Abstract
Pseudosuccinea columella snails transmit the trematode Fasciola hepatica, but in Cuba, six naturally occurring populations successfully resist parasite infection. Here, we present an updated distribution of P. columella in Cuba; 68 positive sites with the earliest records more abundant in west-central Cuba and with east-central populations generally corresponding to the newest samples. No records were found farther east. The IPA site reported 10.5% prevalence of F. hepatica-infected snails. Population genetics, studied through microsatellites, showed low allelic and multilocus genotypic richness (MLGT), mainly in susceptible populations, strong deviations from panmixia and high self-fertilization rates. Susceptible individuals were grouped in one major cluster containing the majority of MLGT, and two independent clusters grouped the MLGT of resistant individuals from western and central populations, respectively. From these, we propose that several introductions of P. columella occurred in Cuba, primarily in the west, with the early arrivals deriving on the resistant populations. A more recent introduction of susceptible P. columella carrying MLGT T and Y may have occurred, where the latter spread quickly through the island and possibly increase the risk of parasite transmission in Cuba since all snails naturally infected with F. hepatica were carriers of the MLGT Y. Interestingly, even though resistant populations are highly diverse and are likely the oldest within Cuba, they are only found in six localities characterized by soft (total hardness, TH = 6.3 ± 1.03°d) and slightly acidic (pH = 6.2 ± 0.12) waters with low richness in snail species (3.2 ± 1.02). This tendency was also observed in a two-year follow-up ecological study that was conducted on a farm where both phenotypes occurred in sympatry; colonization events by resistant over susceptible snails coincided with a reduction in the pH and TH of the water. A comparison of life traits in susceptible and resistant isolates reared at two different pH/TH conditions (5.9/4°d or 7.8/14°d) showed that low pH/TH negatively affects P. columella, irrespective of the phenotype. However, evidence of higher tolerance (higher survival, life expectancy, egg viability) to such conditions was observed in resistant isolates. Finally, we speculate that the limited distribution of resistant populations might be related to a better exploitation of sites that are less suitable to snails (thus, with lower competition), rather than to a differential ecological restriction to specific environmental conditions from susceptible P. columella.
Similar content being viewed by others
Introduction
Freshwater snails of the family Lymnaeidae (also known as pond snails) inhabit tropical, temperate and cold regions, from sea level to high altitudes1. Many of the nearly 100 species recently attributed to this family2 have become the target of taxonomical and parasitological studies over the past few years, due to their important roles as intermediate hosts of several medically relevant parasites, including the globally distributed liver fluke Fasciola hepatica (e.g.3,4). Within Lymnaeidae, several species have been incidentally carried out of their native range and introduced elsewhere (see the cases of Galba cubensis and Austropeplea viridis in Europe;5,6). One of the most interesting examples is that of Pseudosuccinea columella (Say, 1817), a globally introduced species with a considerable invasive ability7. The transmission of F. hepatica by P. columella is well-documented in its native range (North America; see8) and in places where it has been introduced such as Brazil9, Argentina10 and Australia11. This species is also thought to enhance transmission in remote regions where it has invaded, like in Egypt12 and the Pacific islands, including Hawaii and French Polynesia13,14.
In Cuba, the first report of P. columella dates back to 1858 in the western region and the latest malacological surveys depict a western-central distribution15. Although this species is thought to play a secondary role in F. hepatica transmission in Cuba (with Galba cubensis being the main host; see16,17), it is found naturally infected in the field18. Important investigations have been carried out on P. columella in recent decades due to the existence of a resistant phenotype against F. hepatica infection in certain natural Cuban populations19,20. Experimental exposures of these resistant snails to different sympatric and allopatric fluke isolates always result in the failure of parasite larval stage development19,20,21,22 which is associated with an effective encapsulation of the parasite by snail immune cells19.
Interestingly, thus far, all resistant P. columella can be easily differentiated from susceptible individuals in the field though the use of a phenotypic marker consisting of a belt-like pattern of small, sharp and whitish spots in the mid-region of the mantle with bigger spots scattered on the upper and lower sides19. Attempts to genetically differentiate susceptible from resistant individuals have independently clustered both phenotypes by RAPD markers21. More recently, Cuban resistant populations were segregated from P. columella populations found elsewhere, using mitochondrial haplotypes and microsatellites markers7. It is important to stress that the observed molecular and phenotypic differences are not a reliable mean of separating resistant populations as a different species. Resistant snails displayed the same reliable features of the shell and the same reproductive system anatomy defined for P. columella with only a slight difference in two-nucleotide changes (0.17%) within the ITS1 and ITS2 when ribosomal genes were sequenced and compared23. From an ecological point of view, previous comparative studies on demographic dynamics in the laboratory between susceptible and resistant snails have suggested the existence of a trade-offs against reproduction of the latter24,25.
The natural occurrence of populations that are susceptible and resistant to a parasite within the same host species provides a unique model that can be addressed from several disciplines, including evolutionary biology and host-pathogen interaction, environmental and health sciences and snail control. Here, for the first time, we present a global overview of the ecology of P. columella in Cuba, where both phenotypes are considered separately by the use of different approaches. We present its updated distribution, study its infection status and its genetic structure by microsatellites markers, and elucidate the ecological patterns associated with the occurrence of resistant and susceptible populations through the analysis of field data and experimental life history traits. From these, we gain insight into the history, colonization and population structure of P. columella in Cuba, its relationship with F. hepatica and the increasing risk of parasite transmission represented by certain widespread genotypes. In addition, we provide further knowledge related to habitat preferences of P. columella and the environmental conditions that contribute to its occurrence in the wild. All of these aspects are essential to understand and to predict the distribution pattern and further spreading of P. columella snails in Cuba. Findings from this study may play an important role in conservation biology, parasitology and epidemiology and to devise intermediate host control strategies based on the potential application of P. columella resistance.
Material and Methods
Distribution of P. columella in Cuba
The updated distribution of P. columella was obtained from the National Reference Malacological Database for freshwater snails in Cuba, held by the Laboratory of Malacology of the Institute of Tropical Medicine in Havana, through the revision of 496 sampled sites ( = populations) from 1982 to 2018. Each record includes the presence of a given freshwater snail, geographic coordinates, habitat type, date and collector. Nationwide malacological surveys are carried out by our laboratory on a 10-year sampling period basis complemented with annual expeditions covering smaller areas. All sites positive for P. columella were plotted on a map differentiating each record according to a 11-year time interval, to account for new and historical data, using MapInfo v.15 (Pitney Bowes Software Inc., New York, USA, 2015). The frequency of each habitat type where P. columella was observed was used as a measure of habitat preference.
Differentiation of resistant and susceptible P. columella populations to F. hepatica infection were primarily based on the presence or absence of a characteristic band of small sharp spots in the mid-region of the mantle, as its occurrence is associated with the resistant phenotype19. Additionally, both phenotypes, and particularly that of the resistant populations, were double-checked in the laboratory by experimental infection using different panels of potentially sympatric (retrieved from infected cattle reared at the site or in nearby areas) and allopatric F. hepatica isolates (e.g.20,21,22). The latter confirmed the reliability of the mantle pigmentation pattern as a distinctive phenotypic marker for F. hepatica resistance in P. columella. Experimental infections were carried out following the methodology previously described20, exposing 30 snails per population to a dose of five F. hepatica miracidia.
