Abstract
Upon inositol trisphosphate (IP3) stimulation of non-excitable cells, including vascular endothelial cells, calcium (Ca2+) shuttling between the endoplasmic reticulum (ER) and mitochondria, facilitated by complexes called Mitochondria-Associated ER Membranes (MAMs), is known to play an important role in the occurrence of cytosolic Ca2+ concentration ([Ca2+]Cyt) oscillations. A mathematical compartmental closed-cell model of Ca2+ dynamics was developed that accounts for ER-mitochondria Ca2+ microdomains as the µd compartment (besides the cytosol, ER and mitochondria), Ca2+ influx to/efflux from each compartment and Ca2+ buffering. Varying the distribution of functional receptors in MAMs vs. the rest of ER/mitochondrial membranes, a parameter called the channel connectivity coefficient (to the µd), allowed for generation of [Ca2+]Cytoscillations driven by distinct mechanisms at various levels of IP3 stimulation. Oscillations could be initiated by the transient opening of IP3 receptors facing either the cytosol or the µd, and subsequent refilling of the respective compartment by Ca2+ efflux from the ER and/or the mitochondria. Only under conditions where the µd became the oscillation-driving compartment, silencing the Mitochondrial Ca2+ Uniporter led to oscillation inhibition. Thus, the model predicts that alternative mechanisms can yield [Ca2+]Cyt oscillations in non-excitable cells, and, under certain conditions, the ER-mitochondria µd can play a regulatory role.
Similar content being viewed by others
Introduction
In non-excitable cells exposed to submaximal agonist levels, inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release from the endoplasmic reticulum (ER) is known to initiate1,2,3 and, in some cases, sustain oscillations of the cytosolic free Ca2+ concentration ([Ca2+]Cyt) without dependence on Ca2+ entry from the extracellular space4,5,6,7. By imparting frequency- and/or amplitude-dependent information to Ca2+-sensitive kinases and phosphatases, [Ca2+]Cyt oscillations are known to regulate gene expression and, thus, cell function and survival3,5,8,9. Experimental evidence demonstrated that the mitochondria (second largest Ca2+ store), via Ca2+ uptake from and release to the ER, actively participate in shaping and modulating [Ca2+]Cyt oscillations in different cell types6,10,11,12. The evidence was supported by the discovery that the ER and mitochondria form functional complexes called Mitochondria-Associated ER Membranes (MAMs)13,14. During cell stimulation, Ca2+ concentrations in the ER-mitochondria microdomains ([Ca2+]μd) can reach up to 10-fold higher values than in the bulk cytosol, enough to activate the Mitochondrial Ca2+ Uniporter (MCU) and allow for Ca2+ uptake15,16,17. Since the inositol trisphosphate receptor (IP3R) is regulated by Ca2+ in a biphasic manner (stimulatory at low levels/inhibitory at high levels)2,3,18 and functional IP3Rs localize preferentially in MAMs14,19,20, local Ca2+ uptake by mitochondria at the microdomains has the potential to either increase ER Ca2+ release by alleviating the Ca2+-dependent IP3R inactivation6,21,22 or decrease ER Ca2+ release by preventing the positive feedback of Ca2+ on IP3R (also known as Ca2+-induced Ca2+ release; CICR)23. Mitochondrial Ca2+ release to the cytosol (in principle, including the microdomains) via the mitochondrial Na+/Ca2+ exchanger (mNCX) was shown to refill the ER via the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)24,25, and could also affect the ER Ca2+ release by supporting either the Ca2+-dependent IP3R activation (and CICR)10 or the Ca2+-dependent IP3R inhibition21.
Due to the complexity of underlying mechanisms and often conflicting experimental findings, computational modeling has been employed to provide insight into the subcellular processes involved in [Ca2+]Cyt oscillations. Kinetic modeling of the biphasic IP3R response to Ca2+ verified early on that the IP3R is able to induce [Ca2+]Cyt oscillations at constant IP3 levels26,27. Subsequent models of Ca2+ homeostasis included all three subcellular compartments (cytosol, ER, mitochondria), while disregarding the presence of plasmalemmal ion channels (closed-cell), and expressed the dependence of [Ca2+]Cyt on fluxes across the ER and mitochondrial membranes and on binding to cytosolic buffer proteins, but the ER-mitochondria Ca2+ exchange took place only via the cytosol28,29. A direct flux was later introduced between ER and mitochondria, and, in that case, the MCU flux depended on the ER Ca2+ concentration ([Ca2+]ER)30. More recently, a report accounted for the Ca2+ microdomains between IP3R-MCU31: In their closed-cell model, MCU was exposed to [Ca2+]μd, which was calculated based on hemispherical symmetry of Ca2+ diffusion from a point source, a cluster of IP3Rs. MCU flux, [Ca2+]Cyt oscillation amplitude, and [Ca2+]Cyt oscillation frequency varied with the diffusion distance between the cluster of IP3Rs and MCU31.
[Ca2+]Cyt increases in vascular endothelial cells (ECs), a non-excitable cell type, are typically the result of activation of paracrine signaling due to binding of an extracellular agonist (such as ATP, acetylcholine, or histamine) to Gq protein-coupled receptors (GPCR), stimulation of IP3 production, release of Ca2+ from the ER, and, upon ER emptying, Ca2+ influx from the extracellular space via store-operated Ca2+ channels32,33,34. However, experimental evidence suggests that non-excitable cells, including ECs, when exposed to submaximal agonist levels (in the case of ECs, also when exposed to physiological fluid shear stress35) exhibit sustained [Ca2+]Cyt oscillations, at least for short times, without dependence on Ca2+ from the extracellular space (isolating the cell membrane and preventing Ca2+ exchange with the extracellular space was achieved by repeating the experiments in Ca2+-free medium supplemented with La3+)5,6,7,35. Understanding [Ca2+]Cyt dynamics in ECs is essential, because EC [Ca2+]Cyt responses, besides their role in gene expression, are responsible for the production of nitric oxide (NO) and prostanoids, which lead to smooth muscle cell relaxation and vasodilation36,37,38. Fluid mechanical shear stress exerted on cultured ECs is known to cause increases in IP3, due to release of endogenous ATP, and subsequently [Ca2+]Cyt mobilization and NO production39,40,41. [Ca2+]Cyt oscillations were present in ECs of both blood-perfused mouse cremaster muscle arterioles in situ and en face rat, carotid or mesenteric, arteries perfused in saline42,43. Importantly, our earlier experimental work demonstrated a critical role for the ER-mitochondria Ca2+ exchange (and the MCU) in regulating the [Ca2+]Cyt oscillations in cultured ECs exposed to shear stress35,44. The present study on [Ca2+]Cyt oscillations applies to non-excitable cells, including ECs, exposed to low/intermediate chemical agonist concentrations, as well as to ECs exposed to physiological fluid shear stress.
More specifically, our study aims to elucidate the role of the ER-mitochondria µd compartment in Ca2+ dynamics in a closed cell model. To further analyze the role of the µd in [Ca2+] dynamics, we introduced a parameter, termed the connectivity coefficient, for IP3R, MCU, SERCA and mNCX channels. This parameter indicates the fraction of each channel facing the µd. Our approach using connectivity coefficients allowed us to investigate the role of the µd on a spectrum from total isolation to its contiguity with the cytosol. This approach revealed conditions at which sustained oscillations occur and helped identify which subcellular compartment, cytosol or µd, is driving the oscillations under each condition. Interestingly, inclusion of the µd resulted in the presence of two distinct oscillatory regions in the bifurcation diagram of the system with respect to different IP3 levels. Oscillations in these two regions differed mechanistically, and also behaved differently when either the activity of MCU channels was altered or the ER-mitochondria distance (hence, the volume of the µd compartment) was varied. Global sensitivity analysis of model outputs indicated that the model can produce robust and physiological [Ca2+]Cyt oscillations over a wide range of parameter values. In summary, the modeling framework in the present study provides important insight into the intricacies of Ca2+ transport between the µd and each of the other Ca2+ compartments, as well as the effect of these processes on the global Ca2+ response. This information is currently limited and/or difficult to obtain experimentally, although it is critical for an accurate representation of Ca2+ signaling in non-excitable cells, including ECs.
