Abstract
In the Arctic, sea-ice plays a central role in the functioning of marine food webs and its rapid shrinking has large effects on the biota. It is thus crucial to assess the importance of sea-ice and ice-derived resources to Arctic marine species. Here, we used a multi-biomarker approach combining Highly Branched Isoprenoids (HBIs) with δ13C and δ15N to evaluate how much Arctic seabirds rely on sea-ice derived resources during the pre-laying period, and if changes in sea-ice extent and duration affect their investment in reproduction. Eggs of thick-billed murres (Uria lomvia) and northern fulmars (Fulmarus glacialis) were collected in the Canadian Arctic during four years of highly contrasting ice conditions, and analysed for HBIs, isotopic (carbon and nitrogen) and energetic composition. Murres heavily relied on ice-associated prey, and sea-ice was beneficial for this species which produced larger and more energy-dense eggs during icier years. In contrast, fulmars did not exhibit any clear association with sympagic communities and were not impacted by changes in sea ice. Murres, like other species more constrained in their response to sea-ice variations, therefore appear more sensitive to changes and may become the losers of future climate shifts in the Arctic, unlike more resilient species such as fulmars.
Similar content being viewed by others
Introduction
Sea ice plays a central role in polar marine ecosystems; it drives the phenology of primary producers that constitute the base of marine food webs. Primary producers provide the energy which is transferred to successive trophic levels, including zooplankton, fish, seabirds and marine mammals1,2. In the Arctic, primary production includes two consecutive pulses of marine autotrophs: sea-ice algae and phytoplankton3. In early spring, increasing irradiance and rising temperatures enable ice algae to grow. Later in the season when snow and sea ice melt, a phytoplankton bloom develops and follows the ice retreat4,5. Sea ice algae alone contribute 3–25% of the total primary production6, while in the central Arctic Ocean, this contribution could reach 57–83%7,8. In the last decades, the Arctic has changed rapidly as a result of anthropogenic climate change, facing particularly drastic changes in sea ice conditions9. One of the most visible changes in Arctic sea ice is a significant decrease in coverage10,11, with a mean rate of decline in summer extent of 12.8% per decade relative to the 1981–2010 average (National Snow and Ice Data Center), and similar trends are observed for the winter ice extent. Besides changes in coverage, Arctic sea ice has undergone a significant decrease in thickness12 with a shift from largely perennial to seasonal first-year ice cover13. Concurrently, earlier ice break-up14,15 and a prolonged melting season16 have produced changes in the open-water season. With these ongoing trends, climate models predict an ice-free Arctic Ocean in summer by 2050 or by the end of 21st century at the latest9,17. The productive period will thus start earlier and extend until later18, which will have strong implications on the phenology of primary producers19, ultimately impacting the total primary productivity of the Arctic20. For example, the primary production in the Arctic Ocean increased by 30% between 1998 and 201221. Clearly, these changes will deeply modify the structure and the functioning of Arctic marine ecosystems22, in particular large community shifts and changes in range, abundance and growth of several species18,23,24. In such a context, it appears crucial to assess the dependency of key Arctic species on sea ice and its resources and to understand the risks associated with its current decline.
Biomarker approaches enable us to infer the dependency of Arctic consumers on both sympagic (i.e. sea ice-associated) and pelagic resources, by using conservative bioindicators produced by primary producers (ice-algae and phytoplankton respectively), then transferred along the food chain. Trophic biomarkers, such as stable isotopes and Highly Branched Isoprenoids (HBI), thus allow for tracking the trophic fingerprint of both primary producers. Stable isotopes of nitrogen (δ15N) and carbon (δ13C) provide information on the relative trophic level of consumers (i.e. diet) and the feeding habitat (i.e. benthic versus pelagic food chains), respectively25. In polar regions, the distinct δ13C signatures of ice algae (i.e. more enriched in 13C) and pelagic phytoplankton allow for reconstructing foraging on sympagic prey and can hence be considered as an ice proxy in the Arctic26,27. Additionally, HBI biomarkers provide complementary information on the relative contributions of the two primary production pools along with the food web28,29, namely sea ice algae and phytoplankton pools. HBIs are lipid biomarkers synthesized by diatom species almost exclusively belonging to genera Haslea, Navicula, Rhizosolenia and Pleurosigma28. In the Arctic, a restricted number of ice-associated diatom species produce IP25, a mono-unsaturated HBI isomer, which is often associated with a di-unsaturated isomer (diene)30,31. When observed in biological matrices, IP25 and diene provide direct evidence for the contribution of sympagic algae to the diet of the studied organisms. In contrast, a tri-unsaturated HBI (triene) is mostly synthesized by open-water diatom species, representing an indicator of the phytoplankton contribution. Both HBIs and stable isotopes, therefore, constitute powerful analytical tools for assessing environmental effects on marine biota and combining them would enable to better understand how much sea ice and its derived resources are important among Arctic marine ecosystems, from zooplankton consumers up to top predators, such as seabirds.
Seabirds represent a very large biomass32 and play a key role in the functioning of the Arctic marine ecosystems33,34. They represent excellent bioindicators of the marine environment35,36, as they are highly sensitive to changes in oceanographic conditions, food supply or pollution37,38,39, and thus could be largely impacted by changes in sea ice extent40,41,42,43. Further understanding about the role and importance of sea ice for these species is therefore required, especially since investigations involving HBIs and Arctic seabirds are currently missing.
Thick-billed murres (or Brünnich’s guillemot, Uria lomvia) and northern fulmars (Fulmarus glacialis) represent ideal candidates to investigate the importance of current and future Arctic sea ice conditions for seabirds. Both exhibit a near circumpolar distribution; they are amongst the most abundant seabird species in the Arctic during the breeding season and show contrasting feeding ecologies and flight constraints. Both are primarily “income breeders” relying on resources acquired just prior to breeding to form eggs44,45. While thick-billed murres are pursuit-divers feeding mainly on fish and macrozooplankton and present high flight costs46 restricting their large-scale movements, northern fulmars are opportunistic surface-feeders with limited flight constraints42. Given these contrasting characteristics, thick-billed murres and northern fulmars might be affected differently by changes in sea ice in areas surrounding their breeding grounds. Widely distributed in ice-free waters, northern fulmars are not tied to sea ice, whereas thick-billed murres largely breed in waters where sea ice occurs. We, therefore, hypothesize that sea ice would be more important to the breeding ecology of thick-billed murres. Previous studies highlighted negative impacts of a heavy ice cover on thick-billed murre breeding success47,48, resulting in delayed laying and smaller eggs42,49. Hence, the observed decline in Arctic sea ice extent and modifications in phenology could have strong implications on the fate of seabird populations. Surely, sea ice represents a physical barrier for seabirds excluding certain areas for foraging, and thus potentially increasing their energy expenditure to access prey in open water. However, an extensive ice cover does not impede them to access sympagic resources if several leads are present between ice floes that allow them to dive and feed. More importantly, sea ice could provide high-quality sympagic resources for breeding birds, and investigations on the profitability of sea ice are thus required.
In this study, we investigated the sensitivity of thick-billed murres (hereafter ‘murres’) and northern fulmars (hereafter ‘fulmars’) to variations in sea-ice conditions in the vicinity of their breeding grounds. More specifically we used a multi-biomarker approach combining HBIs and stable isotopes to: (i) determine if changes in sea ice conditions influence bird association with ice-derived resources; and (ii) investigate how this association influences bird feeding ecology and egg parameters (proxies of bird investment in the reproduction) of each species.
Results
Sea ice conditions around Prince Leopold Island (PLI) during the study period
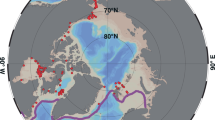
Between 2010 and 2013, both northern fulmars and thick-billed murres encountered contrasting ice conditions around PLI during the entire breeding season (Supplementary Fig. S1), including egg formation (Fig. 1).