Population genetics of P. columella from Cuba
Snail sampling, DNA extraction, amplification and genotyping of microsatellite loci
We sampled 20 populations of P. columella (17 susceptible and 3 resistant, i.e. displaying the reliable phenotypic mantle marker described by Gutiérrez et al.19). When possible, up to 30 individuals were collected from each population, up to a total of 329 snails. The snails were carefully dissected in the laboratory for screening of possible parasite infection. A piece of tissue from the foot was used for DNA extraction to avoid foreign genetic contamination (e.g. sperm from cross-fertilization). The extraction of DNA was performed using Chelex methodology following26 and slightly modified for 96-well plates. In brief, a small portion of tissue was added to a mixture of 100 µL of 5% Chelex®100 (Bio-Rad) and 5 µL of proteinase K (50 mg/mL; Promega) in each well. The plate was mixed in vortex, incubated overnight at 56 °C and placed for 10 min at 95 °C. The mixture was centrifuged at 6000 × g for 6 min and the supernatant containing the DNA was collected and stored at −20 °C until use.
PCR amplifications were performed in a 96-well MJ-Research PTC 100 for all samples. Six microsatellite loci, previously described for P. columella species by Nicot et al.27, were amplified (GenBank Access number): PCO01 (EU04295), PCO02 (EU049296), PCO07 (EU049299), PCO12 (EU049303), PCO13 (EU049304) and PCO20 (EU049309). Each locus was amplified through PCR using 1 µL of extracted DNA in 10 µL of reaction volume containing 2 µL buffer 5 × (Promega), 1 µL of 25 mmol/L MgCl2, 0.5 µL of 2 mmol/L dNTP (Invitro-gen/Life Technology), 0.2 µL of each primer (10 pmol) and 0.2 µL of Taq DNA polymerase (Promega). Thermocycling consisted of 10 min of initial denaturalization at 94 °C, 30 cycles at 94 °C for 30 s, annealing at 55 °C (for each locus) for 30 s, 60 s at 72 °C and a final elongation step at 60 °C for 30 min.
Primers were fluorescently labelled to be used in an ABI automated sequencer (ABI Prism 310 Genetic Analyser, Applied Biosystem, Perkin-Elmer, USA). Amplified products for each individual were used for sequencing where 1 µL of diluted amplicon (1/100) was added to a mix containing 15 µL of Hi-Di Formamide and 0.27 µL of internal size standards (GENESCAN 500 LIZ, Applera). Each allele’s length was read using GeneMapper® v.4.0 software (Applied BiosystemsTM).
Population genetics analysis
Current parameters of population genetics like the mean number of alleles (a), the observed (Ho) and expected (Hs) heterozygosity and FIS were estimated for each locus. Pairwise differentiation between populations (FST) was also tested. Calculations were computed using FSTAT v2.9.3.228 and Bonferroni corrections were applied for multiple tests29. The selfing rate (s) for each population was calculated using the equation s = 2FIS/(1 + FIS)30. Identical multilocus genotypes (MLGT) were identified based only on individuals with all microsatellite loci amplified and coded using a combination of letters (one letter or letters’ combination = one MLGT = unique combination of alleles across all analysed loci). Nei’s31,32 genetic distances between populations were estimated using Genetix33. The obtained distance matrix was used to build a neighbour-joining network of all MLGT in SplitsTree434. The genotypic diversity was calculated in each population with an adaptation of the Simpson index using GENCLONE 2.035.
Ecological patterns of resistant and susceptible P. columella
The ecological patterns of the two phenotypes of P. columella were explored using three approaches: laboratory field records, field study and experimental life tables.
Revisiting laboratory field records of resistant and susceptible P. columella populations
A thorough literature review which included published papers and 37 years of unpublished data from our laboratory’s field notes, was conducted. We gathered data for species composition of the mollusc assemblage and field abiotic factors (phosphates, nitrates, dissolved oxygen, pH and total hardness) measured for resistant and susceptible populations investigated within this study.
Ecological follow up of resistant and susceptible populations of P. columella
We performed a two-year follow-up ecological study on the La Coca (=locality La Coca) cattle farm located in south-western Havana, as it harbours resistant and susceptible populations of P. columella (see Fig. 1A). The region is characterized by a lowland meadow that is commonly flooded during the rainy season, which extends for nearly 10 km2. The area includes several channels used for irrigation and livestock watering, usually no more than 60 centimetres in depth. Gramineous vegetation is abundant but aquatic plants in the channels are scarce. These features contribute to the presence of several water bodies (sites) scattered throughout the locality where freshwater snail populations thrive. The P. columella species is present at five sites from the La Coca locality, four of which harbour susceptible populations. The fifth site is known as Segundo Potrero and is characterized by the occurrence of a resistant P. columella population named La Coca after the locality.
(A) Overview of the locality La Coca showing the two sites, i.e. Segundo Potrero and Negrines, sampled during the two-year follow-up ecological study where the resistant (R) La Coca population and the susceptible (S) Negrines population of Pseudosuccinea columella occurred. (B) Localities from western Cuba where the four populations of resistant (1: La Palma, 2: La Coca) and susceptible (2: Negrines within La Coca locality, 3: Aurora) P. columella used in the life table experiments were collected from.
Thus, based on these characteristics, we selected two sites from the locality La Coca for the ecological follow-up: (i) Segundo Potrero, due to the presence of the resistant P. columella population (La Coca population) and (ii) a nearby site called Negrines, 500 m away and connected by one of the channels, where a population of susceptible individuals (Negrines population) occurs. Each site was sampled at monthly intervals from January 2011 to December 2012 always by the same person during the early hours of the morning, using soft forceps and sieves (1 mm mesh). We used the capture per unit of effort method over a 15 minute interval36 and relative abundance was measured as individuals/15′ for each species. Aquatic and shore vegetation were swept for every sample. Resistant snails were easily identified in the field by the phenotypic marker of mantle pigmentation19. Abiotic factors including pH, temperature, total hardness (TH), carbonate hardness (CH), dissolved oxygen, phosphates and nitrites were measured using a field kit (Merck). Species diversity was calculated for each site using the Simpson index37 and a canonical correspondence analysis38 was done to detect unimodal response of mollusc species abundances to ecological factors.
Additionally, all collected lymnaeid snails were brought to the laboratory and dissected to account for F. hepatica larval stage infection.