Methods
Model
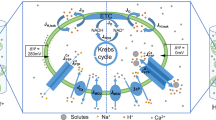
The cell model (Fig. 1) contains four compartments: cytosol (Cyt), ER, mitochondria (Mt), and µd. Ca2+ dynamics in each compartment is governed by a balance of Ca2+ fluxes, leaks, and buffering processes. Since the µd is in reality a part of the Cyt, several µd parameters (i.e. relating to buffer processes, leaks, and channel kinetics) were kept the same as for the Cyt. Temporal changes in [Ca2+] in each compartment is represented using the following ordinary differential equations:
where Ji is flux of Ca2+ ions (i indicates Cyt, ER, Mt, µd, or leak), and Voli is the compartment volume (i indicates the respective compartment Cyt, ER, Mt, or µd). Subscript µd in fluxes through the IP3R, MCU, SERCA, and mNCX channels denotes that the channel is facing the µd; if no subscript, then the channel is facing the Cyt. Leak fluxes (Jleak) are represented as leak from superscript to subscript compartment. All fluxes are defined with respect to VolCyt; thus, Eqs 2–4 are multiplied by volume ratios to adjust for the volume of the respective compartment.
Schematic representation of the cell model. Either chemical stimulation or flow-induced shear stress results in increased intracellular [IP3]. IP3 binds to IP3R triggering ER Ca2+ release. Ca2+ is pumped back into the ER via the SERCA or is taken up by the Mt via the MCU. Ca2+ is also extruded from the Mt into the intracellular space via the mNCX. IP3R, MCU, SERCA and mNCX channels face either the µd or Cyt (channels inward to the respective compartment are filled with color; outward channels are not filled). All fluxes, including leaks, are shown. Each compartment contains Ca2+ buffering proteins (not shown). This schematic diagram was created using Microsoft PowerPoint (https://products.office.com/en-us/powerpoint).
Buffering in each compartment is accounted for using a fast buffering approximation45. The buffering factor for each compartment is described as:
where θi is the buffering factor, BPi is the concentration of buffering proteins, and Ki is the buffer rate constant ratio (where i indicates the respective compartment Cyt, ER, Mt, or µd).
ER membrane fluxes
Efflux from the ER into the Cyt or µd through IP3R channels is defined as:
where VIP3R represents the maximum total flux through IP3R channels (in s−1)31. PoIP3R and \({{\rm{P}}}_{{\rm{oIP}}3{{\rm{R}}}_{{\rm{\mu }}{\rm{d}}}}\) are the open probabilities of IP3R channels facing the Cyt and µd, respectively (Supplementary Fig. S1). The connectivity coefficient CIP3R is the proportion of IP3R channels facing the µd. Mathematical formulations of IP3R dynamics were adopted from Qi et al.31. Thus, PoIP3R and \({{\rm{P}}}_{{\rm{oIP}}3{{\rm{R}}}_{{\rm{\mu }}{\rm{d}}}}\) are defined as:
where Sact and \({{\rm{S}}}_{{{\rm{act}}}_{{\rm{\mu }}{\rm{d}}}}\) are sigmoidal functions of [IP3] and [Ca2+] expressing the probability of a subunit being active, and h is the slow inactivation gating variable expressed as:
and for the µd:
where a2, d1, d2, d3, and d5 are parameters defined in Supplementary Table S1.
Ca2+ from the Cyt and µd is transported into the ER through SERCA pumps. Flux through the SERCA into the Cyt is defined as:
and for the µd:
where VSERCA is the maximal flux through SERCA, and kSERCA is the Ca2+ activation constant for SERCA (Supplementary Fig. S1). The connectivity coefficient CSERCA is the proportion of SERCA channels facing the µd.
Leaks
Ca2+ leak through the ER membrane is driven by concentration gradients between the ER and either the Cyt or µd. Leak from the ER into the Cyt is defined as:
and leak from the ER into the µd is defined as:
Although the µd is not a membrane-bound compartment (Fig. 1), we similarly defined the leak from the µd into the Cyt as:
The terms \({{\rm{k}}}_{{\rm{Cyt}}}^{{\rm{ER}}}\), \({{\rm{k}}}_{{\rm{\mu }}{\rm{d}}}^{{\rm{ER}}}\), and \({{\rm{k}}}_{{\rm{Cyt}}}^{{\rm{\mu }}{\rm{d}}}\) are rate constants defined in Supplementary Table S1.
Mitochondrial membrane fluxes
In Mt, Ca2+ is released via the mNCX that exchanges 1 Ca2+ ion for 3 Na+ ions. Flux through mNCX channels facing the Cyt is defined as:
and for mNCX channels facing the µd:
where [Na+]Cyt and [Na2+]μd are concentrations of Na+ in Cyt and µd, respectively, VmNCX is the maximal flux through the mNCX, and kNa and kmNCX are Na+ and Ca2+ activation constants for mNCX, respectively (Supplementary Fig. S1). The connectivity coefficient CmNCX is the proportion of mNCX channels facing the µd.
Ca2+ is transported into the Mt via the MCU. Flux through the MCU to the Cyt is defined as:
and to the µd:
where \({{\rm{V}}}_{{\rm{MCU}}}={{\rm{V}}}_{{{\rm{MCU}}}_{0}}\Delta \Phi \); and \(\Delta \Phi =\frac{{\rm{bF}}(\Psi -{\Psi }_{0})}{{\rm{RT}}}{{\rm{e}}}^{\frac{{\rm{bF}}(\Psi -{\Psi }_{0})}{{\rm{RT}}}}\,\sinh \,\frac{{\rm{bF}}(\Psi -{\Psi }_{0})}{{\rm{RT}}}\). \({{\rm{V}}}_{{{\rm{MCU}}}_{0}}\) represents the maximal flux through MCU, and ΔΦ is the voltage driving force. Ψ is the inner mitochondrial membrane voltage (150~180 mV, negative inside); F, R, and T are the Faraday constant, the universal gas constant, and temperature in Kelvin, respectively; b and Ψ0 are fitting parameters obtained from Qi et al.31. In the simulations performed in this paper, consistent with31, we assume a constant Ψ of 170 mV, as experimental evidence suggests that Ψ does not change significantly in response to transient cytosolic [Ca2+] increases produced by IP3-generating agonists46,47,48. kMCU is the Ca2+ activation constant for MCU, and the connectivity coefficient CMCU is the proportion of MCU channels facing the µd.
Effective cytosol
Since the µd is in reality a part of the cytosol, we defined the [Ca2+] of an “effective” cytosolic compartment, \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\), as the volume-weighted average of [Ca2+] within the combined Cyt and µd compartments.
Microdomain volume
We assumed that each mitochondrial object is a sphere with 20% of its surface area (SA) in close proximity to the ER49,50. Experimental data in ECs51 provided a range of mitochondrial object diameters between 0.5–1.5 µm, which was used to compute the SA, and an approximate number of mitochondrial objects per cell (N) of ~200. Thus, the volume of the µd compartment was estimated based on the SA of a single mitochondrion, their total number (N), and the ER-Mt distance (D):
Global sensitivity analysis
Robustness of the model was assessed using a global sensitivity analysis of model parameters. Each parameter of the model was allowed to vary within a lower and upper bound (±40% of the control values; Supplementary Table S1), and the Latin hypercube sampling (LHS) method52,53 with uniform distribution was used to select 250,000 random parameter sets. The model was solved for each set and the partial rank correlation coefficient (PRCC) analysis was performed to identify parameters that exerted statistically significant positive or negative influences on [Ca2+]Cyt oscillations. For all the analyses, 95% confidence interval was chosen for statistical significance.
A graphical user interface (GUI)-enabled MATLAB implementation of the model is available online (see https://tsoukias.fiu.edu/models/mitocaldynamics).