Ice conditions around Prince Leopold Island (inner red circle) in Barrow Strait (Nunavut, Canada) during egg formation of northern fulmars (left) and thick-billed murres (right) between 2010 and 2013. Satellites images (NASA Worldview, https://worldview.earthdata.nasa.gov/) highlight sea ice distribution in mid-May (for fulmars; https://worldview.earthdata.nasa.gov/?v=-1661127.7614493677,-1444657.9073818922,-856313.78481856,-1066353.4209707966&p=arctic&t=2010-05-18-T14%3A45%3A31Z) and in mid-June (for murres; https://worldview.earthdata.nasa.gov/?v=-1661127.7614493677,-1444657.9073818922,-856313.78481856,-1066353.4209707966&p=arctic&t=2010-06-15-T14%3A45%3A31Z). The outer red circle represents the 100 km radius circle around the colony, in which daily ice concentrations (center) were calculated for the entire breeding season (May to September). Red dots correspond to dates presented in Supplementary Fig. S1 for each reproductive event (i.e. colony attendance, egg-laying, egg-hatching).
Once they attended the colony from early May, fulmar females form their single egg in mid-May, which is then laid in early-June. During mid-May, which thus corresponds to the egg formation period, ice concentrations ranged from 80% to 99% between 2010 and 2013 (Table 1a). In 2010, surrounding waters were covered by vast ice floes (2–10 km) of thick, first-year ice separated by numerous leads (i.e. access to water) (Fig. 1). In 2011, open-water was accessible between multiple big ice floes (500–2000 m) of thick first-year ice (>120 cm), but sea ice was relatively present. In 2012, consolidated fast ice combining thick first-year ice (>120 cm) and new ice (<10 cm) surrounded the island. The ice margin was located far away (>250 km to the east). In 2013, a dense and thick first-year fast ice (>120 cm) was present around PLI but open water remained accessible near the colony (~50 km).
Murres only arrive at the colony between early- and mid-June and lay their single egg at the end of June or start of July (i.e. approximately one month later than fulmars). In mid-June, which corresponds to the egg formation period, sea ice concentrations ranged from 29% to 99% during the study period (Table 1b). In 2010, patches of sea ice were present around the colony, but patches of open water were easily accessible. Vast ice floes (2–10 km) of thick first-year ice (>120 cm) were surrounded by large leads (Fig. 1). In contrast, open-water conditions were present in the entire region in 2011. Fast ice was only present in coastal areas close to Devon Island shores, as medium-sized (100–500 m) but sometimes large ice floes (2–10 km) of thick first-year ice (>120 cm). In 2012, the colony was still surrounded by a dense and consolidated fast ice (medium and thick first-year ice, 70− 120+ cm) and the ice edge was still located more than 250 km away to the east (Lancaster Sound Polynya). Finally, in 2013, although the area was surrounded by dense fast ice with thick first-year ice (>120 cm), the ice edge was easily accessible located 50 km away from PLI.
Thus, we distinguished two major ice regimes (Fig. 1): (1) 2010 and 2012 represented heavy ice years, with a late ice break-up (i.e. in July) and an extensive ice cover, and (2) 2011 and 2013 were defined by an early ice break up (i.e. in May) and a restricted sea ice cover or an easy access to open water. However, we noticed a gradient over the years from icy to open-water conditions, gradually from 2012 to 2010, 2013 and 2011 for murres (mid-June) and from 2012 to 2010, 2013 and 2011 for fulmars (mid-May).
Biological markers as proxies for sea ice association and use of ice-derived resources
Ice use index
In murres, the ice use index differed significantly across years (F3,40 = 12.1, p < 0.001, ANOVA), with 2010 (1.0 ± 1.1 SD) and 2012 (0.7 ± 1.0) differing from 2011 (−1.1 ± 1.1) and 2013 (−0.7 ± 0.7), and increased linearly with increasing ice concentrations (p < 0.001, R² = 0.32; Fig. 2). In fulmars, the ice use index was significantly different across years (F3,52 = 8.8, p < 0.001, ANOVA), with 2013 (−1.1 ± 1.1) differing from 2010 (0.6 ± 1.0), 2011 (0.6 ± 1.2) and 2012 (−0.0 ± 1.0), and did not vary linearly with ice concentrations (p > 0.05).
Highly branched isoprenoids
The three HBI isomers (i.e. IP25, diene, triene) were present in all samples, but their concentrations were highly variable between individuals (Supplementary Fig. S2). HBI concentrations were very low in fulmar eggs (IP25 = 2.5 ± 2.7 ng.g−1 sample, diene = 0.4 ± 2.7 ng.g−1 standard, triene = 0.4 ± 0.6 ng.g−1 standard) compared to those observed in murre eggs (IP25 = 7.6 ± 11.2 ng.g−1 sample, diene = 3.4 ± 6.7 ng.g−1 standard, triene = 4.1 ± 4.5 ng.g−1 standard) (Supplementary Fig. S3).
For murres, HBI concentrations varied among years, ranging overall from 0 to 38.2 (ng.g−1 standard) and H-Print differed significantly across years (F3,40 = 8.9, p < 0.001, ANOVA; Fig. 3), with 2010 standing out from all other years with significantly lower values. In 2010, ice specific isomers were abundant (IP25 = 18.9 ± 17.4 ng.g−1 sample, diene = 9.7 ± 11.3 ng.g−1 standards; Supplementary Fig. S2a), with the lowest H-Print of all years (28.1 ± 20.8%, Table 2). In 2011, the H-Print was the highest (82.5 ± 18.8%) reflecting the high triene abundance (6.4 ± 6.5 ng.g−1 standard). In 2012, ice association differed between two groups (p < 0.001, t11 9 = −8,4, Welch two-sample t-test): one group with higher amounts of ice biomarkers (H-Print = 34.1 ± 10.9%, n = 8) and a second group with higher amounts of pelagic biomarker (H-Print = 83.0 ± 10.2%, n = 7). Finally, in 2013 eggs exhibited an intermediate H-Print (63.4 ± 18.1%).
For fulmars, the H-Print exhibited a significant inter-annual variability (F3,52 = 6.6, p < 0.001, ANOVA; Fig. 3), with 2013 differing from 2010 and 2011, where ice biomarkers were more abundant (i.e. lower H-Print, 33.9 ± 28.1% in 2010 and 27.3 ± 23.9% in 2011). In 2012 samples, H-Print values were intermediate (47.6 ± 33.1%) while in 2013 triene was more dominant in the eggs (i.e. higher H-Print, 67.6 ± 29.7%).
There was a significant positive linear relationship between H-Print and ice concentrations around the colony (p < 0.001, R² = 0.28) in murres, in contrast to fulmars for which no relationship was found (p > 0.05).
Stable isotopes
Stable isotope results confirmed the distinct feeding ecology of each species with murres exhibiting higher δ15N values (15.6 ± 0.67‰) than fulmars (13.2 ± 0.33‰) (p < 0.001, t72.3 = −23.9, Welch two-sample t-test; Supplementary Fig. S4).
For both species, we identified distinct isotopic niches (MANOVA; murres: F3,40 = 5.8, p < 0.001; fulmars: F3,56 = 7.2, p < 0.001). For murres, δ13C values were significantly different between years (Table 2; F3,40 = 18.5, p < 0.001, ANOVA), with 2010 and 2012 differing from 2011 and 2013. δ15N were also different between years (F3,40 = 4.0, p = 0.015, ANOVA), with 2012 differing from 2011. In icy years, murre eggs were enriched in both 15N (16 ± 0.5‰ in 2010, 15.8 ± 0.5‰ in 2012) and 13C (−19.7 ± 0.4‰ in 2010, −19.3 ± 0.4‰ in 2012) compared to years characterized by open-water presence (δ15N = 15.3 ± 0.7‰, δ13C = −20.3 ± 0.5‰ in 2011; δ15N = 15.4 ± 0.8‰, δ13C = −20.3 ± 0.3‰ in 2013). For this species, δ15N values reached a maximum when the ice use index was intermediate (quadratic regression, p < 0.001, R² = 0.58; Fig. 4).