Laboratory life tables of resistant and susceptible P. columella
Four natural populations of P. columella; two composed of susceptible (Negrines, 22.9577°N; −82.4649°W and Aurora, 23.0789°N; −81.9176°W) and two of resistant (La Coca, 22.9554°N; −82.4598°W and La Palma, 22.7695°N; −83.5443°W) individuals (see Fig. 1B), were sampled and reared under laboratory conditions for one generation. Given the results from field data concerning the ecological patterns related to the occurrence of both phenotypes, two different conditions of pH/total hardness (TH) were set up: common (7.6/14°d; according to Perera36, those conditions are the most commonly found for Cuban freshwater habitats) and lower (5.9/14°d) conditions. Snails were then reared in the two conditions using separate 25 L aquariums. Five-day-old snails from each isolate (n = 30 per population at each condition) were placed in the aquariums and this moment of transfer was considered as time zero for the experiment. All aquariums were provided with 50 mg each of CaCO3 and MgCO3 to contribute to shell formation. Water acidification down to pH 5.9 (low pH condition) was achieved through an automatic CO2 pump connected to a setter pH meter. Total hardness conditions were adjusted to the required range for each aquarium by differentially mixing aged tap water with distilled water. Both pH and TH were systematically checked along with other abiotic parameters (Cl−, NO3−, NO2−, CH) with a commercial aquarium kit to guarantee optimal and reproducible conditions during the experiment. Temperature was always maintained at 25–26 °C and the snails were fed three times per week with a mixture of algae cultured for this purpose following Sánchez et al.39. All populations were followed until the death of all individuals. Live and dead snails, the number of eggs, egg masses and newly hatched snails (NHS) were counted at weekly intervals. At the end of the experiment, the following life table parameters were determined according to Margalef40: proportion of living individuals (lx), probability of survival (px), life expectancy (ex), fecundity rate (mx), net reproduction rate (Ro), and intrinsic (r) and finite (λ) rates of natural increase.
Snail survival curves were constructed based on the percentage of live snails at each time point and pairwise log-rank tests of Kaplan-Meier curves were performed to assess the statistical differences on survivorship data. The factorial ANOVAs followed by the post hoc multiple comparison Tukey test were carried out to assess statistical significance between the mean values of eggs, egg masses and egg/egg masses per population. The overall percentage of viable eggs (i.e. viable eggs = NHS/number of eggs) per strain at each condition was compared through a Fisher’s Exact Test. Similarly, the percentage of NHS from the first, second and third week after egg laying was determined for each strain and compared between experimental settings to assess possible delays. Statistica v.12 (StatSoft. Inc., Tulsa, OK, USA 2014) software was used for calculations and differences were considered significant when P < 0.05.
Ethics approval
French laboratory holds permit #A66040 for experiments on animals, which was obtained from the French Ministry of Agriculture and Fisheries and the French Ministry of National Education, Research and Technology. The housing, breeding and care of the utilized animals followed the ethical requirements of France. The experimenter possesses an official certificate for animal experimentation from both of the above-listed French ministries (Decree #87–848, October 19, 1987). The various protocols used in this study have been approved by the French Veterinary Agency of the DRAAF Languedoc-Roussillon (Direction Régionale de l′Alimentation, de l′Agriculture et de la Forêt), Montpellier, France (authorization # 007083). The Cuban laboratory of Malacology holds permit for experiments on animals obtained from the Cuban government. The housing, breeding and care of the utilized animals followed the ethical requirements of Cuba. The experimenter possesses an official certificate for animal experimentation from the Cuban government.
Results
Distribution of P. columella in Cuba
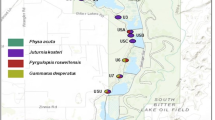
Knowing the distribution of a snail species is particularly important to assess its epidemiological significance and to attempt its effective control. The National Reference Database contains nearly 500 records in Cuba (Fig. 2A), from which 68 verified sites harbour P. columella species (Fig. 2B). The distribution of P. columella ranges from the westernmost region (Pinar del Río province) to the east-central (Camagüey province) region of Cuba (Fig. 2B). No records exist for the easternmost provinces. Notably, 59% of sampled populations were documented in the last 15 years. Overall, earliest records are more abundant in the west-central region while populations from east-central Cuba correspond mostly to newer samplings. From the 68 verified P. columella populations, only six are acknowledged as resistant to F. hepatica infection (i.e. snails displaying the characteristic mantle pigmentation pattern described by Gutiérrez et al.19 and showing no record of parasite development following exposure to different F. hepatica isolates). Populations of resistant individuals are mostly recorded in the west (Pinar del Río – Havana provinces) with the exception of Babiney, in Cienfuegos (central Cuba; Fig. 2B).
(A) Distribution of all registered records in the National Malacology Reference Database accounting for freshwater mollusc species from 1982–2018. (B) Updated distribution of Pseudosuccinea columella from Cuba. Positive sites were plotted with different points representing 11-year time intervals to account for historical and newly recorded data. Resistant populations are named in the map, consistently with previous published works. The year of first record of resistant populations is also indicated. (C) Number of records of P. columella per type of habitat. (Laboratory of Malacology, IPK).
Concerning habitat type preferences, permanent ponds, rivers and dams harbour 62.5% of all P. columella records, with up to six different habitats concentrating the remaining populations (Fig. 2C). We did not find a clear pattern of habitat types for resistant populations which were observed in permanent ponds (2), flooded lands (1), springs (1), ditches (1) and canals (1). Globally, the number of populations recorded in open or connected water systems (e.g. rivers, streams, canals) is fewer than those of closed habitats (e.g. dams, ponds, flooded lands; Fig. 2C).
Population genetic structure of P. columella in Cuba
Population genetic studies on P. columella could give insights into important questions for biological and health sciences; its evolutionary history in Cuba, its pattern of colonization, the genetic structure of its populations and its role in F. hepatica transmission. Thus, from the distribution of P. columella presented above, we selected 20 populations (see Fig. 3 for details) to analyse the genetic structure of this species in Cuba. Records of P. columella infected with different trematode species in Cuba exist in our laboratory but, in the present study, dissection of 329 snails only evidenced infection of F. hepatica in two individuals from IPA population (10.5% prevalence, IPA site). No other infections, by F. hepatica or other trematode species, were recorded at any other site.
(A) Map showing the distribution of the different multilocus genotypes found (MLGT; one letter or letters’ combination = one MLGT = unique combination of alleles across all analysed loci) on sampled Pseudosuccinea columella populations genotyped through 6 microsatellite loci (3, 7, 10: *resistant P. columella populations). (B) MLGT of P. columella clustered following a joining-neighbour network using Nei’s genetic distance from each MLGT (different filling pattern correspond to different clusters). 1: Tio Pancho, 2: SJM, 3: El Azufre*, 4: Río Hondo, 5: IPA, 6: Pilón, 7: La Coca*, 8: Parque Lenin, 9: V7, 10: Babiney*, 11: Vega Grande, 12: El Antojo, 13: El Cacao, 14: Río Manaquita, 15: Río Yayabo, 16: Puesto de Mando, 17: Guillén, 18: Río Central, 19: Matadero Aves, 20: Arroyo.
The six microsatellite loci explored were polymorphic in P. columella, especially PCO12 which displays seven different alleles in Cuba. Population genetic parameters are presented in Table 1. Allelic richness is very low in the 20 studied populations (mean 2.034 ± 0.646 SD) and five populations (Pilón, El Antojo, Puesto de Mando, Guillén and Arroyo) are completely monomorphic at the six loci. The highest allelic richness was observed in the two resistant populations of La Coca and Babiney (2.17 ± 0.75 SD). However, the analysis of the third resistant population (El Azufre) showed a lower allelic richness (1.17 ± 0.41 SD). Expected heterozygosis (mean 0.1 ± 0.09 SD) and observed heterozygosis (0.03 ± 0.056 SD) were very low. Most populations had high and significant values of FIS after Bonferroni correction (mean 0.667 ± 0.313 SD) for which estimated selfing rates were also high ( > 64%). Although an overall value of FST of 0.339 (P > 0.05) was observed, significant differentiation was only recorded in resistant populations (La Coca, Babiney and El Azufre) that not only differed from susceptible populations but also from each other (Supplementary Table S1).