Results
Effect of Mt and µd compartments on the dynamics of Ca2+ oscillations
Bifurcation diagrams show the effect of the Mt and µd compartments on the IP3-induced \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillatory dynamics (Fig. 2A) for a set of control parameter values (Supplementary Table S1). In the absence of Mt and µd compartments (blue curve), the model predicts a single large amplitude (i.e., amplitudes can reach several µM) oscillatory region between two critical IP3/Ca2+ concentration levels (i.e., Hopf bifurcation points). Inclusion of the Mt compartment (red curve) resulted in a slight decrease in the predicted oscillatory amplitude, while the [IP3] window for oscillations (0.1–2 µM) and the corresponding \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) window for oscillations (0.2–1.7 µM) were not significantly affected. Interestingly, addition of the µd compartment resulted in the appearance of two distinct oscillatory domains (labeled regions α and β) in the bifurcation diagram. A small-amplitude oscillatory region appears (Fig. 2A inset) at low levels of IP3 (region α) while oscillations with larger amplitudes are predicted at intermediate levels of stimulation (region β). The presence of the µd reduced the IP3/Ca2+ concentration range for oscillations and the oscillation amplitudes at a given level of stimulation (compare black curve with red and blue ones). Temporal traces of \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) in response to a step increase in [IP3] in regions α, β, and γ, are shown in Fig. 2B–D. Consistent with bifurcation diagrams in Fig. 2A, panels B (region α) and C (region β) showed \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillations at low and intermediate [IP3], respectively, while panel D (region γ) showed damped oscillations with elevated \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) following IP3 stimulation. The amplitude of oscillations is lower (up to hundreds of nM) and the frequency is higher (~4 oscillations/min) at low levels of stimulation (region α) (Fig. 2B) compared to oscillations with μM amplitudes and slightly lower frequencies (~3 oscillations/min) at higher levels of stimulation (region β) (Fig. 2C). These differences suggest that distinct mechanisms may be giving rise to oscillations at different IP3 levels.
Bifurcation diagrams with/without the Mt and µd. (A) Bifurcation diagrams without Mt and µd (blue curve), with Mt and without µd (red curve), and with both Mt and µd (black curve). Compartments were inactivated by setting respective fluxes entering and leaving the compartment equal to zero. Addition of the µd resulted in the emergence of an oscillatory region at low levels of IP3 (magnified in inset of A). (B–D) Traces of \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) vs. time are shown with [IP3] of 0.2, 0.7, and 3 µM (corresponding to regions α, β, and γ, respectively) applied at 50 s. Sustained oscillations occurred following IP3 stimulation at levels corresponding to regions α (B) and β (C), whereas damped oscillations with elevated [Ca2+] levels occurred at high IP3 levels outside the oscillatory domains (region γ) (D).
Ca2+ shuttling between compartments
Temporal profiles of [Ca2+] in the subcellular compartments (ER, Mt, µd) before and after a step increase in IP3 levels were superimposed onto the simulated \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) profiles for regions α (Fig. 3A) and β (Fig. 3B). Ca2+ oscillations showed slightly shorter period (higher frequency) in region α compared to region β. While resting Ca2+ levels prior to IP3 stimulation remained the same for both regions, IP3 stimulation resulted in higher [Ca2+]Mt and lower [Ca2+]ER in region α compared to β. The shuttling of Ca2+ content between different compartments upon stimulation is better depicted in Fig. 3C,D. Under resting conditions, the ER stores the vast majority of total Ca2+ content (free and buffered). Following IP3 stimulation, ER Ca2+ is channeled to both the Cyt and the µd. At low level of IP3 stimulation (region α), the mitochondria pick up a larger portion of Ca2+ released by the ER through the µd compared to the portion picked up by the Cyt (Fig. 3C inset). However, at higher levels of IP3 stimulation (region β), this portion is reduced, as more of ER-released Ca2+ is picked up by the Cyt (Fig. 3D inset).
Ca2+ oscillation profiles and mass content in each subcellular compartment at different levels of IP3 stimulation. (A,B) Ca2+ oscillations in ER, Mt, and effective Cyt due to an IP3 stimulus of 0.2 µM (A; region α) and 0.7 µM (B; region β) applied at 50 s. Initial/basal Ca2+ levels in each compartment were the same in regions α and β. (C,D) Total Ca2+ content in each compartment (in femtomoles) is shown for region α (C) and β (D) and percentages following IP3 stimulation are reported in corresponding insets. Prior to IP3 stimulation, the majority of total Ca2+ content is stored in the ER. Following IP3 stimulation, ER-released Ca2+ is channeled to the Cyt and Mt at percentages that depend on the IP3 level.
Oscillations driven by different pools of IP3Rs
We examined the role of the two pools of IP3Rs (i.e., receptors facing either the Cyt or the µd) in the generation of Ca2+ oscillations. Simulations were performed under control conditions and after clamping the Ca2+ flux through each pool of IP3Rs, those facing the Cyt or those facing the µd, to its time-averaged value over a cycle (effectively abolishing the periodic opening of IP3R and maintaining the same average Ca2+ efflux from the ER). When the IP3R flux into the Cyt was clamped (JIP3R Clamped), \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillations were preserved in region α (their amplitude was minimally decreased; red curve in Fig. 4A), but were abolished in region β (red curve in Fig. 4B). Conversely, clamping of the IP3R flux into the µd (JIP3Rµd Clamped) resulted in loss of oscillations in region α (blue curve in Fig. 4A), while oscillations were maintained in region β (and their amplitude was increased; blue curve in Fig. 4B). These results suggest that the \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillations in region α are driven by the activity of IP3Rs facing the µd, whereas the oscillations in region β depend on the activity of IP3Rs facing the Cyt.
Driving of oscillatory activity by Cyt-facing vs. µd-facing IP3Rs. Upon IP3 stimulation (t = 50 s), flux through each of the two pools of IP3Rs was clamped to its time-averaged value. \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) profiles following an IP3 stimulus of 0.2 µM (region α; A) and 0.7 µM (region β; B) are shown for control (black curve), after clamping IP3Rs facing the Cyt (red curve), or after clamping IP3Rs facing the µd (blue curve). The activity of IP3Rs facing the µd is required for oscillatory activity at low [IP3] (blue curve in A), whereas the Cyt-facing IP3R activity drives the oscillations at higher [IP3] (red curve in B).
Oscillatory modes at different levels of IP3 stimulation
To probe the underlying mechanisms of oscillatory activity, we examined the timing of the filling and emptying phases in each compartment. Figure 5A,B depict the evolution of [Ca2+] in ER, Mt, μd and Cyt during an oscillatory cycle, at low (region α) and intermediate (region β) levels of IP3 stimulation. To better compare Ca2+ traces in compartments with significantly different Ca2+ content, we opted to normalize the ranges that concentrations span in each compartment (from the basal, prior to IP3 stimulation, level to the extreme level during stimulation) to be between 0 and 1. The slopes of Ca2+ traces in Fig. 5A,B are plotted in Fig. 5C,D, respectively. Regions with positive slope (above the grey dashed line) indicate that the net Ca2+ flux is inward in that compartment (i.e., “Refilling” phase for the compartment), whereas negative slopes (below the dashed line) indicate an outward net Ca2+ flux from the respective compartment (i.e., “Emptying” phase). Figure 5 shows the timing of [Ca2+] peaks in each compartment, and also provides information on how Ca2+ is shuttled between compartments to enable them to refill and sustain oscillatory activity. Upon stimulation, oscillatory activity is initiated when [Ca2+] in either Cyt or µd increases to sufficient levels, causing the IP3Rs facing these compartments to open, resulting in a Ca2+ burst/spike (µd, green line in Fig. 5A; Cyt, black line in Fig. 5B). During this time, the ER Ca2+ efflux through IP3Rs also charges the Mt. The high local Ca2+ levels achieved in µd and Cyt inactivate the IP3Rs and Ca2+ levels eventually decrease and the bursting activity subsides. Following the termination of a Ca2+ burst (i.e., during the interspike interval), the oscillation-driving compartment (μd in Fig. 5A or Cyt in Fig. 5B) is replenished and its [Ca2+] increases to adequate levels to reopen the IP3Rs. However, the compartment that feeds the oscillation-driving compartment (µd or Cyt) during the interspike interval varies depending on the level of IP3 stimulation, thus, yielding different modes of oscillatory activity. During the interspike interval of \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillations in the low stimulatory scenario (Fig. 5A), the µd is refilled primarily by the Mt (via local mNCX activity) with only a small contribution from the Cyt. At higher [IP3] stimulation (Fig. 5B), the oscillation-driving compartment, i.e., Cyt, is refilled primarily by the ER and to a smaller extent by the Mt (Fig. 5C,D). The different oscillatory modes affect the timing that [Ca2+] peaks in each compartment. During the oscillatory cycle in Fig. 5A, [Ca2+] in the µd peaks first followed by peaks in the Cyt and the Mt (Fig. 5A). Ca2+ oscillations in the ER are in antiphase with oscillations in the Mt (Fig. 5A). In Fig. 5B, [Ca2+] in the Mt is peaking before [Ca2+] in the Cyt, and oscillations in the Cyt are in antiphase with ER oscillations (Fig. 5B).