For fulmars, δ13C values were different across years (Table 2; F3,52 = 6.8, p < 0.001, ANOVA), with 2013 differing from all other years (−19.7 ± 0.2‰ versus −19.3 ± 0.2‰ in 2010, −19.4 ± 0.3‰ in 2011 and −19.4 ± 0.2‰ in 2012). δ15N values also differed across years (F3,52 = 7.0, p < 0.001, ANOVA), with 2012 (13.4 ± 0.3‰) different from 2010 (13.0 ± 0.3‰) and 2011 (13.0 ± 0.30‰). For this species, however, δ15N did not linearly respond to varying ice use index (p = 0.9).
Sea ice and seabird egg parameters
To investigate how variations in sea ice conditions and sea ice use influenced bird investment in the reproduction and egg quality, egg volumes (for both species) and egg energy contents (only for murres) were both compared to the ice use index. First, the egg volume did not differ significantly across years for murres (190.0 ± 17.0 cm3; F3,56 = 2.5, p = 0.07, ANOVA; Fig. 5). However, when analyzed by ice regime, eggs were on average larger in icy years (191.9 ± 12.8 cm3 in 2010 and 198.4 ± 18.7 cm3 in 2012) than in years of open-water (186.4 ± 16.1 cm3 in 2011 and 183.2 ± 17.6 cm3 in 2013) (p = 0.02, t57.9 = 2,5, Welch two-sample t-test). Egg volume of murres increased linearly with increasing ice use index (p < 0.01, R² = 0.13; Fig. 6). For 2012, unlike for the H-Print, murres did not form distinct groups for their egg volume (p = 0.08, t11 8 = 1.9). For fulmars, egg volume remained stable across years (177.1 ± 14.0 cm³; F3,44 = 0.7, p = 0.54, ANOVA; Fig. 5) and did not linearly respond to changing ice use (p = 0.71; Table 3).
The energy content of murre eggs differed significantly across years (F3,24 = 4.6, p = 0.01, ANOVA), with higher energetic content in icier years (127.5 ± 21.8 kcal in 2010 and 156.1 ± 21.2 kcal in 2012) than in open-water years (107.2 ± 38.4 kcal in 2011 and 107.2 ± 22.4 kcal in 2013) (p = 0.02, t29 1 = 3.3; Fig. 5), and with 2012 differing from 2011 and 2013. As for the egg volume, the two distinct groups of murres in 2012 did not show distinct egg energetic content (p = 0.8, t3.4 = −0.3). The energetic content linearly increased as the ice use index increased (p < 0.01, R2 = 0.21; Fig. 6). There was also a linear positive relationship between energetic content and δ15N values (p = 0.005, R2 = 0.23; Supplementary Fig. S5). Finally, the energetic content linearly increased with increasing egg volume (p = 0.02, R2 = 0.16; Supplementary Fig. S6).
Discussion
Sea ice phenology, by shaping the pulses of primary productivity in the Arctic, may control the ecology and reproductive success of seabird species50. In this study, variations in the abundance and distribution of HBIs51 and stable isotopes in eggs were used to determine the influence of sea ice on diet and breeding investment of two key Arctic seabirds. We confirmed that HBIs acquired via food are transferred into eggs during their production by female birds. This transfer was previously reported for two iconic Antarctic bird species, Adelie penguins (Pygoscelis adeliae) and snow petrels (Pagodroma nivea)52. Our results, therefore, provide additional insights into HBI transfer to the highest trophic levels across the marine food web in the Arctic, even if the metabolism, the assimilation and the elimination processes still remain poorly understood. The presence of IP25 and diene in egg samples reflects the contribution of ice-derived organic matter in the diet of females while the contribution of the organic matter produced in open waters is represented by the triene. With ice- and plankton-derived isomers observed in all samples (Supplementary Fig. S2), our investigations demonstrate that both species rely on both primary production pools. However, the large interspecific and inter-annual differences (Supplementary Fig. S3) observed reveal that thick-billed murres and northern fulmars differed significantly in their relationship to sea ice and its associated resources.
Bird relationship to sea ice
During icy years, murres laid eggs with biomarker (HBI and carbon stable isotopes) patterns suggesting high ice use, in contrast to biomarker patterns from low ice years. The positive linear relationships observed between ice concentrations and ice use index suggest that murres strongly respond to changes in sea ice and would thus be more sensitive to potential impacts of climate change.
Amongst the four years studied, 2012 was the iciest year. The entire region around Prince Leopold Island (PLI) was covered by a dense, thick and unfragmented fast ice that remained until very late in the season. With the highest ice use indices during 2012 on average, birds were relying the most on sea ice for feeding (Fig. 2). High ice use indices are also observed in 2010 samples and sea ice was also very abundant in the area that year. This further confirms the strong relationship between murres and sea ice around PLI. Interestingly, HBIs were up to six times more abundant in eggs collected in 2010 than in those from 2012 (Supplementary Fig. S2). In fact, in 2012 the ice margin was located at the entrance of Lancaster Sound until mid-July. Access to open water was thus limited and birds had to travel longer distances to access their feeding grounds (conditions previously shown to be deleterious to breeding at this colony42). Also abundant in 2010, sea ice was however fragmented in relatively large and mobile floes separated by numerous leads. This configuration provided easy access to water and its resources for murres in the vicinity of the colony (e.g. murres could forage for sympagic prey on the undersides of sea ice). As such, while ice use indices and to some extent HBI distributions are correlated to sea ice concentration around PLI, it seems that HBI abundances reflect the accessibility of prey to murres which, at least in this study, are associated to ice “type” and configuration. In 2012, the distribution of HBIs in murre eggs highlighted two distinct groups: one made by birds more associated with sea ice and one where eggs contained more of the phytoplankton-derived triene. Like earlier in 200242, 2012 can be considered as an extreme year regarding ice conditions, with no access to open water except at the ice edge located more than 250 km away to the east. The only other access to open water was a small polynya located approximately 130 km southwest from PLI (Fig. 1). Our results thus suggest that some birds might have headed south to this small polynya while other birds might have headed east to feed in open waters out of Lancaster Sound and the north Baffin Bay. This reflects contrasting responses of birds to adverse foraging conditions. Such plasticity in bird behaviour was already reported by Pratte et al.53 who showed that, facing such extreme ice conditions, seabirds can adopt different strategies. Grémillet and co-workers54 also showed that the great plasticity of little auk (Alle alle) feeding behaviour was allowing them to maintain their fitness levels and attenuate effects of climate change.