Multilocus genotyping at the six microsatellite loci showed the existence of 36 MLGT. Observed mean number of MLGT in susceptible populations was 2.764 (±1.562 SD) while a higher average of 6.333 (±3.785 SD) was attained in resistant populations. In particular, differences in MLGT between each group of populations were statistically significant (t value (df: 18) = 3.58; P = 0.002). Mean genotypic diversity was 0.397 (±0.357) with the highest values in the resistant populations of La Coca (0.893) and Babiney (0.836). In the case of resistant individuals, 18 MLGT were observed for which only MLGT E is shared in two populations (El Azufre and La Coca). Figure 3A shows the distribution of the 36 MLGT in Cuba with the MLGT Y widespread in most sampled sites and the only one observed in susceptible monomorphic populations. This MLGT Y is absent in resistant populations (Fig. 3A). Interestingly, the two infected individuals found in the IPA population and the population from Pilón previously reported as infected (see Gutiérrez et al.18), shared the same MLGT Y. A different MLGT (T) was observed only in the easternmost populations (Río Manaquita, Río Central and Matadero Aves) within several individuals. The rest of the MLGT are considered rare and differ from one another by a single or few alleles. The obtained MLGT network from Nei’s genetic distance displays three majoritarian clusters (Fig. 3B). Susceptible MLGT gathered within one major cluster. Two different major clusters and one small cluster contain most of the MLGT from resistant individuals (Fig. 3B).
Ecological patterns associated with the occurrence of resistant and susceptible P. columella in Cuba: field and laboratory observations
The higher allelic and MLGT richness of resistant populations presented herein contrasts with their restricted distribution, as they have been identified in only six of 68 sites harbouring P. columella. In this sense, studies depicting more general ecological interactions of both susceptible and resistant P. columella in the mollusc assemblage and their interaction with the abiotic factors, which could explain the differential distribution observed, are lacking. Considering that such resistance could hold promise as a control strategy to disengage F. hepatica transmission in the field, it is important to account for its ecological or demographical patterns. Thus, we analysed the occurrence of each phenotype in the wild in association with different environmental factors and we carried out experimental life tables to address this topic.
Reviewing data from natural populations
Since no apparent pattern concerning the type of habitat was found in either resistant or susceptible P. columella (see section 3.1), we aimed to identify specific ecological features associated with the occurrence of each phenotype. For this, we used data from 14 sites harbouring P. columella mostly concerning different abiotic factors (see Table 2). It can be noted, that both susceptible and resistant populations seem to prefer water with low nitrite and ammonium concentrations. Interestingly, the recorded ecological data showed a tendency for resistant populations to settle in waterbodies with lower pH (6–6.5), TH (4–9°d) and mollusc richness (see Table 2).
Ecological factors affecting the abundance of resistant and susceptible P. columella in the La Coca locality
We found the two distinct phenotypes (resistant and susceptible) of P. columella occurring in the La Coca locality, but with a marked degree of ecological segregation depending on the particular sampled site, with Segundo Potrero only harbouring resistant snails (La Coca resistant population). G. cubensis (Lymnaeidae), Physa acuta (Physidae), Drepanotrema anatinum and Drepanotrema lucidum (Planorbidae) were recorded in Negrines. In this site, P. acuta and D. anatinum showed similar variations while G. cubensis performed two ephemeral colonisations with rapid extinctions (Supplementary Fig. 1). Physa acuta was dominant in Negrines but it did not seem to affect the population dynamic of P. columella (Supplementary Fig. 1). In contrast, the scenario at Segundo Potrero was completely different with a very low snail species richness marked by low abundances of D. anatinum and Gundlachia radiata, and a considerable dominance by resistant P. columella snails (Supplementary Fig. 1).
The ecological follow-up was only carried out in two sites, which limits replication. However, the closeness and constant water flow interchange between both sites and the ecological variations recorded on some variables in the Negrines site allowed us to observe an interesting pattern. Concerning the different phenotypes of P. columella, we should note that, while in Segundo Potrero only resistant individuals are found (i.e. La Coca population), in Negrines susceptible individuals occurred in sympatry with resistant snails at certain times of the study (Fig. 4B). The canonical correspondence analysis performed with all the ecological data recorded at both sites showed a particular relationship between the abundances of resistant and susceptible individuals with variations in pH and both TH and CH (Fig. 4A). We found a strong negative association between resistant snails and pH increase demonstrating a certain tolerance for slightly acidic waters. The remaining factors had a minor effect on species abundances. In this sense, while in Negrines the mean values of pH and TH were around 7.5 and 14°d respectively, Segundo Potrero showed lower values (pH: 5.5–6; TH: 2°d-4°d) with an annual stability and a high abundance of resistant P. columella. However, in Negrines, the population of P. columella remained stable at low effective size but with peaks of resistant individuals when susceptible snails decreased or disappeared. Interestingly, such colonization events by the resistant snails in Negrines matched a drop in pH and TH values highlighting their particular association with water acidity and TH observed in the canonical analysis (Fig. 4) and with the overall pattern recorded from the previous analysis (section 3.3.1). Globally, susceptible snails were more abundant when pH ranged between 7 and 8.
(A) Canonical correspondence analysis of the two-year follow up study in the La Coca locality (sampled sites: Negrines and Segundo Potrero). (B) Monthly relative abundances of Pseudosuccinea columella and dynamics of pH and water hardness in the two sampled sites within the La Coca locality. AV: aquatic vegetation, CH: Carbonate hardness, D: Simpson’s Diversity index, S: susceptible P. columella, R: resistant P. columella, T: temperature, TH: Total hardness.
Notably, we failed to detect F. hepatica infection from P. columella during the two-year ecological follow-up study in La Coca. However, while susceptible snails from Negrines were experimentally infected, it was not possible to infect resistant snails with different F. hepatica isolates.
Influence of pH and total hardness on experimental life history traits of resistant and susceptible P. columella
Given that the evidences gathered from the historical field notes and from the population dynamics in the La Coca locality suggested a differential relation between the occurrence of susceptible and resistant individuals concerning the pH/TH (i.e. positive association of resistant populations with acidic and soft waters with low species richness), we performed an experimental life table study to assess the effect of these abiotic factors on several life-history traits of P. columella. We measured survival and reproductive traits as shell size showed no significant differences between P. columella phenotypes in a previous study24. Age-independent parameters, and life expectancy and fecundity rates for weeks 0, 5, 9 and 14; considering the start of the experiment, sexual maturity, middle and late snail developmental stages respectively, are shown in Table 3. To facilitate comprehension and to elucidate patterns, we will address the results from two different levels of analysis: (a) the overall effects on the species and (b) the differential effects on the resistant/susceptible phenotypes.
Overall life-history traits for Pseudosuccinea columella – common pH/TH conditions seem better suited to P. columella species: Survival of P. columella was highest during the first eight weeks with a lifespan ranging from 15 to 23 weeks (3 to 5 months). Sexual maturity was reached between the third and fifth weeks, depending on the population and experimental setting. Reproductive traits showed a steady increase since first egg laying until reaching a peak between week 13 and week 16 (see Table 3 and Fig. 5 for details).