Relative changes in Ca2+ levels of each compartment during an oscillation cycle. (A,B) Normalized relative changes in [Ca2+] are plotted following stimulation with low [IP3] (region α; A) and intermediate [IP3] (region β; B) during the duration of one oscillation cycle with peaks labeled for each compartment. The µd peaked first in both regions (green line in A,B). The Cyt peaked before the Mt in region α (black and cyan lines in A), while it peaked after the Mt in region β (black and cyan lines in B). (C,D) The slopes of the curves of relative [Ca2+] changes for regions α and β indicate refilling or emptying of compartments as positive and negative, above or below the grey dashed line, respectively. The interspike interval of the oscillation-driving compartment is labeled (µd in region α (A,C) and Cyt in region β (B,D)). Emptying and refilling phases are also labeled (C,D), in order to show which compartment is refilling the oscillation-driving compartment during the interspike interval. The µd is refilled primarily by the Mt in region α, and the Cyt is refilled primarily by the ER in region β.
Modulation of oscillations by the MCU
The above differences in subcellular mechanisms that drive [Ca2+] oscillations at low vs. intermediate IP3 stimulation may have implications in how the system responds to alterations in mitochondrial function. We used the model to predict cell responses when the mitochondrial Ca2+ uptake is altered. Figure 6A,B depict the \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillation frequency as the activity of the MCU (VMCU) changes at low and intermediate levels of IP3 stimulation. Oscillation frequency was highly sensitive to changes in VMCU in the μd-driven oscillations (region α; Fig. 6A); frequency increased within a certain VMCU range, but oscillations were lost for larger changes from the control value. Conversely, oscillation frequency in Cyt-driven oscillations (region β; Fig. 6B) was less sensitive to changes in VMCU, showing a slight reduction as VMCU increased (Fig. 6B). [Ca2+] traces of the oscillation-driving compartments, µd for region α and Cyt for region β, at two different values of VMCU are shown in Fig. 6A,B insets, respectively. In region α, the burst duration decreased at a higher value of VMCU (orange curve of Fig. 6A inset). In region β, the burst duration was mostly unaffected, but the interspike interval was increased as VMCU was increased (inset in Fig. 6B). Overall, mitochondrial uptake affects the system differently between regions, markedly increasing the \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillation frequency in region α, while maintaining/slightly decreasing the frequency in region β. Knocking out the MCU channel (MCU KO), by setting VMCU = 0, resulted in loss of oscillations in region α (Fig. 6C), but oscillatory activity was maintained in region β (Fig. 6D). These findings suggest that Ca2+ uptake by the Mt via the MCU (i.e., the MCU channel activity) is critical for \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillations in region α, but only has a modulatory effect on oscillations in region β (i.e., small changes in frequency and amplitude). This agrees with the Mt being responsible for refilling the oscillation-driving compartment in region α (Fig. 5A) and not being as critically required in region β, where oscillations depend mainly on Ca2+ shuttling between the ER and the Cyt (Fig. 5B).
Effect of MCU activity on Ca2+ dynamics. \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillation frequency as a function of VMCU is plotted for regions α (A) and β (B) with insets in each panel showing oscillations at two different values of VMCU (indicated with colored arrows on the x-axis). Oscillations in region α were highly sensitive to changes in VMCU (A), while in region β, frequency changed slightly with increasing VMCU (B). Oscillatory cycles shown indicate that frequency is modulated primarily by the burst duration in region α (A inset), whereas it is modulated primarily by the duration of the interspike interval in region β (B inset). (C,D) Temporal changes in [Ca2+] are plotted for effective Cyt (black), Mt (cyan), and ER (red) following MCU KO (VMCU = 0) and IP3 stimulation (t = 50 s) in regions α (C) and β (D). (C) In region α, MCU KO resulted in loss of oscillations in all compartments. At steady-state, \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) remained elevated and [Ca2+]ER was slightly decreased compared to their corresponding basal levels. (D) In region β, MCU KO allowed for sustained \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) and [Ca2+]ER oscillations with increased amplitude compared to their corresponding control traces (Fig. 3B). Mt curves in either region show [Ca2+] depletion due to lack of Ca2+ influx (and continued Ca2+ efflux). Notice that [Ca2+]Mt is multiplied by a factor of 10 (C,D).
Effect of MCU and IP3R channel distribution on Ca2+ oscillation frequency
The influence of the μd on Ca2+ oscillations depends on the relative distribution of MCU and IP3Rs between the μd and the Cyt (i.e., connectivity coefficients CMCU and CIP3R). Figure 7A examines how the oscillation frequency (color coded) varies as a function of CMCU and [IP3]. The heat map highlights conditions (i.e., CMCU and [IP3] values) where oscillations occur and could be decomposed into two areas by clamping either the Cyt-facing (Fig. 7B) or the μd-facing (Fig. 7C) IP3Rs to their time-averaged values, as described earlier (Fig. 4). At [IP3] ranging from ~0.1–0.25 µM (i.e., levels of stimulation corresponding to the µd-driven oscillatory region α), high CMCU values are required for oscillations and the frequency depends on CMCU (Fig. 7B). At [IP3] > ~0.25 µM (i.e. levels of stimulation corresponding to the Cyt-driven oscillatory region β), oscillations appear independently of the MCU presence in the μd (CMCU = 0) and oscillation frequency did not change substantially with respect to CMCU and remained at ~3 oscillations/min (Fig. 7C). Similarly, Fig. 7D,E examine how the oscillation frequency (color coded) varies as a function of CIP3R and CMCU at two different [IP3]. At low level of stimulation, CMCU > 0.5 and 0.3 < CIP3R < 0.6 promote μd-driven oscillations (Fig. 7D). Cyt-driven oscillations that are only slightly modified with CMCU are predicted at both low and high IP3 stimulation provided that a sufficient percentage of IP3Rs is facing the Cyt (CIP3R < 0.3 in Fig. 7D; CIP3R < 0.6 in Fig. 7E). Overall, the behavior of the system in response to changes in CIP3R and CMCU within each region agrees with the mechanistic differences predicted by the model.
Dependence of Ca2+ oscillation frequency on CMCU and CIP3R. (A) Heatmap of oscillation frequency as a function of CMCU and [IP3]. At low [IP3] (corresponding to region α, the µd-dominant region), oscillations were present only at select values of CMCU. At higher [IP3] (corresponding to region β, the Cyt-dominant region), the oscillation frequency remained almost unchanged with respect to CMCU. (B) Clamping of JIP3R resulted in loss of oscillatory activity in the Cyt-dominant region, but sustained activity in the µd-dominant region. (C) Clamping of JIP3Rµd resulted in loss of oscillations in the µd-dominant region, but activity was sustained in the Cyt-dominant region. (D,E) Oscillation frequency was computed as a function of CMCU and CIP3R at two [IP3]. (D) In region α, the highest oscillation frequency occurred with CMCU close to 1 and CIP3R of ~0.5. Higher values of CIP3R resulted in loss of oscillations. (E) In region β, the oscillation frequency remained almost unchanged with respect to CMCU, but oscillations were lost at CIP3R > ~0.6.
Effect of ER-Mt distance and channel distribution on Ca2+ oscillation frequency
Variations in ER-Mt distance D alter the Volµd (Eq. (27)) and, thus, D plays an important role in Ca2+ dynamics. Figure 8 examines \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) oscillation frequency as a function of D and channel connectivity coefficients. D was not allowed to be <10 nm due to the size of IP3R54. At [IP3] = 0.2 µM (Fig. 8A,B), increasing D reduced the oscillation frequency until eventually abolishing them at D > ~150 nm; this relationship was most prominent within narrow ranges of connectivity coefficients (CIP3R = 0.3–0.5 in Fig. 8A; CMCU > 0.8 in Fig. 8B). Simulations also show lower frequency regions at CIP3R < ~0.3 with [IP3] = 0.2 µM, which did not vary significantly with D (Fig. 8A). These low frequency regions resemble those seen in Fig. 7D. At [IP3] = 0.7 µM, variations in D did not substantially alter the oscillation frequency (Fig. C,D); in fact, oscillations were sustained at all distances and connectivity values except when CIP3R > ~0.75.