While HBIs were quite abundant in murre eggs, the analysis of those laid by fulmars revealed much lower HBI abundances with, on average, concentrations approximately five times lower than those observed in murres (Supplementary Fig. S2). Cusset and collaborators (unpublished) recently analysed different tissues (muscle, liver, blood) from fulmars and murres and, although HBIs were a bit less abundant in fulmars, they did not observe such extreme differences. It may be possible that HBI transfer to eggs in fulmars is not as efficient as in murres, but several factors may be involved. A plausible explanation would lie in the different breeding phenology of each species, with fulmars laying their egg a few weeks earlier than murres. In fact, fulmars form their egg at a time when phytoplankton just start to bloom, meaning that only very low amounts of triene are available to grazers and their predators. Also, although sympagic algae are blooming, these are not released from the ice and therefore IP25 and diene are relatively unavailable to higher trophic levels. Indeed, high abundances of HBIs were reported in copepods collected in spring in Beaufort Sea55, but a three week lag between the sympagic bloom and HBI accumulation in zooplankton was observed. Further, even in the middle of winter (January), HBIs were observed in most compartments of the Ripfjorden ecosystem except those from above 75 m deep56. Since the highly mobile and opportunistic fulmars are restricted to surface waters, it is likely that only a small amount of HBIs are available during their pre-laying period. As such, the large differences in HBI abundances in murre and fulmar eggs most likely originate from their distinct and contrasting trophic ecologies. Fulmars are capable of travelling over large distances57 (>250 km) to reach their feeding grounds, and in our study region they can fly >500 km one way to feed58. Furthermore, during the pre-laying period, fulmars undertake their pre-laying exodus, during which they leave the colony and probably travel out of Lancaster Sound to the eastern Baffin Bay or potentially the North Water Polynya58, where open-water is more predictable across years and algal blooms may occur earlier. Thus, fulmars are easily able to reach open waters even during extreme years, and this could explain the lack of interannual differences in this species. In this case, studying GPS-equipped birds would help refining their actual feeding grounds and adjusted analyses of ice conditions in this particular area would help clarify the influence of sea ice for northern fulmars.
Thick-billed murres are pursuit-divers, diving down to 200 m depth to feed mainly on fish, especially the ice-associated Arctic cod (Boreogadus saida, Lepechin), and macrozooplankton47. Their feeding range is much shorter than the fulmar’s and typically restricted to approximately 150 km from their colony59,60. Murres are often observed near ice edges61 where they can easily dive under sea ice to forage on sympagic prey. Northern fulmars are opportunistic surface-feeders relying on fish such as arctic cod, but also on copepods, mysids, and gelatinous prey62,63,64,65,66. Given such differences in their feeding ecology, it is not surprising to see that H-Print and the ice use index differ between the two species. For thick-billed murres, both indices were closely linked to local ice concentrations and varied linearly with mid-June ice concentrations within a 100 km radius circle from the colony. For northern fulmars, neither H-Print nor ice use index were influenced by ice conditions. However, these interspecific differences might be explained by the fact that ice concentrations are defined in mid-May and mid-June for fulmars and murres, respectively. Ice concentrations exhibited much higher interannual variability during mid-June (29–99%) than during mid-May (80–99%). Hence, in our study fulmars did not experience years with reduced ice cover during egg formation. It is thus reasonable to expect smaller variations in HBI concentrations and distributions and, therefore, a weaker correlation to sea ice conditions for fulmars. Besides, fulmars have a longer breeding period, and Mallory and Forbes67 suggested that the high Arctic is near the environmental limit at which fulmars can complete their breeding. In contrast, thick-billed murres naturally lay eggs at a time closer to seasonal ice retreat and are hence already exposed to larger variations in ice conditions. Thus, the much lower interannual variations in sea ice cover might be below the threshold for northern fulmars to be influenced by sea ice variations (as they are constrained to breed early and fly far to complete chick-rearing before migration), and maybe that only extremely early open-water conditions (e.g. as early as in mid-May) would lead to observable impacts.
Do seabirds change their feeding habits to cope with changes in ice conditions?
Variations in sea ice conditions clearly influenced the feeding ecology of both species. However, as for the usage of ice-derived resources, there appear to be large interspecific differences (Supplementary Fig. S3). As ice concentrations increased, eggs laid by murres exhibited a higher δ13C content and therefore probably reflected the fact that birds were relying more on sympagic resources. With increasing sea ice, murres also seemed to feed on prey from higher trophic levels (i.e. higher δ15N). The average increase of 0.7‰ in δ15N observed between 2011 and 2010 (i.e. years with the lowest and highest values on average, respectively), however, is too low to reflect a change in trophic level but rather suggests a higher percentage of prey of higher trophic level in their diet. Higher δ15N values are correlated to low H-Print. The presence of ice in the vicinity of the colony favors murre access to prey belonging to higher trophic levels and exhibiting a stronger relationship to sea-ice derived resources. Inversely, in years with reduced ice coverage, murres prey on organisms from lower trophic levels such as hyperiid amphipods (Parathemisto spp.), mysids or copepods63,68 that are more associated with pelagic resources. Our data confirm that murres take advantage of sea ice for feeding and thus any variation in ice cover has an influence on its accessibility to prey. Interestingly, δ15N values obtained from 2012 egg samples are lower than those of 2010, even if 2012 was the iciest year of the study. Again, as with HBI abundances, nitrogen isotopic values suggest that in 2012, ice conditions were too harsh to provide birds with easy access to their preferred fish resources. This suggests that an intermediate ice cover during egg formation represents the optimal breeding (foraging) scenario for murres at this colony. Indeed, arctic cod hide under the ice to avoid predators69, and during years like 2010 where ice was abundant but highly fragmented, birds could easily access their fish prey. In extreme years like 2012, ice precludes access to much of the sympagic prey for murres, and birds would forage more in the open water, spending much energy while focusing on fish, and had also to rely on zooplankton.
In contrast, high and relatively invariable δ13C values in eggs laid by fulmars indicate that birds fed in coastal icy habitat regardless of ice conditions. Lower (than murres) and invariable δ15N values also indicate that fulmars preyed upon lower trophic levels (likely zooplankton), without any influence of ice conditions. This absence of variation in isotopic composition has also been observed recently53 and suggests that every year, birds reach the same “predictable” feeding areas. These are probably located along the western Greenland coast in Baffin Bay, as suggested by tracking of foraging fulmars from a colony farther north on Devon Island58.
Impact of sea ice on seabird egg parameters
In the current context of sea ice decline in the Arctic, studies focusing on the relationships between bird reproductive performances and ice are needed70. Indeed, top-predators such as birds closely synchronize their breeding schedule with their food supply in order to meet the energetic needs of their reproduction71,72. In the Arctic, food supply is closely linked to sea ice since both phytoplankton and sea ice algal blooms are controlled by its presence or absence73. In our study, we used egg size (i.e. egg volume) as a proxy for breeding investment. Indeed, a number of studies showed that although energetically costly to produce, larger eggs produce bigger chicks which then have a greater survival potential74,75,76. For murres and fulmars, Gaston and collaborators42 performed an exhaustive study and monitored colonies of both species at PLI between 2000 and 2003. They showed that in 2001 and 2002 (years relatively similar to 2012) laying was delayed and thick-billed murres produced smaller eggs. This was also reported by Hipfner and colleagues49 who showed that thick-billed murres laid smaller eggs later in the season during years of extensive sea ice in the High Arctic (PLI and Coburg Island). Both studies therefore showed that heavy ice conditions were leading to reduced breeding success, delayed laying date and decreased body condition/growth of chicks. In our study spanning very contrasting ice conditions, we observed an opposite effect of sea ice on murre breeding investment, with eggs on average larger in icy years than in years of open water. In previous studies, sea ice was only considered as a physical barrier that forces birds to commute over larger distances and involves higher energetic costs for breeding adults42. Hence, ice conditions were defined only according to the distance between colony and ice margin and did not consider the type of ice and how it was distributed (e.g. fast ice versus pack ice) as in the present study. Here, ice concentration around PLI, the position of the margins and the breakout period in 2010 and 2012 were relatively similar. However, as described above, the situation is extremely different when it comes to accessibility to food resources. In 2010, the very fragmented ice pattern provided murres with easy access to open water. In contrast, in 2012, birds had to travel over very long distances to reach open water. This situation prevented them from fully relying on ice-associated resources. The correlation between egg size and ice use index illustrate these differences in resource accessibility and use. Larger eggs were more associated with ice-derived resources (i.e. lower H-Print and higher ice use index) and therefore sea-ice conditions impacted the size of thick-billed murres eggs by modulating the availability of sympagic prey. Goutte et al.77 reported similar findings from their study on Adélie penguins (Pygoscelis adelia). They also highlighted a positive impact of a higher contribution of ice-derived resources on the breeding success of this species during years of extensive ice cover. Like murres, the Adelie penguin is an ice-associated diver that feeds mainly on Antarctic krill (Euphausia superba) and fish such as Pleurogramma antarcticum78, in waters covered by 20 to 80% ice as well as in the open sea, under pack ice or under coastal fast ice79. Guen et al.80 showed that intermediate ice coverage (20% ice), provided optimal breeding for Adelie penguins since krill juveniles rely exclusively on sympagic flora and the presence of sea ice promotes higher abundances of krill81. However, Emmerson and Southwell82 also showed that, as for murres, sea ice and, in particular, the presence of fast ice close to the shore could negatively impact reproduction performance of Adelie penguins by reducing their access to prey items. Thus, prey availability (accessibility and/or quality) clearly influences the breeding performance of various seabird species.