(A) Survival curves of resistant (R) and susceptible (S) Pseudosuccinea columella populations in which pairwise comparisons of snail survival between population at each experimental setting, performed by log-rank Tests, are also shown. (B) Egg production data of experimentally reared P. columella snails at pH and total hardness (pH/TH) of 5.9/5°d or 7.6/14°d. Arrows within the egg production data indicate the week at which 100% mortality was observed for the given population at each condition. Results of Factorial ANOVAs were P < 0.05.
Overall, P. columella performed better at common pH/TH conditions irrespective of the phenotype (see Table 3; Fig. 5). Low pH/TH values had a negative impact on snail survival with the lowest life expectancies compared to common conditions (Table 3). In particular, with the exception of La Palma, the other populations considerably decreased their life expectancy at birth (La Coca: 1.7-fold, Aurora: 1.5-fold, Negrines: 2.9-fold). Regarding mortality, this trait peaked at week 1 for Negrines and around week 7 for the remaining populations (i.e. all populations but La Palma were reduced by more than half; Fig. 5A, Table 3). Furthermore, fertility traits were also negatively affected under these conditions. A significant delay in snail hatching within the first week following egg laying was recorded at low pH/TH for all populations but La Palma (NHS within the first week in Table 3). Additionally, several egg masses laid and incubated at 5.9/4°d needed up to three weeks to hatch. The combination of these later effects also affected population increases (r and λ) which were lower at low pH/TH. Net reproductive rate was also lower at 5.9/4°d except for La Coca which presented a two-fold higher Ro (Table 3). All these impairments on survival and reproductive parameters of P. columella reared at low pH/TH strongly suggests that common conditions of 7.6/14°d are better suited to this species, irrespective of the phenotype.
Interestingly, overall higher fecundity rate was observed at 5.9/4°d (Table 3) probably to compensate for the reduction in self-maintenance and reproduction recorded on P. columella under these conditions. However, a significant increase of the mean number of eggs, egg masses and egg per egg masses was only attained by La Coca and Aurora snails at low pH/TH (Fig. 5B; Tukey’s Test post hoc, P < 0.05).
Differential effects of pH and TH at the resistant/susceptible phenotype level – higher tolerance to low pH/TH conditions is possibly associated with the resistant phenotype: Despite the overall decrease in self-maintenance and reproduction of P. columella at 5.9/4°d presented above, differences can be seen between phenotypes that points at a higher tolerance for low pH/TH of resistant versus susceptible populations. In general, higher survival (Log rank Tests, P < 0.05; Fig. 5A) and life expectancy at birth (Table 3) were observed in resistant snails at low pH/TH conditions. In addition, we recorded a higher percentage of viable eggs in resistant compared to susceptible populations at low pH/TH (Table 3; Fisher’s Exact Test, P < 0.05). No significant variation in this trait in resistant P. columella at common versus low pH/TH (Table 3; Fisher’s Exact Tests, P > 0.05) was recorded. Conversely, an overall significant decrease in the proportion of viable eggs was observed on susceptible populations at 5.9/4°d compared to 7.6/14°d (Table 3; Fisher’s Exact Test, P <0.05).
Differences in life traits between populations (irrespective of the experimental setting) with focus on reproductive outputs: It is worth mentioning that different patterns regarding reproductive traits were recorded for each population independent of the pH/TH conditions. In susceptible P. columella, the Aurora isolate was characterized by high values of Ro, r and λ (Fig. 5; Table 3) while a more discrete output of laid eggs per snail was recorded in Negrines for both conditions (Fig. 5B for details). Interestingly, the resistant La Coca population presented an overall lower fecundity rate than both susceptible populations (see Table 3). In addition, egg masses/snail (attempts to reproduce) in this population always peaked the latest (weeks 16–17 at low pH/TH and 19–20 at common pH/TH) with the exception of Negrines at low pH/TH that instead of a marked peak, showed a relatively steady production since early stages. In the case of resistant La Palma we observed that, regardless of the experimental condition, around 30% of their eggs needed more than one week to hatch (see NHS in Table 3). Even at 7.6/14°d, where the reproductive output of this population was higher (high values of Ro, r and λ), this delay in egg hatching remained unaltered (Fisher’s Exact Test, P > 0.05; Table 3). Additionally, even after the increase of Ro, r and λ observed at 7.6/14°d (see Table 3), the age-independent reproductive traits of La Palma, although outperforming Negrines, were lower to the susceptible population of Aurora.
Discussion
Distribution and population genetics of P. columella in Cuba: insights into its history and colonization and its relationship with Fasciola hepatica transmission
Pseudosuccinea columella is native to North America but has been widely introduced outside of its native range due to natural (e.g. aquatic birds, flooding) or human-mediated invasion7. The date of introduction of P. columella in Cuba is unknown, but it was first reported in 1858 by Poey under the name of Lymnaea francisca. It is thus plausible that a first introduction in the beginning of the 19th century allowed P. columella to settle in the western part of Cuba, the region where the oldest and abundant known documentations have been described. The close proximity of Cuba to the North American continent provides an easy opportunity for natural introductions, particularly in the westernmost region. Our results reveal several facts suggesting that successive introductions of P. columella occurred successfully in Cuba, mainly in the west but also in the central region. A sluggish invasion of this species to the eastern region of the island, mostly by more recently introduced invasive genotypes (e.g. MLGT Y), is presumed to be ongoing. Perhaps the most obvious evidences to support this assumption are: (1) the increased number of records in recent years from the east-central region and its complete absence in the easternmost region; (2) the higher allelic and multilocus genotypic richness in the west (particularly in resistant populations); (3) the existence of three major clusters of MLGTs and the presence of some MLGTs only found in the central region of Cuba, which contrasts with (4) the similar number of MLGTs shared by most susceptible P. columella populations (with five that were completely monomorphic) and the widespread distribution of MLGT (Y) in Cuba. The overall low genetic structuration and diversity found in P. columella can be explained by recent genetic bottlenecks that usually occur after introduction events, followed by the expansion of certain invasive genotypes such as MLGT Y (see Lounnas et al.7).
In particular, previous studies showed that resistant P. columella populations differ from those which are susceptible. Gutiérrez et al.23 detected different RAPD profiles using 17 different primers. Another study by Calienes et al.21 was the first to attempt to detect clear segregation when comparing polymorphic DNA of three resistant populations (La Palma, El Azufre, Babiney) and nine susceptible populations from Cuba using RAPD profiles. In the present study, we found that the genetic structure of the three resistant populations analysed (El Azufre, La Coca and Babiney) is significantly different in terms of presence and amount of MLGTs from susceptible populations, with higher genotypic diversity. The clear segregation into different susceptible and resistant MLGT associated clusters observed here, support the idea of an early, different and detached history from susceptible P. columella populations. In contrast to highly susceptible populations, resistant MLGTs from western and central populations cluster separately from each other, and it is thus likely that they originated after different introduction events. However, we should note that we have only tested three out of the six known resistant populations, and thus the three others remain unexplored.