Dependence of Ca2+ oscillation frequency on the ER-Mt distance. Heatmaps of oscillation frequency at two different levels of IP3 stimulation ([IP3] = 0.2 µM in (A,B) [IP3] = 0.7 µM in C,D) as a function of D and either CIP3R (A,C) or CMCU (B,D). Oscillation frequency at higher levels of IP3 (C,D) remained close to ~3 oscillations per minute, showing less dependence on D. At lower levels of IP3 stimulation (A,B), oscillation frequency was sensitive to variations in D; the frequency decreased as D increased with complete loss of oscillatory activity when D > ~150 nm. However, oscillations that were completely insensitive to variations in D occurred at low stimulation with CIP3R < ~0.3 (dark red region in lower part of A).
Sensitivity analysis and model robustness
Robustness of model output in response to changes in parameter values was assessed using a global sensitivity analysis. Figure 9A,B show the statistically significant parameters for peak amplitude and frequency, respectively, of [Ca2+]Cyt oscillations. The PRCC value (i.e., slope of a fitted line to model output as a function of the parameter of interest) indicates the relative magnitude of the influence of the parameter (only parameters with PRCC > 0.05 are shown). The sign indicates whether the parameter is positively or negatively correlated to output. For example, CIP3R is negatively correlated to the amplitude and frequency of oscillations owing to a reduction of the fraction of IP3R efflux that enters the Cyt. D is also negatively correlated to the amplitude and frequency of oscillations, whereas the level of IP3 is positively correlated. Overall, Fig. 9A,B show that different parameters, especially those determining the IP3R, MCU and SERCA channel activities, have differential effects on the peak amplitude and frequency of [Ca2+]Cyt oscillations. Figure 9C–G show the probability distribution of the peak Ca2+ amplitude in each compartment (Fig. 9C–F) and frequencies (Fig. 9G) from 250,000 parameter sets analyzed. It is noteworthy that, despite the wide range of parameter values, model responses remain within physiological levels following IP3 stimulation with peak amplitudes near 2 µM for [Ca2+]Cyt and [Ca2+]Mt, and 50 µM for [Ca2+]μd, and frequencies near 3 oscillations/min, in agreement with experimental observations in non-excitable cells, including ECs35,43,55,56.
Robustness and sensitivity of model output to variations in parameters. (A,B) Partial Rank Correlation Coefficient (PRCC) values of statistically significant parameters taken from 250,000 parameter sets obtained from Latin Hypercube Sampling with uniform distribution. Each parameter was allowed to vary ±40% of its control value in Supplementary Table S1. Out of 250,000 parameter sets, oscillations occurred in ~60,000 sets (~25% of total). Sensitivity of parameters to peak amplitude (A) and frequency (B) are shown. (C–F) Histograms of peak amplitude of [Ca2+] oscillations in each compartment. (G) Histogram of frequency of [Ca2+]Cyt oscillations.
Ca2+ shuttling between compartments produces alternative oscillatory modes
Simulations using our control parameter values revealed two distinct oscillatory mechanisms under low and intermediate stimulation conditions. At low [IP3], µd-driven oscillations relied on Ca2+ refilling by the Mt. At higher [IP3], simulated oscillations depended on opening of the Cyt-facing IP3Rs and refilling of the Cyt by the ER. However, exploration of the parameter space revealed that oscillations can occur when either the ER, the Mt, or a combination of the two (Fig. 10A–C, respectively) refills the Cyt. Figure 10 depicts the normalized changes in [Ca2+] in Cyt, ER, and Mt (as described in Fig. 5) from representative simulations using different sets of parameters (Supplementary Table S2). Figure 10A shows a decline in [Ca2+]ER, while [Ca2+]Cyt and [Ca2+]Mt increase during the interspike interval. This indicates that the ER refills the system bringing the [Ca2+]Cyt above the critical level necessary to initiate the subsequent oscillation (ER feeding). In Fig. 10B, oscillations occur with Mt refilling the Cyt during the interspike interval (Mt feeding; see decreased [Ca2+]Mt and increased [Ca2+]Cyt and [Ca2+]ER slopes). In Fig. 10C, efflux from both the ER and the Mt increases [Ca2+]Cyt up to the critical level. Thus, mechanistically-distinct modes of oscillations can be generated based on the contribution of different model components.
Additional modes of Ca2+ oscillations captured by the model. Three parameter sets (chosen from the set of 250,000 obtained from the global sensitivity analysis) illustrate additional modes of oscillations that can be captured by the model (the chosen parameter sets are shown in Supplementary Table S2). Colored arrows indicate sign of the slope for each compartment (black for Cyt, red for ER, blue for Mt). Oscillatory modes are defined based on which compartments refill the Cyt during the interspike interval (shown by a dashed line); modes include: ER feeding (A), Mt feeding (B), and both ER and Mt feeding (C).
Discussion
In the present study, we investigate the mechanisms underlying [Ca2+]Cyt oscillatory activity in non-excitable cells, such as vascular ECs, when exposed to submaximal agonist stimulation or fluid shear stress. A mathematical model of IP3-induced Ca2+ mobilization in non-excitable cells was developed that accounts for all major subcellular Ca2+ compartments (i.e., Cyt, ER, Mt), their respective Ca2+ channels and buffering, and also considers explicitly the ER-Mt Ca2+ microdomains as a distinct Ca2+ pool (µd). The model was able to produce physiologically relevant [Ca2+] in all compartments, at rest and following stimulation, and exhibited robust oscillations. Simulations highlighted the role of the mitochondria as regulators of [Ca2+]Cyt oscillatory activity and the importance of the ER-Mt µd in the process.
Mitochondria are able to uptake and store significant amounts of Ca2+. Their buffering potential allows them to modulate Ca2+ mobilization in the cytosol including oscillatory dynamics. For example, model simulations in Fig. 2A showed only a slight reduction in oscillatory amplitude after incorporating the Mt compartment in the model, in agreement with57. Furthermore, simulations in Fig. 6B,D suggested that changing Mt Ca2+ uptake has a minimal effect on oscillatory frequency and that oscillations can be sustained in an in silico MCU KO cell. Hence, Ca2+ buffering by Mt does not necessarily establish their critical regulatory role in cell function11,58. We postulated that the presence of the ER-Mt µd may shape the [Ca2+]Cyt oscillatory dynamics and uncover the critical regulatory role of Mt in [Ca2+]Cyt oscillations, in agreement with experimental observations6,10,12.
A mathematical model by Penny et al.59 previously explored the role of the ER-lysosome µd in driving [Ca2+]Cyt dynamics in stimulated fibroblasts by varying the distribution of two-pore Ca2+ channels on lysosomes. They showed that the distribution and density of the Ca2+ channels on lysosomes that face the ER-lysosome µd regulate the IP3R-dependent [Ca2+]Cyt oscillations59. To the best of our knowledge, the only mathematical study that has explored the role of the ER-Mt µd prior to our study is the one by Qi et al.31, where they assumed Ca2+ diffusion from a point source, a cluster of IP3Rs to the MCU, and varied the distance between the ER and Mt compartments (MCU activity depended on the local [Ca2+], while JIP3R was only a function of [Ca2+]Cyt; their Eqs 5–7). Their study found that there is an optimal IP3R-MCU distance for effective Ca2+ transfer (~30 nm) and generation of physiologically relevant [Ca2+]Cyt oscillations, and abnormally high [Ca2+]Cyt arises when the distance is greater than the optimal one31. In contrast, in the present model, we incorporated an explicit µd compartment with a variable volume which depended on the ER-Mt distance (Eq. 27). This approach allowed us to separate IP3Rs, as well as MCU, SERCA, and mNCX channels, into two distinct pools; one pool of IP3Rs that is facing the μd and, thus, their activity depends on [Ca2+]μd, and another pool of IP3Rs that are facing the Cyt and are exposed to [Ca2+]Cyt. This resulted in identifying conditions where the µd and the Mt play a regulatory role, aside from their modulatory role, in [Ca2+] oscillatory dynamics. More specifically, incorporation of the µd and the channel connectivity coefficients for the above four channels led to the emergence of two distinct oscillatory regions at low and intermediate [IP3] (Fig. 2A). Oscillations at low levels of IP3 stimulation were predominately driven by the opening of IP3Rs facing the µd, whereas oscillations at higher levels of IP3 stimulation were driven by opening of Cyt-facing IP3Rs (Figs. 4 and 5). Simulation results also demonstrated the important role of the ER-Mt distance, in combination with channel connectivity coefficients, on the frequency and sustainment of oscillations, more notably at low levels of IP3 stimulation (Fig. 8). Global sensitivity analysis also revealed that the frequency of cytosolic Ca2+ oscillations is negatively correlated with the ER-Mt distance (Fig. 9), in agreement with Qi et al.31.