In subarctic areas, like in Hudson Bay, a decrease in the breeding success of murre colonies resulted from an increase in relative abundances of capelin (Mallotus villosus) in southern Arctic waters at the expense of Arctic cod41. A switch from Arctic cod to demersal sculpins, resulting from shifts from arctic to subarctic regimes, was also observed in black guillemots (Cepphus grille mondtii) in the Beaufort Sea and associated with a decrease in chick condition83. For seabird species laying a single egg, quality and quantity of food delivered by parents to the chick has a strong effect on the reproductive success84. In pigeon guillemots (Cepphus columba), chick growth and survival decreased as the proportion of the lipid-rich Pacific sand lance (Ammodytes hexapterus) decreased and as the proportion of low-lipid demersal fish increased85. Here, we provide evidence that ice-derived resources influence murre egg size (proxy for breeding investment) and further investigations including other breeding parameters (such as chick growth rate and survival, breeding success) would help to confirm the observed trends and understand murre vulnerability to sea ice declines in the Arctic.
The energy content of murre eggs was greater with higher use of ice resources and when the birds fed at a higher trophic level, suggesting higher investment in eggs during icier years. Furthermore, larger eggs contained more energy for the chick’s development, ultimately providing nestlings with higher chances of survival. Barrionuevo and Frere74 showed that yolk-area was a strong predictor of nestling survival in another diving seabird, the Magellanic penguin (Sheniscus magellanicus), with yolk-area positively related to nestling survival, and nestling body size positively impacted by egg volume. Larger eggs with larger yolk contain more lipids and more energy for the chick’s development, producing ultimately a larger chick with higher chances of survival86. Therefore, sea ice represents a real and valuable asset for ice-associated seabirds, like murres, that might be more sensitive to future changes in Arctic sea ice.
For northern fulmars, egg size remained stable (Fig. 5), without any impact of either sea ice concentrations or ice-derived resources, consistent with previous results (same laying date, same egg size)42. Unlike murres, fulmars thus appear less sensitive to sea ice variations which do not seem to impact their investment in reproduction. As such, northern fulmars are more likely to be dramatically affected by a « tipping point » in Arctic sea ice conditions at the time of egg development in females; a point at which previously small changes become significant enough to impact their feeding ecology and reproductive investment. Further investigations on fulmars during years of highly variable ice conditions are thus required to fully understand the influence of sea ice for this species.
Conclusion
Our study examined the importance of sea ice and ice-derived resources for breeding Arctic seabirds in a context where the Arctic might become ice-free in summer within the next decades. Instead of considering the sea ice cover as a physical barrier preventing seabirds to access their prey, we highlight the importance of sea ice via the resources it provides to marine predators. Our multi-biomarker approach, combining highly branched isoprenoids and stable isotopes, showed how important sea ice is for seabirds and how its use conditions different aspects of their biology, including their egg characteristics. For the fulmar, which has exceptional ability to fly long distances but must breed early to complete its long breeding period, variation in sea ice had limited effects, presumably because birds have adapted to fly long distances to open water remote from the colony but predictably open early in the season. However, the influence of sea ice is especially important for species such as diving thick-billed murres, perhaps more constrained in their response to sea ice variations, that rely heavily on local environmental conditions. At least for this colony, murres appear to have “optimal” ice conditions when ice cover is heavier but still broken up providing feeding locations; ice-associated biomarkers and proxies of reproductive effort are reduced at higher or lower ice conditions. Overall, we provide new and essential knowledge to comprehend the consequences of current and future climate changes on the fate of Arctic seabird populations. We also emphasise the importance of combining different biomarkers to better understand the importance of sympagic resources for top predators within changing Arctic marine ecosystems.
Methods
Sample collection
As part of a long-term monitoring program supported by Environment and Climate Change Canada and the Northern Contaminants Program, eggs of murres and fulmars were collected during four consecutive years (2010–2013) from nests on the eastern and southern cliffs at the multi-species colony on Prince Leopold Island in Lancaster Sound (74°N, 90°W; Nunavut). For consistency, murre and fulmar eggs were sampled at the same period every year (late June – early July). One egg per nest was randomly sampled shortly after laying for murres and at mid-incubation for fulmars (murres and fulmars generally lay only one egg). Eggs were either taken by hand or by using an extension pole holding a small cup. In the field, eggs were kept cool and shipped to the National Wildlife Research Center (Ottawa) for processing. All eggs were taken under appropriate annual research and collection permits (e.g. Nunavut Wildlife Research Licence 2012–040; Environment Canada NUN-SCI-12-04). Egg length (mm), width (mm) and weight (g) were measured, and a volume index (cm³) was calculated according to previously published methodology42,47 (Eq. 1).
Egg contents were homogenized and stored frozen at −40 °C in acid-rinsed polyethylene vials. One- or two-gram aliquots (15 eggs per species per year) were sent to Laval University (Quebec City) where HBI and caloric content analyses were performed.
Environmental conditions
As murres have a local feeding range (0–150 km59), we described environmental conditions by characterizing the presence/absence of sea ice, its type (size, thickness, presence of leads) and concentration in a 100 km radius circle around Prince Leopold Island. To determine the presence and the type of sea ice around the colony, regional ice maps (i.e. Eastern Arctic; weekly total concentrations; week including 20 June) were downloaded from Canadian Ice Service Archives for the 2010–2013 period. Ice maps for the 2000–2003 period were also downloaded in order to compare our data with a previous study42. As these maps gather weekly data at a regional scale, we used satellite images from NASA Worldview dataset (EOSDIS, https://worldview.earthdata.nasa.gov/) to determine sea ice distribution and concentration at a finer temporal and spatial scale. The proportion of sea ice around the colony was calculated using MODIS 500 m resolution raster images and only images with clear sky were considered. Pixels were first extracted in a circle of 100 km radius around the colony. Land pixels were then masked out using Open Street Map mask (http://openstreetmapdata.com/data/water-polygons). The remaining pixels were classified into two categories (open water or sea ice) using a K-means clustering procedure. The clustering has been performed using the RGB values of the pixels. A maximum of 100 iterations was allowed for convergence. The proportion between pixels classified as open water and sea ice was then calculated and used as a proxy for the proportion of sea ice around the colony.