Concerning parasite transmission, the observed distribution pattern (range and number of populations) in P. columella compared to the other lymnaeid G. cubensis (over 100 recorded populations all over the Cuban archipelago; see15) supports the presumed secondary role for this species as an intermediate host of F. hepatica in Cuba. A recent global-scale genetic study on P. columella considered one of our recorded MLGT (T) as highly invasive after it was detected in Colombia, Peru, Venezuela, South Africa and the Indian Ocean7. Surprisingly we only found this MLGT in two eastern populations in Cuba (Río Central and Matadero Aves) suggesting that it may be a recent introduction and may pose a risk of further spread in Cuba. Additionally, MLGT Y which is only found in monomorphic populations, is most likely the result of a recent introduction and may in fact present itself as ecologically adaptable (found in 15 out of 20 analysed populations) and highly compatible with F. hepatica. For instance, the five individuals found naturally-infected with F. hepatica in Cuba correspond to this specific MLGT (two individuals from IPA and three from Pilón). From this we could expect that if populations with a tendency to become infected matched those that present a high ecological plasticity, a highly favourable scenario for F. hepatica transmission in Cuba would result, since it would increase the probability for the parasite to find a suitable host. However, this hypothesis would benefit from the identification of more infected individuals that share the same GTML. In any case, the lack of genetic diversity has already been recognised as an advantage for parasites to proliferate in a given host population41, and overall, susceptible populations of P. columella showed no significant differentiation (low FST, P > 0.05).
In the present study, the low allelic richness per population as well as the strong deviations from panmixia suggest a high self-fertilization rate in this species, as also demonstrated by Lounnas et al.7. Other lymnaeid snails such as G. truncatula42 and Omphiscola glabra43 also prefer self-fertilization as a reproductive mode. Similarly, other studies observed the same low allelic richness pattern in P. columella that we found27. However, here, three populations of P. columella (IPA, Río Central and Río Arimao) showed lower self-fertilization rates than those previously reported. Several studies indicated that cross-fertilization may represent a selective advantage when populations are under severe parasitic pressures44,45. Conversely, self-fertilization can be selected in stochastic environments46 securing reproduction even if only one individual survives the harsh conditions. Thus, we should expect lower self-fertilization rates in stable habitats or endemic areas for parasite transmission. The IPA site matches both criteria since it was previously suggested as a fasciolosis transmission site by Gutiérrez et al.47, and consists of a permanent pond used to stock water. In fact, the two infected individuals belonged to this population. The other two sites (Rio Central and Rio Arimao) are recognised as fasciolosis areas as high bovine prevalence are reported48.
While the establishment of resistant populations decreases the chances of transmission of the liver fluke, the observed distribution pattern of such phenotype even with a high genetic diversity and an older presence of the resistant population in Cuba suggests that there is an ecological cost for resistance. The latter was previously proposed by Gutiérrez et al. after performing experimental history traits following F. hepatica infection24 and under competition25. Thus, a deeper look into the ecological patterns associated with the occurrence of this phenotype in nature is necessary in the pursuit of using this phenomenon to develop novel control strategies for F. hepatica transmission.
Ecological patterns associated with resistant and susceptible P. columella from Cuba: insights into the cost of resistance
Lymnaeid snails are known to occur worldwide in an extensive range of habitat types1. In Cuba, it has been previously shown that P. columella does not exhibit particular preferences regarding natural or transformed habitats and contrasts with its relative G. cubensis, commonly established in anthropic sites15. Pseudosuccinea columella is highly aquatic and consequently could be more severely affected by the physical and chemical factors of the water, a fact that could be linked to its overall preference for closed freshwater systems and, in particular, to habitats like permanent ponds as recorded herein. A previous study by Gutiérrez49 in Pinar del Río, Cuba, showed a positive correlation between the abundances of P. columella, the pH and the concentrations of nitrites. Here, we reveal a different effect when the overall pattern of occurrence of resistant and susceptible individuals and the ecological dynamics of those living in sympatry, are considered separately. As presented here, the conditions for occurrence of susceptible individuals in terms of pH, TH and the concentration of nitrites and phosphates are similar to those reported by Perera36 elsewhere in Cuba. However, resistant snails present a marked association to lower values of water pH and TH and a tendency to colonize sites with low species richness. This result could be explained by two hypotheses:
(1) One hypothesis could be that these conditions are essential for the survival of resistant snails in the field (i.e. resistant snails could not tolerate other conditions), suggesting that they have a different ecology than susceptible snails. Such marked ecological segregation might lead us to think in a strong differentiation between phenotypes that, over time, would result in speciation. However, we have successfully kept resistant P. columella in our laboratory for more than 20 years at pH/TH conditions ranging between 7–8/12–18°d. In addition, invasive F. hepatica-susceptible P. columella populations have been reported in France at sites characterized by acidic soils and soft waters50,51. Both facts suggest that, as a single species, both P. columella phenotypes can tolerate pH and TH variations rather than be differentially-restricted to specific values of these parameters. Our experimental life-history traits also support that since P. columella, regardless of the phenotype, can survive within the same range of pH/TH conditions (5.9–7.6/4–12°d). However, while the species seems to be better suited to 7.6/12°d conditions, an overall higher performance (i.e. higher tolerance) at low pH/TH conditions, was observed for resistant individuals compared to susceptible populations, in terms of self-maintenance (survival and life expectancy) and reproductive (egg viability) traits.
(2) Thus, a second hypothesis could be that low pH and TH are much less suitable for other species (with narrower tolerance levels) and that these low values directly reduce competition. In this sense, our results demonstrate that such conditions negatively affect some demographic traits of P. columella in favour of resistant phenotypes. It is therefore difficult to provide an explanation for how resistant populations are maintained in ‘neutral’ (where common pH/TH levels are present) sites unless colonisation events by competitor snails are rare and that resistant individuals were the only ones to settle. This scenario was observed within our field results since the colonization of Negrines by resistant snails coincided with a decrease of pH and TH, and lower abundances of susceptible individuals (Fig. 4). This hypothesis can also be supported by the observed pattern of low richness of snail species in slightly acidic/soft water-sites where resistant populations are found. In a colonisation event of suitable sites by susceptible P. columella a replacement of the resistant snails might be expected. In fact, under experimental conditions, Gutiérrez et al.25 reported that resistant P. columella are less competitive than susceptible snails in terms of reproductive traits (reduced Ro when raised in the presence of susceptible snails). The latter contrasted with an increase in shell growth and Ro on susceptible P. columella when reared in competition with resistant individuals25. To summarise, the restricted distribution and the overall negative correlation of resistant P. columella and species diversity may be interpreted as an ecological cost of resistance, explained by a less competitive potential of resistant snails.
Assessing the competitive potential of resistant isolates in relation to susceptible P. columella and other freshwater snail species could be experimentally challenging and a demonstration of a definitive cost of resistance will require rearing in competition with one another, both P. columella phenotypes in the presence and absence of the parasite. However, the present study takes an important step forward in advancing our understanding of the suggested cost of resistance of P. columella from previous laboratory studies24,25, by recording a natural pattern and integrating field data and new experimental results into a plausible hypothesis. In this sense, while we cannot assure that susceptible populations outperform resistant isolates, evidence of possible trade-offs against reproduction in resistant populations were also found here (La Coca: lower fecundity rates compared to both susceptible isolates, late reproductive peaks); La Palma: delay of egg hatching regardless of the pH/TH condition, lower Ro compared to Aurora snails). Trade-offs are expected to occur from selecting advantageous features (e.g. resistance to parasite, ecological tolerance) and thus contribute to their low occurrence in the wild. In this sense, a previous study similarly observed reproductive constraints for La Palma (resistant) snails compared to other susceptible P. columella populations, particularly in terms of lower Ro24. Even considering the discrete performance of Negrines, the limited reproductive output whether in quantity (low fecundity rates) or in time (late peak of egg production or delay of egg hatching) observed here in resistant isolates, may negatively affect their success in colonizing new and highly suitable habitats. While several factors could account for the preclude of a natural dispersion of this phenotype in Cuba where the most common conditions of Cuban freshwater bodies include pH and TH ranging from 7–8 and 12–18°d36, identifying potential trade-offs associated with this phenotype might also bring forward some understanding of all possible outcomes of the suggested cost of resistance.