The series of events that result in initiation and maintenance of [Ca2+]Cyt oscillations at low [IP3] (region α) is as follows: A small increase in [IP3] activates the IP3R causing ER Ca2+ release into the µd and Cyt. The elevation in [Ca2+] in each of these compartments increases the IP3R open probability causing further release of Ca2+ from the ER (i.e., CICR). High receptor density in the small µd volume results in a substantial increase in [Ca2+]μd, which further activates the µd-facing IP3Rs and results in a Ca2+ burst and the initiation of oscillations (Fig. 5A). The increase in [Ca2+]μd causes a secondary increase in [Ca2+]Cyt through Ca2+ leak fluxes from the µd and efflux from mNCX (i.e., stimulation level is not sufficient to initiate a burst of Ca2+ release from the IP3R channels facing the Cyt). As a result, [Ca2+]Cyt increases slower and cytosolic oscillations are delayed compared to those in the µd (Fig. 5A). In contrast to region α, the higher level of [IP3] in region β causes an increased efflux from Cyt-facing IP3Rs, which brings the [Ca2+]Cyt to sufficient levels for initiation of Ca2+ bursting. High [Ca2+]μd, on the other hand, inactivates the µd-facing IP3Rs at high levels of stimulation. Thus, in region β, periodic opening of Cyt-facing IP3Rs governs the oscillations of \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\). The temporal evolution of [Ca2+] in subcellular compartments in Figs. 5 and 10 suggests that the oscillatory activity can be further classified based on the exchange of Ca2+ between compartments in each cycle. As the Ca2+ burst subsides, [Ca2+] in the compartment driving the oscillatory activity (µd or Cyt) decreases. For the next oscillatory cycle to occur, this compartment needs to be replenished, so the IP3Rs can be reopened by sufficient [Ca2+] levels. Simulations suggest that fluxes from the ER (Figs. 5B and 10A) and/or the Mt (Figs. 5A and 10B) can play this role depending on stimulus strength and/or channel distribution. Thus, alternative oscillatory modes are predicted depending on the compartment that drives the oscillatory activity and the compartment that feeds it between Ca2+ spikes. Simulations (Supplementary Fig. S2) also depict the effect of the µd on [Ca2+] in the Mt, as well as the Cyt, at different levels of IP3. In the absence of the µd, [Ca2+]Mt increases as IP3 levels increase (Fig. S2B); however, the increase is small and follows/is a result of the corresponding increase in [Ca2+]Cyt. In the presence of the µd (Fig. S2A), the Mt Ca2+ uptake dissociates from [Ca2+]Cyt and a significant [Ca2+]Mt increase is observed as [IP3] increases between 0–0.25 µM. This result highlights the importance of locally high [Ca2+]μd on Mt Ca2+ uptake, as suggested by the dissociation of [Ca2+]Mt and [Ca2+]Cyt in the presence of IP3 buffering proteins, in the study by Lin et al.60.
The predicted mechanistic differences in the generation and maintenance of oscillations can result in different cell responses to MCU inhibition. MCU KO resulted in loss of oscillations when the oscillation-driving compartment (i.e., the µd) was refilled by Mt (region α; Fig. 6C). In contrast, simulations suggested only a modulatory role of MCU in a Cyt-driven and ER-replenished system (region β; Fig. 6D). Partial inhibition of MCU activity can reduce the oscillation frequency or even stop the oscillations in the Mt refilling system (region α; Fig. 6A). Specifically, decreased MCU activity resulted in prolonged emptying of the µd, thus, increasing the period of oscillations and decreasing the frequency (notice the falling phase of [Ca2+]μd in blue vs. orange traces; Fig. 6A inset). On the contrary, in region β, the oscillation frequency slightly increases with decreasing MCU activity (Fig. 6B). Specifically, decreased MCU activity results in less Ca2+ being picked up by the Mt and, hence, more Ca2+ remaining in the Cyt. Consequently, [Ca2+]Cyt during the interspike interval reaches the threshold level needed for the subsequent oscillatory cycle faster (notice the interspike interval in blue vs. orange traces; Fig. 6B inset), and the oscillation frequency slightly increases (Fig. 6B). This result is expected, since oscillations in region β mainly depend on refilling of the Cyt from the ER; hence, loss of MCU activity only has a modulatory effect on Ca2+ oscillations (Figs. 5 and 6).
The model predictions on the effect of MCU KD on the oscillation frequency are in agreement with experimental findings: There is ample evidence in the literature that in non-excitable cells stimulated by low/intermediate levels of agonists, e.g. 1–10 µM histamine, as well as in ECs exposed to physiological levels of shear stress, MCU KD decreases the frequency of [Ca2+]Cyt oscillations compared to control (non-transfected or transfected with a scramble siRNA) cells exposed to the same treatment6,7,35,61. Our findings in region α corroborate these observations: At certain levels of low [IP3] (<0.25 µM), the oscillation frequency was shown to decrease with either decreasing VMCU (Fig. 6A) or CMCU (Fig. 7A). At higher levels of chemical stimulation, e.g. 100 µM histamine (which may be region β), in agreement with the model findings, Hoffman NE et al.62 (their Supplementary Fig. 3B,C and personal communication with Dr. M. Madesh) found no significant effect of MCU KD on the [Ca2+]Cyt oscillation frequency in HeLa cells (they observed an increase in basal [Ca2+]Cyt, due to the reduced Ca2+ uptake by Mt62).
Identifying the compartment that refills the oscillation-driving compartment during the interspike interval allows us to classify the types of oscillations generated in previous modeling (and limited experimental) studies on Ca2+ dynamics in non-excitable cells, and compare those types to the ones generated by the present model. Simulated [Ca2+] traces in Wacquier et al. (their Fig. 2B)61 and in Pecze et al. (their Fig. 2E)7 suggest that the Cyt is mainly refilled by the Mt during the interspike interval, while, in Qi et al. (their Fig. 2A)31, the Cyt is refilled by the ER. The current model consolidates these previous findings and shows that, depending on the choice of parameter values that regulate Ca2+ fluxes between compartments (including the µd), we can achieve different feeding mechanisms to maintain [Ca2+]Cyt oscillations. Different parameter value sets produce oscillations where the Cyt is fed by only the ER, only the Mt, or a combination of both (Fig. 10 and Supplementary Table S2). Furthermore, stimulus strength may switch oscillatory modes, as shown for our control parameter set (Fig. 5 and Supplementary Table S1). Our findings may provide an explanation for the discrepancies observed in the system response to changes in MCU activity among previous modeling studies (e.g., in agreement with the feeder compartment identified in their model, Pecze et al.7 found a significant reduction in oscillation frequency in MCU KD, compared to control, mouse mesothelial cells following stimulation; their Fig. 3B). Experimental validation of the feeding mechanism is currently limited, because very few groups have recorded [Ca2+]Cyt oscillations together with either [Ca2+]ER or [Ca2+]Mt oscillations from the same cell, and even fewer superimposed them (on the same time axis)6,10. The experimental work by Ishii et al.10, where they transduced HeLa cells with retroviruses expressing fluorescence Ca2+ indicators targeted to the Cyt, ER and Mt, clearly showed that, during histamine (10 µM) stimulation, [Ca2+]Cyt and [Ca2+]Mt oscillate almost in phase with [Ca2+]Cyt leading, while [Ca2+]ER is in anti-phase with them, and the feeder compartment is the Mt (their Figs. 2B and 3B), further supporting the role of the µd as the key compartment driving the [Ca2+]Cyt oscillations.