HBI analyses
HBIs were analysed following the procedure initially described in Belt et al.87. Samples were freeze-dried for 48 h and the water content determined. 7-hexylnonadecane and 9-octylheptadec-8-ene (10 µL; 9,68 µg.mL−1 each) were added as internal standards to dried aliquots (~0.1 g to 0.3 g dry weight). Total lipids were extracted four consecutive times with dichloromethane-methanol mixture (2:1, 15 min sonication). Delipidized samples were dried overnight at 45 °C (12 h) and stored frozen (−20 °C) for isotopic analyses. Total lipid extracts were then dried under a gentle stream of N2 and subjected to saponification (4 mL, 5%, MeOH/H2O, 90/10, 90 °C, 1 h). Non-Saponifiable Lipids (NSL) were extracted from saponification mixture by liquid-liquid extraction (Hexane, 3 × 2 mL), evaporated (nitrogen stream <35 °C) and purified using open column chromatography (SiO2 50 g.g−1 NSL; Hexane) to yield an apolar lipid fraction containing HBIs. These apolar fractions were then analysed via GC-MS following the procedure described in Belt et al.87. Briefly, the abundance of IP25, diene and triene were determined to integrate 350.3, 348.3 and 346.3 responses, respectively. The ratio of these responses against those of internal standards (m/z 266) was normalized to the mass of the sample and multiplied by the mass of standard added to the sample. For IP25, response factors were determined by injection of authenticated standards and results are expressed in ng.g−1. For other HBI isomers, in the absence of pure standards results are expressed in ng of standard equivalent.g−1.
Once individual HBIs were identified and quantified, the H-Print index was calculated for each sample using Eq. (2) from Brown et al.31:
where 0, 50 and 100% correspond respectively to a sympagic (i.e. ice algae), a mixed (i.e. ice algae and phytoplankton) and a pelagic (i.e. phytoplankton) association.
Stable isotope analyses
Stable isotope analyses were performed on aliquots of the delipidized fractions resulting from HBI analyses and loaded into tin cups (0.2 to 0.8 mg dry weight). An elemental analyser (Flash EA 1112, Thermo Fisher) coupled in continuous flow mode to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Fisher, Bremen, Germany) was used to determine stable isotope abundances. Results were expressed in δ notation as the deviation from standards in parts per thousand (‰), according to Eq. (3):
where X is 13C or 15N, and R is the corresponding ratio 13C/¹²C or 15N/14N. Standard values were obtained from Vienna Pee Dee Belemnite (VPDB) and atmospheric N2 (air) for C and N respectively. Stable isotopes analyses were performed at the Littoral, Environment and Societies institute (LIENSs, France). Replicate measurements of laboratory standards (USGS-61 and USGS-62) indicated that the measurement accuracy was <0.2% for both δ15N and δ13C values (analytical precision <0.15%). δ15N and δ13C isotopic values provide information on the relative trophic level of birds (i.e. diet) and their feeding habitat (i.e. carbon source) respectively88,89. Based on distinct δ13C signatures of ice algae and phytoplankton (i.e. more enriched ice algae), δ13C is also considered as an ice proxy in the Arctic26,27.
Egg caloric content analyses
Aliquots of dried homogenized samples (n = 32) were compressed to form pellets and analysed in a Parr 6300 Automatic Isoperibol-Oxygen Bomb Calorimeter. A Benzoic Acid standard was added to each sample (0.3–0.7 g) due to low sample weight (<0.2 g). Instrument precision was determined by running Benzoic Acid standards (6318 cal.g−1) prior to the samples. Results were expressed in both cal.g−1 dry and wet sample. The energy available per egg (kcal) was calculated by multiplying cal.g−1(wet) with the total egg weight. Unfortunately, the weight of the shell of each egg was not determined prior to homogenization and we, therefore, make the assumption that the variation amongst individuals is negligible.
Statistical analysis
A principal component analysis (PCA) was performed using the two sea-ice tracers (H-Print and δ13C) in order create a single variable representing bird-ice association (hereafter named Ice Use Index). The first principal component (PC1) explained 78.4% and 79.3% of the total variance in murres and fulmars, respectively, representing a range of sea-ice use, on which individual coordinates were projected. To investigate the influence of ice association on bird feeding ecology and reproductive parameters, we tested linear or quadratic regressions of δ15N, egg volume and energetic content with the Ice Use index. Normality of residuals and homoscedasticity were tested prior to interpretation using the Shapiro-Wilk normality test and a Breusch-Pagan test, respectively, and data were log-transformed when assumptions were not respected.
For each species, a Multivariate Analysis of Variance (MANOVA) was performed to test whether there were significant differences in the isotopic niche (δ13C, δ15N) of birds between years. One-way ANOVA then enabled to test significant differences of each variable between years, and posthoc Tukey HSD tests were performed to locate these differences. Residuals normality and variance homogeneity were tested prior analysis with tests described above, and data were log-transformed when these assumptions were not respected.
Due to small sample sizes, we used Welch-modified t-test to distinguish groups of murres in 2012 (H-Print and egg volume), for interspecific differences (δ15N) and for inter-annual differences in egg volume and energetic content between reduced-ice (i.e. 2011 and 2013) and extensive-ice years (i.e. 2010 and 2012). All statistical analyses and figures were computed in R90 (Version 3.5.0).
Ethical approval
All applicable institutional and national guidelines for the care and use of animals were followed and methods were carried out in accordance with relevant guidelines and regulations, including appropriate scientific and land use permits. All eggs were taken under appropriate annual research and collection permits (e.g. Nunavut Wildlife Research Licence 2012–040; Environment Canada NUN-SCI-12-04). The local indigenous community was consulted and had representatives participate in the work in most years.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on a reasonable request.
References
Darnis, G. et al. Current state and trends in Canadian Arctic marine ecosystems: II. Heterotrophic food web, pelagic-benthic coupling, and biodiversity. Clim. Change 115, 179–205 (2012).
Welch, H. E. et al. Energy flow through the marine ecosystem of the Lancaster Sound region, Arctic Canada. Arctic 45, 343–357 (1992).
Horner, R. & Schrader, G. C. Relative contributions of ice algae, phytoplankton, and benthic microalgae to primary production in nearshore regions of the Beaufort Sea. Arctic 35, 485–503 (1982).
Perrette, M., Yool, A., Quartly, G. D. & Popova, E. E. Near-ubiquity of ice-edge blooms in the Arctic. Biogeosciences 8, 515–524 (2011).
Tremblay, J.-É. et al. Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim. Change 115, 161–178 (2012).
Legendre, L. et al. Ecology of sea ice biota. Polar Biol. 12, 429–444 (1992).
Boetius, A. et al. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430–1432 (2013).
Gosselin, M., Levasseur, M., Wheeler, P. A., Horner, R. A. & Booth, B. C. New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 44, 1623–1644 (1997).
IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change. Core Writing Team, R.K. Pachauri and L. A. Meyer (eds). 151 (IPCC, 2014).
Comiso, J. C., Parkinson, C. L., Gersten, R. & Stock, L. Accelerated decline in the Arctic sea ice cover. Geophys. Res. Lett. 35 (2008).
Serreze, M. C., Holland, M. M. & Stroeve, J. Perspectives on the Arctic’s shrinking sea-ice cover. Science 315, 1533–1536 (2007).
Kwok, R. & Rothrock, D. A. Decline in Arctic sea ice thickness from submarine and ICESat records: 1958–2008. Geophys. Res. Lett. 36 (2009).
Kwok, R. Near zero replenishment of the Arctic multiyear sea ice cover at the end of 2005 summer. Geophys. Res. Lett. 34 (2007).
Comiso, J. C. Large decadal decline of the Arctic multiyear ice cover. J. Clim. 25, 1176–1193 (2011).
Stroeve, J. & Notz, D. Changing state of Arctic sea ice across all seasons. Environ. Res. Lett. 13, 103001 (2018).
Markus, T., Stroeve, J. C. & Miller, J. Recent changes in Arctic sea ice melt onset, freezeup, and melt season length. J. Geophys. Res. Oceans 114 (2009).
Johannessen, O. M. et al. Arctic climate change: observed and modelled temperature and sea-ice variability. Tellus Dyn. Meteorol. Oceanogr. 56, 328–341 (2004).
Wassmann, P. & Reigstad, M. Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. (2011).