Concluding Remarks
The introductions of vectors and intermediate hosts are significant issues because of the epidemiological risks they pose. In fact, recently introduced populations suffer strong genetic bottlenecks that drag off former genetic diversity preventing them from acquiring resistance to local parasites41. Genetic flow could act as a force that increases this diversity but, in contrast to what may be occurring in the liver fluke populations from Cuba (high cross-fertilization and bad management of cattle that mixes the strains;52), it is unlikely to take place at effective rates in P. columella snails. However, as discussed above, populations carrying some highly invasive MLGTs are colonizing a number of available sites in Cuba, one of which (MLGT Y) was also directly related to F. hepatica transmission in the present study. Thus, it is plausible that the ability of P. columella individuals with this MLGT to invade represents a true danger regarding transmission of the liver fluke. In any case, it is of major importance to maintain an active surveillance of parasite prevalence in P. columella and of potentially new introductions of this species in different and distant regions of Cuba and the world.
On the other hand, while the spread of resistant P. columella could definitively aid in the effective control of F. hepatica transmission, our results suggest that a resistance-related cost exists, as previously discussed by Gutiérrez et al.24,25. It is, thus, highly improbable that genotypes with invading abilities also correspond to those resistant to F. hepatica. The present work is a step forward towards the rational application of P. columella resistance as a potential variant to tackle parasite transmission. Our results provide insights into the ecological cost of resistance and the patterns associated with its occurrence in nature that could be used for planning a human-mediated introduction of resistant snails in particular high-risk transmission foci. Other strategies to incorporate resistant features into susceptible populations by out-crossing could be challenging if the high self-fertilization rates of P. columella showed herein and elsewhere7 are considered. On the other hand, other approaches like genetic manipulation, the controlled selection of resistant features or induction of resistance-mediated mechanisms in wild vector populations might turn out to be feasible. However, for any of these approaches to be applied, a deeper and wider comprehension of the molecular and immunological bases that mediate the natural resistance of P. columella to F. hepatica is required.
References
Hubendick, B. Recent lymnaeids, their variation, morphology, taxonomy, nomenclature and distribution. Kungliga Svenska Vetenskapsakademiens Handlingar 4, 1–223 (1951).
Strong, E., Gargomini, O., Ponder, W. & Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595, 149–166 (2008).
Bargues, M. D. & Mas-Coma, S. Reviewing lymnaeid vectors of fascioliasis by ribosomal DNA sequence analyses. J Helminthol 79, 257–267 (2005).
Vázquez, A. A. et al. Lymnaeid snails hosts of Fasciola hepatica and Fasciola gigantica (Trematoda: Digenea) worldwide: a practical review. CAB Reviews., in press (2018).
Schniebs, K. et al. A new alien species in Europe: First record of Austropeplea viridis (Quoy & Gaimard, 1833) (Mollusca, Gastropoda, Lymnaeidae) in Spain. J Conchol. 42, 357–370 (2017).
Schniebs, K., Glöer, P., Quiñonero-Salgado, S., Lopez-Soriano, J. & Hundsdoerfer, A. K. The first record of Galba cubensis (L. Pfeiffer, 1839) (Mollusca: Gastropoda: Lymnaeidae) from open fields of Europe. Folia Malacol. 26, 3–15 (2018).
Lounnas, M. et al. Self-fertilization, long-distance flash invasion and biogeography shape the population structure of Pseudosuccinea columella at the worldwide scale. Mol Ecol. 26, 887–903 (2017).
Cruz-Reyes, A. & Malek, E. Suitability of six limneaid snails for infection with Fasciola hepatica. Vet Parasitol. 24, 203–210 (1987).
Oliveira, S., Fujii, T., Filha, E. & Martins, A. Ocorrencia de Lymnaea columella Say, 1817 infectada naturalmente por Fasciola hepatica (Linnaeus, 1758), no vale do Ribeira, Sao Paulo, Brasil. Aquivos Instituto Biologia, Sao Paulo 1, 29–37 (2002).
Prepelitchi, L. et al. First report of Lymnaea columella Say, 1817 (Pulmonata: Lymnaeidae) naturally infected with Fasciola hepatica (Linnaeus, 1758) (Trematoda: Digenea) in Argentina. Mem Inst Oswaldo Cruz 7, 889–891 (2003).
Molloy, J. B. & Anderson, G. R. The distribution of Fasciola hepatica in Queensland, Australia, and the potential impact of introduced snail intermediate hosts. Vet Parasitol. 137, 62–66 (2006).
Dar, Y., Vignoles, P., Rondelaud, D. & Dreyfuss, G. Role of the lymnaeid snail Pseudosuccinea columella in the transmission of the liver fluke Fasciola hepatica in Egypt. J Helminthol. 89, 699–706 (2015).
Cowie, R. Invertebrate invasions on Pacific Islands and the replacement of unique native faunas: a synthesis of the land and freshwater snails. Biol Invasions. 3, 119–136 (2001).
Pointier, J. P. & Marquet, G. Taxonomy and distribution of freshwater mollusks of French Polynesia. Japanese J Malacol. 48, 147–160 (1990).
Vázquez, A. A., Hevia, Y. & Sánchez, J. Distribución y preferencia de hábitats de moluscos hospederos intermediarios de Fasciola hepatica en Cuba. Rev Cubana Med Trop. 61, 248–253 (2009).
Vázquez, A. A., Sánchez, J., Alba, A., Pointier, J. P. & Hurtrez-Boussés, S. Natural prevalence in Cuban populations of the lymnaeid snail Galba cubensis infected with the liver fluke Fasciola hepatica: small values do matter. Parasitol Res, https://doi.org/10.1007/s00436-015-4653-2 (2015).
Alba, A. et al. Assessment of the FasciMol-ELISA in the detection of the trematode Fasciola hepatica in field-collected Galba cubensis: a novel tool for the malacological survey of fasciolosis transmission. Parasite Vect 9, 22, https://doi.org/10.1186/s13071-13016-11303-13071. (2016).
Gutiérrez, A. et al. First report of larval stages of Fasciola hepatica in a wild population of Pseudosuccinea columella from Cuba and the Caribbean. J Helminthol. 85, 109–111 (2011).
Gutiérrez, A., Pointier, J. P., Yong, M., Sánchez, J. & Théron, A. Evidence of phenotypic differences between resistant and susceptible isolates of Pseudosuccinea columella (Gastropoda: Lymnaeidae) to Fasciola hepatica (Trematoda: Digenea) in Cuba. Parasitol Res. 90, 129–134 (2003).
Vázquez, A. A., Sánchez, J., Pointier, J. P., Théron, A. & Hurtrez-Boussès, S. Fasciola hepatica in Cuba: compatibility of different isolates with two intermediate intermediate hosts, Galba cubensis and Pseudosuccinea columella. J Helminthol. 88, 434–440 (2014).
