L. Chelant-Induced Phytoextraction of Heavy Metals from Contaminated Soils: A Review. Polish journal of environmental studies 27(6), 2417–2424 (2018).
Saifullah, M. E. et al. EDTA-assisted Pb phytoextraction. Chemosphere 74(10), 1279–1291 (2009).
Zhao, S. L., Lian, F. & Duo, L. EDTA-assisted phytoextraction of heavy metals by turfgrass from municipal solid waste compost using permeable barriers and associated potential leaching risk. Bioresource Technology 102(2), 621–626 (2011).
Sillanpa, M. E. T., Kurniawan, T. A. & Lo, W. H. Degradation of chelating agents in aqueous solution using advanced oxidation process (AOP). Chemosphere 83(11), 1443–1460 (2011).
Luo, C. L., Shen, Z. G., Lou, L. Q. & Li, X. D. EDDS and EDTA-enhanced phytoextraction of metals from artificially contaminated soil and residual effects of chelant compounds. Environmental Pollution 144(3), 862–871 (2006).
Grcman, H., Vodnik, D., Velikonja-Bolta, S. & Lestan, D. Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. Journal of Environmental Quality 32(32), 500–506 (2003).
Lan, J. C. et al. Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 91(9), 1362–1367 (2013).
Yang, R. L. et al. Effect of citric acid on uranium contaminated soil repaired by Loilum perenne. Atomic Energy Science and Technology 50, 1748–1755 (2016).
Yang, J. et al. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. Journal of Hazardous Materials 264, 498–504 (2014).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon and organic matter. In: Sparks, D. L. et al. (eds). Methods of soil analysis: SSSA, Madison, Wisconsin. pp. 961–1010 (1996).
Shen, J. C., Zhang, Z. H., Liu, R. & Wang, Z. H. Ecological restoration of eroded karst utilizing pioneer moss and vascular plant species with selection based on vegetation diversity and underlying soil chemistry. International Journal of Phytoremediation 20(14), 1369–1379 (2018).
Yang, J., Teng, Y., Zuo, R. & Song, L. Comparison of bioavailable vanadium in alfalfa rhizosphere soil extracted by an improved BCR procedure and EDTA, HCl, and NaNO3 single extractions in a pot experiment with V–Cd treatments. Environmental Science and Pollution Research 22(12), 8833–8842 (2013).
Elrashidi, M. A., Seybold, C. A. & Wysocki, D. A. Effects of Annual Precipitation on Heavy Metals in Runoff from Soils in the US Great Plains. Water, Air, & Soil Pollution 226(12), 417 (2015).
Afshan, S. et al. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environmental Pollution 22(15), 11679–11689 (2015).
Evangelou, M. W., Ebel, M. & Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68(6), 989–1003 (2007).
Li, J. H. et al. Ethyllactate-EDTA composite system enhances the remediation of the cadmium-contaminated soil by Autochthonous Willow (Salix x aureo-pendula CL ‘J1011’) in the lower reaches of the Yangtze River. Journal of Hazardous Materials 181(1-3), 673–678 (2010).
Lux, A., Martinka, M., Vaculík, M. & White, P. J. Root responses to cadmium in the rhizosphere:a review. Journal of Experimental Botany 62(1), 21–37 (2011).
Jagetiya, B. & Sharma, A. Optimization of chelators to enhance uraium uptake from tailings for phytoremediation. Chemosphere 91(5), 692–696 (2013).
Ehsan, S. et al. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicology and Environmental Safety 106, 164–172 (2014).
Moslehi, A., Feizian, M., Higueras, P. & Eisvand, H. R. Assessment of EDDS and vermicompost for the phytoextraction of Cd and Pb by sunflower (Helianthus annuus L.). International Journal of Phytoremediation 21(3), 191–199 (2019).
Chen, L., Wang, D., Zeng, C. & Long, C. Improving Cobalt Phytoextraction by Astragalus Sinicus L. Grown in Co-Contaminated Soils Using Biodegradable Chelators. Soil and Sediment Contamination: An International Journal 28(5), 461––472 (2019).
Farhangi-Abriz, S. & Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicology and Environmental Safety 147(1), 1010–1016 (2018).
Ebrahimi, M. Effect of EDTA and DTPA on phytoremediation of Pb-Zn contaminated soils by Eucalyptus camaldulensis Dehnh and effect on treatment time. Desert. 19, 65–73 (2014).
Quartacci, M. F., Irtelli, B., Baker, A. J. & Navariizzo, F. The use of NTA and EDDS for enhanced phytoextraction of metals from a multiply contaminated soil by Brassica carinata. Chemosphere 68(10), 1920–1928 (2007).
Wei, Z. B., Chen, X. H., Wu, Q. T. & Tan, M. Enhanched Phytoextraction of Heavy Mentals from Contaminated Soils Using Sedum alfredii Hance with Biodegradable Chelate GLDA. Environmental Science 36(5), 1864–1869 (2015).
Ghnaya, T. et al. Implication of organic acids in the long-distance transport and the accumulation of lead in Sesuvium portulacastrum and Brassica juncea. Chemosphere 90(4), 1449–1454 (2013).
Zhang, H. et al. Comparison of chelates for enhancing Ricinus communis L. phytoremediation of Cd and Pb contaminated soil. Ecotoxicology and Environmental Safety 133, 57–62 (2017).
Hu, N. et al. Enhancement of repeated applications of chelates on phytoremediation of uranium contaminated soil by Macleaya cordata. Journal of Environmental Radioactivity 199, 58–65 (2019).
Wan, Q. F. et al. Phytoremediation for Soil Contaminated by Uranium. Acta Chimica Sinica 69(15), 1780–1788 (2011).
Hseu, Z. Y., Jien, S. H., Wang, S. H. & Deng, H. W. Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. Ecotoxicology and Environmental Safety 117, 58–64 (2013).
Lozano, J. C., Blanco, R. P., Tome, F. V. & Calvo, C. P. Enhancing radium solubilization in soils by citrate, EDTA, and EDDS chelating amendments. Journal of Hazardous Materials 198, 224–231 (2013).
Li, C. W. et al. Phytoextraction of uranium from contaminated soil by Macleaya cordata before and after application of EDDS and CA. Environmental Science and Pollution Research 22(8), 6155–6163 (2014).
Li, F., Xiong, Z. & Hu, H. Effects of chelating agents on toxicity of copper to Elsholtzia splendens. Environmental Science 24(6), 96–100 (2003).
Acknowledgements
This work was financially supported by the National Defense Foundation of China (Grant No. 16ZG6101).
Author information
Authors and Affiliations
Contributions
Dan Wang designed the research. Li Chen conducted the experiment and drafed the manuscript. Chan Long and Zhengxu Cui did the statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Wang, D., Long, C. et al. Effect of biodegradable chelators on induced phytoextraction of uranium- and cadmium- contaminated soil by Zebrina pendula Schnizl. Sci Rep 9, 19817 (2019). https://doi.org/10.1038/s41598-019-56262-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56262-9
This article is cited by
-
Synergistic interactions of assorted ameliorating agents to enhance the potential of heavy metal phytoremediation
Stress Biology (2024)
-
Phytoremediation of electroplating wastewater by vetiver grass (Chrysopogon zizanoides L.)
Scientific Reports (2021)
-
Effects of S,S-ethylenediamine disuccinic acid on the phytoextraction efficiency of Solanum nigrum L. and soil quality in Cd-contaminated alkaline wheat soil
Environmental Science and Pollution Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.