Ji, R., Jin, M. & Varpe, Ø. Sea ice phenology and timing of primary production pulses in the Arctic Ocean. Glob. Change Biol. 19, 734–741 (2013).
Kahru Mati, Lee Zhongping, Mitchell B. Greg & Nevison Cynthia D. Effects of sea ice cover on satellite-detected primary production in the Arctic Ocean. Biol. Lett. 12, 20160223 (2016).
Arrigo, K. R. & van Dijken, G. L. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 136, 60–70 (2015).
Tremblay, J.-é et al. Climate forcing multiplies biological productivity in the coastal Arctic Ocean: upwelling and productivity in the Arctic. Geophys. Res. Lett. 38, n/a–n/a (2011).
Eamer, J. et al. Life Linked to Ice. A guide to sea-ice-associated biodiversity in this time of rapid change. (Conservation of Arctic Flora and Fauna (CAFF), 2013).
Walther, G.-R. et al. Ecological responses to recent climate change. Nature 416, 389–395 (2002).
Atwell, L., Hobson, K. A. & Welch, H. E. Biomagnification and bioaccumulation of mercury in an arctic marine food web: insights from stable nitrogen isotope analysis. Can. J. Fish. Aquat. Sci. 55, 1114–1121 (1998).
Hobson, K. A., Ambrose, W. G. Jr & Renaud, P. E. Sources of primary production, benthic-pelagic coupling and trophic relationships within the Northeast Water Polynya: insights from 613C and 615N analysis. Mar. Ecol. Prog. Ser. 128, 1–10 (1995).
Søreide, J. E., Hop, H., Carroll, M. L., Falk-Petersen, S. & Hegseth, E. N. Seasonal food web structures and sympagic–pelagic coupling in the European Arctic revealed by stable isotopes and a two-source food web model. Prog. Oceanogr. 71, 59–87 (2006).
Belt, S. T. et al. Distinctive 13C isotopic signature distinguishes a novel sea ice biomarker in Arctic sediments and sediment traps. Mar. Chem. 112, 158–167 (2008).
Massé, G. et al. Highly branched isoprenoids as proxies for variable sea ice conditions in the Southern Ocean. Antarct. Sci. 23, 487–498 (2011).
Belt, S. T. et al. A novel chemical fossil of palaeo sea ice: IP25. Org. Geochem. 38, 16–27 (2007).
Brown, T. A., Alexander, C., Yurkowski, D. J., Ferguson, S. H. & Belt, S. T. Identifying variable sea ice carbon contributions to the Arctic ecosystem: A case study using highly branched isoprenoid lipid biomarkers in Cumberland Sound ringed seals. Limnol. Oceanogr. 59, 1581–1589 (2014).
Gaston, A. J., Mallory, M. L. & Gilchrist, H. G. Populations and trends of Canadian Arctic seabirds. Polar Biol. 35, 1221–1232 (2012).
Brooke, M. The food consumption of the world’s seabirds. Proc. R. Soc. Lond. B Biol. Sci. 271, S246–S248 (2004).
González-Bergonzoni Ivan et al. Small birds, big effects: the little auk (Alle alle) transforms high Arctic ecosystems. Proc. R. Soc. B Biol. Sci. 284, 20162572 (2017).
Furness, R. W. & Camphuysen, K. C. J. Seabirds as monitors of the marine environment. ICES J. Mar. Sci. 54, 726–737 (1997).
Mallory, M. L., Robinson, S. A., Hebert, C. E. & Forbes, M. R. Seabirds as indicators of aquatic ecosystem conditions: A case for gathering multiple proxies of seabird health. Mar. Pollut. Bull. 60, 7–12 (2010).
Cairns, D. K. Seabirds as indicators of marine food supplies. Biol. Oceanogr. 5, 261–271 (1988).
Provencher, J. F., Gaston, A. J., O’Hara, P. D. & Gilchrist, H. G. Seabird diet indicates changing Arctic marine communities in eastern Canada. Mar. Ecol. Prog.Ser. 454, 171–182 (2012).
Vihtakari, M. et al. Black-legged kittiwakes as messengers of Atlantification in the Arctic. Sci. Rep. 8, 1178 (2018).
Amélineau, F. et al. Arctic climate change and pollution impact little auk foraging and fitness across a decade. Sci. Rep. 9, 1014 (2019).
Gaston, A. J., Gilchrist, H. G. & Hipfner, J. M. Climate change, ice conditions and reproduction in an Arctic nesting marine bird: Brunnich’s guillemot (Uria lomvia L.). J. Anim. Ecol. 74, 832–841 (2005).
Gaston, A. J., Gilchrist, H. G. & Mallory, M. L. Variation in ice conditions has strong effects on the breeding of marine birds at Prince Leopold Island, Nunavut. Ecography 28, 331–344 (2005).
Spencer, N. C., Gilchrist, H. G. & Mallory, M. L. Annual movement patterns of endangered ivory gulls: the importance of sea ice. PLOS ONE 9, e115231 (2014).
Gaston, A. J. & Hipfner, J. M. Thick-billed Murre (Uria lomvia). Birds N. Am. Online, https://doi.org/10.2173/bna.497 (2000).
Mallory, M. L., Hatch, S. A. & Nettleship, D. N. Northern Fulmar (Fulmarus glacialis), version 2.0. Birds N. Am. Online, https://doi.org/10.2173/bna.361 (2012).
Elliott, K. H. et al. High flight costs, but low dive costs, in auks support the biomechanical hypothesis for flightlessness in penguins. Proc. Natl. Acad. Sci. 110, 9380–9384 (2013).
Gaston, A. J. & Nettleship, D. N. The Thick-billed Murre of Prince Leopold Island. (Environment Canada - Canadian Wildlife Service, 1981).
Mallory, M. L., Gaston, A. J., Gilchrist, H. G., Robertson, G. J. & Braune, B. M. Effects of climate change, altered sea-ice distribution and seasonal phenology on marine birds. In A Little Less Arctic 179–195, https://doi.org/10.1007/978-90-481-9121-5_9 (Springer, Dordrecht, 2010).
Hipfner, J. M., Gaston, A. J. & Gilchrist, H. G. Variation in Egg Size and Laying Date in Thick-Billed Murre Populations Breeding in the Low Arctic and High Arctic. The Condor 107, 657–664 (2005).
Ramírez, F. et al. Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. Sci. Rep. 7, 4500 (2017).
Brown, T. A., Yurkowski, D. J., Ferguson, S. H., Alexander, C. & Belt, S. T. H-Print: a new chemical fingerprinting approach for distinguishing primary production sources in Arctic ecosystems. Environ. Chem. Lett. 12, 387–392 (2014).
Goutte, A. et al. Diatom-specific Highly Branched Isoprenoids as biomarkers in Antarctic consumers. PLoS ONE 8, 1–9 (2013).
Pratte, I., Braune, B. M., Hobson, K. A. & Mallory, M. L. Variable sea-ice conditions influence trophic dynamics in an Arctic community of marine top predators. Ecol. Evol. 9, 7639–7651 (2019).
Grémillet, D. et al. Little auks buffer the impact of current Arctic climate change. Mar. Ecol. Prog. Ser. 454, 197–206 (2012).
Brown, T. A. & Belt, S. T. Closely linked sea ice–pelagic coupling in the Amundsen Gulf revealed by the sea ice diatom biomarker IP25. J. Plankton Res. 34, 647–654 (2012).
Brown, T. A., Hegseth, E. N. & Belt, S. T. A biomarker-based investigation of the mid-winter ecosystem in Rijpfjorden, Svalbard. Polar Biol. 38, 37–50 (2015).
Hamer, K. C., Thompson, D. R. & Gray, C. M. Spatial variation in the feeding ecology, foraging ranges, and breeding energetics of northern fulmars in the north-east Atlantic Ocean. ICES J. Mar. Sci. 54, 645–653 (1997).