Calienes, A. F. et al. Detection and genetic distance of resistant populations of Pseudosuccinea columella (Mollusca: Lymnaeidae) to Fasciola hepatica (Trematoda: Digenea) using RAPD markers. Acta Trop. 92, 83–87 (2004).
Alba, A. et al. Fasciola hepatica - Pseudosuccinea columella interaction: effect of increasing parasite doses, successive exposures and geographic origin on the infection outcome of naturally-resistant and susceptible snails from Cuba. Parasite & Vect accepted (2018).
Gutiérrez, A. et al. Fasciola hepatica: identification of molecular markers for resistant and susceptible Pseudosuccinea columella snail hosts. Exp Parasitol. 105, 211–218 (2003).
Gutiérrez, A., Perera, G., Yong, M. & Lin, W. Fasciola hepatica (Trematoda: Digenea): its effect on the life history traits of Pseudosuccinea columella (Gastropoda: Lymnaeidae), an uncommon interaction. Parasitol Res. 88, 535–539 (2002).
Gutiérrez, A., Yong, M., Sanchez, J. & Wong, M. & Pointier, J. P. Competition between Fossaria cubensis and two isolates (susceptible and resistant to Fasciola hepatica) of Pseudosuccinea columella under laboratory conditions. Haliotis 35, 1–11 (2005).
Alba, A. et al. A multiplex PCR for the detection of Fasciola hepatica in the intermediate snail host Galba cubensis. Vet Parasitol. 211, 195–200 (2015).
Nicot, A., Dubois, M. P., Debain, C., David, P. & Jarne, P. Characterization of 15 microsatellite loci in the pulmonate snail Pseudosuccinea columella (Mollusca, Gastropoda). Mol Ecol. 8, 1281–1284 (2008).
FSTAT, a program to estimate and test gene diversities and fixation indices. v. 2.9.3.2, http://www.unil.ch/izea/softwares/fstat.html (2001).
Rice, W. R. Analyzing Tables of Statistical Tests. Evolution. 43, 223–225 (1989).
Hartl, D. L. & Clark, A. G. Principles of population genetics. (Sinauer Associates, Inc., 1997).
Nei, M. Genetic distance between populations. Am Naturalist. 106, 283–292 (1972).
Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 583–590 (1978).
GENETIX v. 4.05.2. (Laboratoire génome, populations, interactions, CNRS UMR, 5000., 2004).
SplitsTree 4 v. 4.13.1. (www.splitstree.org., 2013).
GenClone 2.0: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. v. 2.0, http://si-wagner.ualg.pt/ccmar/maree/software.php?soft=genclon (2007).
Perera, G. Écologie des mollusques d’eau douce d’intérêt médical et vétérinaire a Cuba. Thèse de Doctorat thesis, Université de Perpignan (1996).
Simpson, E. H. Measurement of diversity. Nature. 163, 688 (1949).
ter-Braak, C. J. F. & Verdonschot, P. F. M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sci. 57, 255–289 (1995).
Sánchez, R., Perera, G. & Sánchez, J. Cultivo de Fossaria cubensis (Pfeiffer) (Pulmonata: Lymnaeidae) hospedero intermediario de Fasciola hepatica (Linnaeus) en Cuba. Rev Cubana Med Trop. 47, 71–73 (1995).
Margalef, R. Ecología. 951 (1986).
King, K. C. & Lively, C. M. Does genetic diversity limit disease spread in natural host populations? Heredity. 109, 199–203 (2012).
Meunier, C. et al. Field and experimental evidence of preferential selfing in the freshwater mollusc Lymnaea truncatula (Gastropoda, Pulmonata). Heredity. 92, 316–322 (2004).
Hurtrez-Boussès, S. et al. Comparison between shell morphology and genetic diversity in two sympatric lymnaeid snails, vectors of fasciolosis. Canadian J Zool 83, 1643–1648 (2006).
Lively, C. M. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328, 519–521 (1987).
Agrawal, A. F. & Lively, C. M. Modelling infection as a two-step process combining gene-for-gene and matching-allele genetics. Proc R Soc London B. 270, 323–334 (2002).
Baker, H. B. Support for Baker’s law – as a rule. Evolution. Evolution 21, 853–856 (1967).
Gutiérrez, A., Hernández, D. F. & Sánchez, J. Variation of snail’s abundance in two water bodies harboring strains of Pseudosuccinea columella resistant and susceptible to Fasciola hepatica miracidial infection, in Pinar del Río Province, Cuba. Mem Inst Oswaldo Cruz 100, 725–727 (2005).
Instituto de Medicina Veterinaria, I. Informe Anual del Instituto de Medicina Veterinaria., (La Habana, Cuba, 2013).
Gutiérrez, A. Intéractions Hôtes/Parasites dans le modèle Fasciola/Lymnaeidae: aspects dynamiques et génétiques. PhD thesis, Université de Perpignan, (2004).
Vignoles, P., Dreyfuss, G. & Rondelaud, D. Fasciola hepatica: comparative metacercarial productions in experimentally-infected Galba truncatula and Pseudosuccinea columella. Parasite 22, 1–6 (2015).
Vignoles, P., Dreyfuss, G. & Rondelaud, D. Les effets d’un mollusque invasif, Pseudosuccinea columella sur les limnées locales dans des habitats sur sols acides. Ann Sci Limousin 27, 39–46 (2016).
Vázquez, A. A. et al. Genetic and infective diversity of the common liver fluke Fasciola hepatica (Trematoda: Digenea) from Cuba. J Helminthol. 14, 1–7 (2016).
Perera, G., Sánchez, R., Yong, M., Ferrer, J. R. & Amador, O. Ecology of some freshwater pulmonates from Cuba. Malacol Rev. 19, 99–104 (1986).
Perera, G. Ecologie des mollusques d’eau douce d’intérêt médical et vétérinaire à Cuba. PhD thesis, Université de Perpignan, (1996).
Acknowledgements
This study is set within the framework of the “Laboratoires d’Excellences (LABEX)” TULIP (ANR‐10‐LABX‐41). Partial financial support for this investigation was provided by the subventions granted to AA by the French Embassy in Cuba and to AAV by Institut de Recherche pour le Développement (BEST grant). BG was supported by ANR JCJC INVIMORY (number ANR 13-JSV7-0009) from the French National Research Agency (ANR). The authors would like to thank Dr. Jérémie Vidal-Dupiol, for his suggestions on the experiment of the life history traits. The authors also acknowledge the valuable comments and suggestions made by the anonymous reviewers and the editor that greatly helped in improving the manuscript.
Author information
Authors and Affiliations
Contributions
A.A., AAV designed, performed and analysed the experiments and drafted the manuscript. J.S., M.L., J.P.P., S.H. and B.G. participated in design of the experiments, analysis and the reviewing process. J.S. and M.L. participated in the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alba, A., Vázquez, A.A., Sánchez, J. et al. Patterns of distribution, population genetics and ecological requirements of field-occurring resistant and susceptible Pseudosuccinea columella snails to Fasciola hepatica in Cuba. Sci Rep 9, 14359 (2019). https://doi.org/10.1038/s41598-019-50894-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50894-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.