The present work has certain limitations: In the model we assumed a closed system, based on experimental data showing that Ca2+ influx from the extracellular space is not necessary for initiating and maintaining [Ca2+]Cyt oscillations (although it is thought to become important during prolonged stimulation)5,6,35. Furthermore, the present model builds on earlier work, in particular, the models by Qi et al.31, Wacquier et al.61, and Pecze et al.7, all of which considered a closed cell and demonstrated the generation of physiological [Ca2+]Cyt oscillations based on IP3R regulation by Ca2+ and on ER-Mt Ca2+ transport. However, neglecting transmembrane fluxes may lead to overestimation of Ca2+ levels during oscillations, i.e., \({[{{\rm{Ca}}}^{2+}]}_{{\rm{Cyt}}}^{{\rm{eff}}}\) >2 µM. Thus, inclusion of the transmembrane fluxes may be an avenue for future work.
Our model did not consider the spatial distribution of subcellular organelles and it adopted a simplified approach regarding the presence of functional IP3R, MCU, SERCA and mNCX channels in the MAMs by assigning values between 0–1 to their respective connectivity coefficients. However, the model was still able to provide insights on how varying the above channel activities in the MAMs, at different levels of IP3, influences the role of the µd in regulating Ca2+ signaling and ultimately cell function. Quantitative experimental information on the connectivity coefficients of Mt and ER channels is currently lacking in the literature. Immunofluorescence studies have detected IP3R and SERCA in MAMs and a number of studies found the IP3Rs to be MAM-enriched in certain cell types, but local channel activity levels were not assessed quantitatively (reviewed in14). More recently, De La Fuente et al.63 used size exclusion chromatography to verify the presence of MCU-EMRE complexes (both proteins are essential for functional MCU channels) in the mitochondria associated with the sarcoplasmic reticulum (SR) fraction of heart homogenate, followed by 45Ca2+ isotope uptake assays to measure MCU-mediated Ca2+ uptake activity. They found a 2-fold increase in MCU activity in SR-associated mitochondria compared to non SR-associated ones, and concluded that MCU “hot spots” exist in cardiomyocyte MAMs63. Furthermore, at the current stage, our model does not differentiate among the IP3R isoforms, i.e. IP3R1, 2, and 3. IP3 sensitivity, Ca2+ gating (i.e. feedback inhibition), distribution, and other properties are different among these isoforms, and their relative abundance varies in different cell types. IP3R isoforms were recently found to differentially regulate ER-Mt contacts and the local Ca2+ fluxes54. Their role in Ca2+ dynamics can be accounted for in future modeling studies.
The present model also used an explicit dependence of VMCU on a constant inner mitochondrial membrane voltage (Ψ). This assumption of a constant Ψ is based on experimental evidence that Mt uptake does not significantly alter Ψ46,47,48. More detailed modeling of Ψ kinetics, as demonstrated in Wacquier et al.61, would be an appropriate addition in future modeling efforts. Last, the model utilized several assumptions regarding the shape and number of mitochondria in a non-excitable cell, as well as in the percentage of Mt surface area that faces the µd, in order to relate the Volµd with the ER-Mt distance D and assess the importance of D (which was varied within a physiological range) in Ca2+ oscillations. In conclusion, additional experiments combined with mathematical modeling are needed to better understand how the µd regulates global Ca2+ signaling.
In summary, we developed a compartmental closed-cell mathematical model of Ca2+ dynamics in a prototypical non-excitable cell that includes a Ca2+ µd between ER and Mt. Model results showed robust oscillatory activity using physiologically relevant parameter values, and alternative oscillatory modes were identified depending on stimulus strength and the relative distribution of Ca2+ channels facing each compartment. This enabled us to consolidate previous theoretical and experimental findings and suggested conditions where the mitochondria play a critical regulatory role, rather than simply modulating oscillatory activity, upon stimulation. We provided evidence that the microenvironment between the ER and Mt can play a critical role in Ca2+ dynamics by modulating the activity of IP3Rs, allowing shuttling of Ca2+ between the ER and Mt, and creating oscillations sensitive to the mitochondrial function.
References
Woods, N. M., Cuthbertson, K. S. & Cobbold, P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319, 600–602, https://doi.org/10.1038/319600a0 (1986).
Miyakawa, T. et al. Ca(2+)-sensor region of IP(3) receptor controls intracellular Ca(2+) signaling. Embo j 20, 1674–1680, https://doi.org/10.1093/emboj/20.7.1674 (2001).
Berridge, M. J. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793, 933–940, https://doi.org/10.1016/j.bbamcr.2008.10.005 (2009).
Sneyd, J., Tsaneva-Atanasova, K., Yule, D. I., Thompson, J. L. & Shuttleworth, T. J. Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci USA 101, 1392–1396, https://doi.org/10.1073/pnas.0303472101 (2004).
Parekh, A. B. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 36, 78–87, https://doi.org/10.1016/j.tibs.2010.07.013 (2011).
Samanta, K., Douglas, S. & Parekh, A. B. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS One 9, e101188, https://doi.org/10.1371/journal.pone.0101188 (2014).
Pecze, L., Blum, W. & Schwaller, B. Routes of Ca2+ Shuttling during Ca2+ Oscillations: Focus on the role of mitochondrial Ca2+ handling and cytosolic Ca2+ buffers. J Biol Chem 290, 28214–28230, https://doi.org/10.1074/jbc.M115.663179 (2015).
Zhu, L. et al. Ca2+ oscillation frequency regulates agonist-stimulated gene expression in vascular endothelial cells. J Cell Sci 121, 2511–2518, https://doi.org/10.1242/jcs.031997 (2008).
Smedler, E. & Uhlen, P. Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840, 964–969, https://doi.org/10.1016/j.bbagen.2013.11.015 (2014).
Ishii, K., Hirose, K. & Iino, M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep 7, 390–396, https://doi.org/10.1038/sj.embor.7400620 (2006).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–578, https://doi.org/10.1038/nrm3412 (2012).
De Stefani, D., Rizzuto, R. & Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annual review of biochemistry 85, 161–192, https://doi.org/10.1146/annurev-biochem-060614-034216 (2016).
Csordas, G. et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174, 915–921, https://doi.org/10.1083/jcb.200604016 (2006).
Raturi, A. & Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta 1833, 213–224, https://doi.org/10.1016/j.bbamcr.2012.04.013 (2013).
Csordas, G. et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39, 121–132, https://doi.org/10.1016/j.molcel.2010.06.029 (2010).
Giacomello, M. et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38, 280–290, https://doi.org/10.1016/j.molcel.2010.04.003 (2010).
Williams, G. S., Boyman, L., Chikando, A. C., Khairallah, R. J. & Lederer, W. J. Mitochondrial calcium uptake. Proc Natl Acad Sci USA 110, 10479–10486, https://doi.org/10.1073/pnas.1300410110 (2013).
Bezprozvanny, I., Watras, J. & Ehrlich, B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754, https://doi.org/10.1038/351751a0 (1991).
Hajnoczky, G., Csordas, G., Madesh, M. & Pacher, P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol 529(Pt 1), 69–81 (2000).
Hayashi, T. & Su, T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610, https://doi.org/10.1016/j.cell.2007.08.036 (2007).
Vay, L. et al. Modulation of Ca(2+) release and Ca(2+) oscillations in HeLa cells and fibroblasts by mitochondrial Ca(2+) uniporter stimulation. J Physiol 580, 39–49, https://doi.org/10.1113/jphysiol.2006.126391 (2007).
Olson, M. L., Chalmers, S. & McCarron, J. G. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J Biol Chem 285, 2040–2050, https://doi.org/10.1074/jbc.M109.027094 (2010).
Hajnoczky, G., Hager, R. & Thomas, A. P. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+. J Biol Chem 274, 14157–14162 (1999).
Arnaudeau, S., Kelley, W. L., Walsh, J. V. Jr. & Demaurex, N. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem 276, 29430–29439, https://doi.org/10.1074/jbc.M103274200 (2001).
Malli, R., Frieden, M., Trenker, M. & Graier, W. F. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem 280, 12114–12122, https://doi.org/10.1074/jbc.M409353200 (2005).