Mallory, M., Forbes, M., Ankney, C. & Alisauskas, R. Nutrient dynamics and constraints on the pre-laying exodus of High Arctic northern fulmars. Aquat. Biol. 4, 211–223 (2008).
Gaston, A. J. et al. Modeling foraging range for breeding colonies of thick-billed murres Uria lomvia in the Eastern Canadian Arctic and potential overlap with industrial development. Biol. Conserv. 168, 134–143 (2013).
Mallory, M. L. et al. Identifying key marine habitat sites for seabirds and sea ducks in the Canadian Arctic. Environ. Rev. 1–26, https://doi.org/10.1139/er-2018-0067 (2018).
Bradstreet, M. S. W. Thick-billed murres and black guillemots in the Barrow Strait area, N.W.T., during spring: diets and food availability along ice edges. Can. J. Zool. 58, 2120–2140 (1980).
Bradstreet, M. S. W. Summer feeding ecology of seabirds in eastern Lancaster Sound. (1976).
Bradstreet, M. S. W. & Cross, W. E. Trophic relationships at High Arctic ice edges. Arctic 35, 1–12 (1982).
Hobson, K. A. & Welch, H. E. Observations of foraging Northern Fulmars (Fulmarus glacialis) in the Canadian High Arctic. Arctic 45, 150–153 (1992).
Mallory, M. L. et al. Temporal and spatial patterns in the diet of northern fulmars Fulmarus glacialis in the Canadian High Arctic. Aquat. Biol. 10, 181–191 (2010).
Bradstreet, M. S. W. et al. Aspects of the biology of Arctic cod (Boreogadus saida) and its importance in arctic marine food chains. Can. Tech. Rep. Fish. Aquat. Sci. 1491, 193 (1986).
Mallory, M. L. & Forbes, M. R. Does sea ice constrain the breeding schedules of high arctic northern fulmars? The Condor 109, 894–906 (2007).
Gaston, A. J. & Bradstreet, M. S. W. Intercolony differences in the summer diet of Thick-billed Murres in the eastern Canadian Arctic. Can. J. Zool. 71, 1831–1840 (1993).
Gradinger, R. R. & Bluhm, B. A. In-situ observations on the distribution and behavior of amphipods and Arctic cod (Boreogadus saida) under the sea ice of the High Arctic Canada Basin. Polar Biol. 27, 595–603 (2004).
Grémillet, D. et al. Arctic warming: nonlinear impacts of sea-ice and glacier melt on seabird foraging. Glob. Change Biol. 21, 1116–1123 (2015).
Lack, D. The natural regulation of animal numbers. (Clarendon Press, 1954).
Gaston, A. J., Gilchrist, H. G., Mallory, M. L. & Smith, P. A. Changes in seasonal events, peak food availability, and consequent breeding adjustment in a marine bird: a case of progressive mismatching - cambio de eventos estacionales, de disponibilidad máxima de alimento, y de ajuste consiguiente en reproducción de un ave marina: un caso de desajuste progresivo. The Condor 111, 111–119 (2009).
Søreide, J. E., Leu, E., Berge, J., Graeve, M. & Falk Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Change Biol. 16, 3154–3163 (2010).
Barrionuevo, M. & Frere, E. Parental investment in eggs and its effect on nestling growth and survival in Magellanic Penguins. Emu - Austral Ornithol. 114, 259–267 (2014).
Hipfner, J. M. & Gaston, A. J. The relationship between egg size and posthatching development in the thick-billed murre. Ecology 80, 1289–1297 (1999).
Williams, T. D. Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol. Rev. 69, 35–59 (1994).
Goutte, A. et al. Importance of ice algal production for top predators: new insights using sea-ice biomarkers. Mar. Ecol. Prog. Ser. 513, 269–275 (2014).
Ainley, D. G. et al. Diet and foraging effort of Adélie penguins in relation to pack-ice conditions in the southern Ross Sea. Polar Biol. 20, 311–319 (1998).
Cottin, M. et al. Foraging strategies of male Adélie penguins during their first incubation trip in relation to environmental conditions. Mar. Biol. 159, 1843–1852 (2012).
Guen, C. L. et al. Reproductive performance and diving behaviour share a common sea-ice concentration optimum in Adélie penguins (Pygoscelis adeliae). Glob. Change Biol. 24, 5304–5317 (2018).
Nicol, S. Krill, currents, and sea ice: Euphausia superba and its changing environment. BioScience 56, 111–120 (2006).
Emmerson, L. & Southwell, C. Sea ice cover and its influence on Adélie penguin reproductive performance. Ecology 89, 2096–2102 (2008).
Divoky, G. J., Lukacs, P. M. & Druckenmiller, M. L. Effects of recent decreases in arctic sea ice on an ice-associated marine bird. Prog. Oceanogr. 136, 151–161 (2015).
Durant, J. M., Anker-Nilssen, T. & Stenseth, N. C. Ocean climate prior to breeding affects the duration of the nestling period in the Atlantic puffin. Biol. Lett. 2, 628–631 (2006).
Litzow, M. A., Piatt, J. F., Prichard, A. K. & Roby, D. D. Response of pigeon guillemots to variable abundance of high-lipid and low-lipid prey. Oecologia 132, 286–295 (2002).
Carey, C. Avian Energetics and Nutritional Ecology. (Springer US, 1996).
Belt, S. T. et al. A reproducible method for the extraction, identification and quantification of the Arctic sea ice proxy IP25 from marine sediments. Anal. Methods 4, 705–713 (2012).
Hobson, K. A., Piatt, J. F. & Pitocchelli, J. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 63, 786–798 (1994).
Kelly, J. F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 78, 1–27 (2000).
R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2008).
Acknowledgements
We are grateful to the field assistants who helped with egg collections over the years. We thank Gaël Guillou and the ‘Isotopic Spectrometry’ plateform for conducting stable isotope analyses at the LIENSs. Funding for sample collections was provided by Environment and Climate Change Canada, the Northern Contaminants Program of Indigenous and Northern Affairs Canada, and Acadia University. Logistical support for the fieldwork out of Resolute Bay was provided by the Polar Continental Shelf Program of Natural Resources Canada. We acknowledge the use of imagery from the NASA Worldview application (https://worldview.earthdata.nasa.gov/), part of the NASA Earth Observing System Data and Information System (EOSDIS). FC benefited from scholarships from Natural Sciences and Engineering Research Council (Discovery grant to GM). This study represents a contribution to the French Polar Institute ADACLIM research program (IPEV Pgr 388).
Author information
Authors and Affiliations
Contributions
F.C., J.F., G.M. and M.M. contributed to conception and design. F.C. performed the experiments and acquired the data, while P.M. analysed the ice conditions. F.C., J.F., G.M., M.M., P.M. and B.B. contributed to analysis and interpretation of data. F.C., J.F. and G.M. drafted the article. All authors revised the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cusset, F., Fort, J., Mallory, M. et al. Arctic seabirds and shrinking sea ice: egg analyses reveal the importance of ice-derived resources. Sci Rep 9, 15405 (2019). https://doi.org/10.1038/s41598-019-51788-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51788-4
This article is cited by
-
Year-round utilization of sea ice-associated carbon in Arctic ecosystems
Nature Communications (2023)
-
Complementary diet analyses reveal intraspecific and temporal variation in ringed seal (Pusa hispida) foraging in the Canadian high arctic
Polar Biology (2022)
-
Cold comfort: Arctic seabirds find refugia from climate change and potential competition in marginal ice zones and fjords
Ambio (2022)
-
Sea ice extent and phenology influence breeding of high-Arctic seabirds: 4 decades of monitoring in Nunavut, Canada
Oecologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.