De Young, G. W. & Keizer, J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci USA 89, 9895–9899 (1992).
Li, Y. X. & Rinzel, J. Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J Theor Biol 166, 461–473, https://doi.org/10.1006/jtbi.1994.1041 (1994).
Marhl, M., Schuster, S. & Brumen, M. Mitochondria as an important factor in the maintenance of constant amplitudes of cytosolic calcium oscillations. Biophysical chemistry 71, 125–132 (1998).
Schuster, S., Marhl, M. & Hofer, T. Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signalling. European journal of biochemistry/FEBS 269, 1333–1355 (2002).
Szopa, P., Dyzma, M. & Kazmierczak, B. Membrane associated complexes in calcium dynamics modelling. Physical biology 10, 035004, https://doi.org/10.1088/1478-3975/10/3/035004 (2013).
Qi, H., Li, L. & Shuai, J. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Scientific reports 5, 7984, https://doi.org/10.1038/srep07984 (2015).
Busse, R., Pohl, U. & Luckhoff, A. Mechanisms controlling the production of endothelial autacoids. Zeitschrift fur Kardiologie 78(Suppl 6), 64–69 (1989).
Hu, Q., Deshpande, S., Irani, K. & Ziegelstein, R. C. [Ca(2+)](i) oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem 274, 33995–33998 (1999).
Raqeeb, A., Sheng, J., Ao, N. & Braun, A. P. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49, 240–248, https://doi.org/10.1016/j.ceca.2011.02.008 (2011).
Scheitlin, C. G. et al. Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress. Am J Physiol Cell Physiol 310, C479–490, https://doi.org/10.1152/ajpcell.00171.2015 (2016).
Nilius, B. & Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81, 1415–1459 (2001).
Michel, T. & Vanhoutte, P. M. Cellular signaling and NO production. Pflugers Archiv: European journal of physiology 459, 807–816, https://doi.org/10.1007/s00424-009-0765-9 (2010).
Tsoukias, N. M. Calcium dynamics and signaling in vascular regulation: computational models. Wiley interdisciplinary reviews. Systems biology and medicine 3, 93–106, https://doi.org/10.1002/wsbm.97 (2011).
Prasad, A. R., Logan, S. A., Nerem, R. M., Schwartz, C. J. & Sprague, E. A. Flow-related responses of intracellular inositol phosphate levels in cultured aortic endothelial cells. Circ Res 72, 827–836 (1993).
Yamamoto, K. et al. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci 124, 3477–3483, https://doi.org/10.1242/jcs.087221 (2011).
Wang, S. et al. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. The Journal of clinical investigation 125, 3077–3086, https://doi.org/10.1172/jci81067 (2015).
Duza, T. & Sarelius, I. H. Localized transient increases in endothelial cell Ca2+ in arterioles in situ: implications for coordination of vascular function. Am J Physiol Heart Circ Physiol 286, H2322–2331, https://doi.org/10.1152/ajpheart.00006.2004 (2004).
Wilson, C., Lee, M. D. & McCarron, J. G. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol 594, 7267–7307, https://doi.org/10.1113/JP272927 (2016).
Alevriadou, B. R., Shanmughapriya, S., Patel, A., Stathopulos, P. B. & Madesh, M. Mitochondrial Ca(2+) transport in the endothelium: regulation by ions, redox signalling and mechanical forces. J R Soc Interface 14, https://doi.org/10.1098/rsif.2017.0672 (2017).
Higgins, E. R., Cannell, M. B. & Sneyd, J. A buffering SERCA pump in models of calcium dynamics. Biophys J 91, 151–163, https://doi.org/10.1529/biophysj.105.075747 (2006).
Csordas, G. & Hajnoczky, G. Plasticity of mitochondrial calcium signaling. J Biol Chem 278, 42273–42282, https://doi.org/10.1074/jbc.M305248200 (2003).
Chalmers, S. & McCarron, J. G. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J Cell Sci 121, 75–85, https://doi.org/10.1242/jcs.014522 (2008).
McCarron, J. G., Olson, M. L. & Chalmers, S. Mitochondrial regulation of cytosolic Ca(2)(+) signals in smooth muscle. Pflugers Archiv: European journal of physiology 464, 51–62, https://doi.org/10.1007/s00424-012-1108-9 (2012).
Rizzuto, R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998).
de Brito, O. M. & Scorrano, L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J 29, 2715–2723, https://doi.org/10.1038/emboj.2010.177 (2010).
Giedt, R. J., Pfeiffer, D. R., Matzavinos, A., Kao, C. Y. & Alevriadou, B. R. Mitochondrial dynamics and motility inside living vascular endothelial cells: Role of bioenergetics. Ann Biomed Eng 52, 348–356, https://doi.org/10.1007/s10439-012-0568-6 (2012).
Marino, S., Hogue, I. B., Ray, C. J. & Kirschner, D. E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254, 178–196, https://doi.org/10.1016/j.jtbi.2008.04.011 (2008).
McKay, M. D., Beckman, R. J. & Conover, W. J. Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 21, 239–245 (1979).
Bartok, A. et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nature communications 10, 3726, https://doi.org/10.1038/s41467-019-11646-3 (2019).
Bononi, A. et al. Mitochondria-associated membranes (MAMs) as hotspot Ca(2+) signaling units. Advances in experimental medicine and biology 740, 411–437, https://doi.org/10.1007/978-94-007-2888-2_17 (2012).
Socha, M. J., Domeier, T. L., Behringer, E. J. & Segal, S. S. Coordination of intercellular Ca(2+) signaling in endothelial cell tubes of mouse resistance arteries. Microcirculation (New York, N.Y.: 1994) 19, 757–770, https://doi.org/10.1111/micc.12000 (2012).
Li, X. et al. Dynamic analysis on the calcium oscillation model considering the influences of mitochondria. Biosystems 163, 36–46, https://doi.org/10.1016/j.biosystems.2017.12.002 (2018).
Giorgi, C., Marchi, S. & Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19, 713–730, https://doi.org/10.1038/s41580-018-0052-8 (2018).
Penny, C. J., Kilpatrick, B. S., Han, J. M., Sneyd, J. & Patel, S. A computational model of lysosome-ER Ca2+ microdomains. J Cell Sci 127, 2934–2943, https://doi.org/10.1242/jcs.149047 (2014).
Lin, X. et al. Control of calcium signal propagation to the mitochondria by inositol 1,4,5-trisphosphate-binding proteins. J Biol Chem 280, 12820–12832, https://doi.org/10.1074/jbc.M411591200 (2005).
Wacquier, B., Combettes, L., Van Nhieu, G. T. & Dupont, G. Interplay Between Intracellular Ca(2+) Oscillations and Ca(2+)-stimulated Mitochondrial Metabolism. Scientific reports 6, 19316, https://doi.org/10.1038/srep19316 (2016).
Hoffman, N. E. et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell reports 5, 1576–1588, https://doi.org/10.1016/j.celrep.2013.11.026 (2013).
De La Fuente, S. et al. Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle. J Biol Chem 291, 23343–23362, https://doi.org/10.1074/jbc.M116.755496 (2016).
Acknowledgements
We would like to acknowledge many useful conversations with Madesh Muniswamy, PhD, University of Texas Health San Antonio. We would also like to acknowledge Richard Buckalew, PhD, and Christopher Scheitlin, PhD, for preliminary work in the early stages of this project, and Chavier Laffitte, for assistance with literature reviews. This work was supported by National Institutes of Health grant R01 HL142673 (B.R.A.).
Author information
Authors and Affiliations
Contributions
B.R.A. and N.M.T. conceived this study. A.M., B.A., N.M.T. and B.R.A. designed this study. B.A. and A.M. performed the simulations. A.M., B.A., N.M.T. and B.R.A. analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moshkforoush, A., Ashenagar, B., Tsoukias, N.M. et al. Modeling the role of endoplasmic reticulum-mitochondria microdomains in calcium dynamics. Sci Rep 9, 17072 (2019). https://doi.org/10.1038/s41598-019-53440-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53440-7
This article is cited by
-
Modeling Reactive Hyperemia to Better Understand and Assess Microvascular Function: A Review of Techniques
Annals of Biomedical Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.