Abstract
Plant-derived lignans have numerous biological effects including anti-tumor and anti-inflammatory activities. Screening of purified constituents of Rubia philippinensis from human glioblastoma cells resistant to TNF-related apoptosis-inducing ligand (TRAIL) has suggested that the lignan pinoresinol was a highly active TRAIL sensitizer. Here we show that treatment with nontoxic doses of pinoresinol in combination with TRAIL induced rapid apoptosis and caspase activation in many types of glioblastoma cells, but not in normal astrocytes. Analyses of apoptotic signaling events revealed that pinoresinol enhanced the formation of TRAIL-mediated death-inducing signaling complex (DISC) and complete processing of procaspase-8 within the DISC in glioblastoma cells, in which caspase-8 was inactivated. Mechanistically, pinoresinol downregulated the expression of cellular FLICE-inhibitory protein (cFLIPL) and survivin through proteasome-mediated degradation, without affecting death receptors or downstream intracellular apoptosis-related proteins. Furthermore, the sensitization of TRAIL-mediated apoptosis by pinoresinol strictly depended on the expression level of cFLIPL, which was regulated through de novo protein synthesis, rather than by NF-κB or p53 signaling. Taken together, our results indicate that pinoresinol facilitates DISC-mediated caspase-8 activation by targeting cFLIPL in an early event in apoptotic signaling, which provides a potential therapeutic module for TRAIL-based chemotherapy.
Similar content being viewed by others
Introduction
The use of TNF-related apoptosis-inducing ligand (TRAIL) in cancer therapy has long been thought as an attractive strategy because it can selectively target cancer cells without affecting the majority of normal human cells1. The anti-cancer activity of TRAIL is attributable to its ability to elicit apoptosis through binding of its functional receptors, death receptors 4 and 5 (DR4 and DR5), and subsequent association with an adaptor protein, Fas-associated death domain (FADD)2. During apoptosis, FADD recruits the initiator caspases (procaspase-8 and/or procaspase-10) for the assembly of a death-inducing signaling complex (DISC). Within the DISC, the oligomerization and cleavage of the initiator caspases are the critical upstream events for activation of either the executioner caspase-3 cascade or mitochondrial-dependent apoptotic pathway via Bid cleavage, leading to sufficient apoptosis upon TRAIL treatment, depending on the specific cell type3,4. Importantly, genetic lesions in the components of TRAIL signaling have been found in human malignant cancers, suggesting that TRAIL functions in immune surveillance against developing cancers and metastasis5,6,7. Indeed, mice null of TRAIL receptor were shown to susceptible to inflammation and tumorigenesis with apoptotic defects8. Consistent with this possibility, currently evaluated TRAIL agonistic antibodies have demonstrated a significant therapeutic efficacy in a number of preclinical studies9,10. However, therapeutic benefits in clinical trials have been rather limited due to the appearance of cancer cells that escape the cytotoxicity induced by TRAIL-targeted therapy11,12. Thus, the discovery of a therapeutic strategy module that can eradicate cancer cells without restoring resistance has been pending in the field of TRAIL-based chemotherapy.
The genus Rubia (family Rubiaceae), a perennial herb, is widely distributed worldwide. It is one of the most attractive plant resources because of its potent and wide spectrum of in vivo and in vitro biological activities, which include anti-cancer, anti-inflammatory, and anti-angiogenic effects13,14,15. In a recent phytochemical study of Rubia philippinensis Elmer, we isolated several compounds, including derivatives of anthraquinones, pentacyclic triterpenoids, cyclopeptides, and lignans16,17,18. Although several studies have reported the anti-cancer effects of Rubia species, the effects of the principle constituents of R. philippinensis on DR-mediated cell death, particularly during TRAIL sensitization, have not yet been determined. As part of our ongoing search to identify potential therapeutic approaches for sensitizing TRAIL-mediated cell death, we tested 33 compounds isolated from R. philippinensis and found that nontoxic doses of pinoresinol, a lignan, drastically sensitized cancer cells against TRAIL-induced apoptosis. Pinoresinol facilitated DISC formation to trigger a caspase-8-dependent apoptotic cascade activation in TRAIL-resistant glioblastoma cells. Moreover, our findings revealed novel evidence that the prominent sensitizing effects of pinoresinol against TRAIL-mediated apoptosis involved the downregulation of levels of cellular FLICE-inhibitory protein (cFLIPL) by a mechanism involving de novo protein synthesis.
Results
IIdentification of pinoresinol from R. philippinensis as a TRAIL sensitizer in TRAIL resistant glioma cells
We characterized a set of major compounds obtained from R. philippinenesis to identify active constituents that synergistically sensitized the cytotoxic effects of TRAIL in TRAIL-resistant glioblastoma cells (Supplementary Table S1, Supplementary Figs 1–33). Treatment of LN428 cells with 50–200 ng/mL TRAIL alone induced a limited number of cell deaths (<5%) over 24 h (data not shown). In the screening assay, LN428 cells were sequentially treated with the purified compounds and 50 ng/mL TRAIL, followed by an ATP-based cell viability assay. In parallel, we tested the cytotoxicty of each compound on LN428 cells as single agents. Of the compounds screened, the lignin pinoresinol was a potent sensitizer of TRAIL-mediated cytotoxicity (Fig. 1A,B). It eliminated the survival of LN428 cells but only in the presence of TRAIL; it had only marginal growth inhibitory effects as a single agent (Fig. 1C). Consistently, no cell death was observed when cells were treated with pinoresinol alone at concentrations up to 1 μM over 24 h. By contrast, combined treatment with the same concentrations of pinoresinol and TRAIL resulted in a drastic increase in cell death (Fig. 1D), thus confirming that this combination resulted in extensive cell death at low concentrations (0.2–1 μM) of pinoresinol.
Identification of pinoresinol as a potent TRAIL sensitizer from the constituents of R. philippinensis. (A) Screening of the principle constituents of R. philippinensis for potential cytotoxic enhancer in TRAIL resistant glioblastoma cells. LN428 cells were pretreated with a series of constituents (5 μM) for 30 min, followed by 50 ng/ml TRAIL for 24 h. Cell death was quantified by using Cell Titer-glo Luminescent cell viability assay kit as described as Methods. (B) Chemical structure of pinoresinol (PINO). (C,D) LN428 cells were pretreated with indicated concentrations of PINO, followed by 50 ng/ml TRAIL. After 24 h, cells were fixed, stained and photographed. (C) Cell death was quantified as in A. (D) Data were normalized to the rate of spontaneous cell death occurring in untreated cells. Data represents the mean ± SE of three independent experiments. Statistical difference (*p < 0.05) compared with the PINO only-treated group are indicated.
Sensitization of TRAIL-induced killing by pinoresinol is associated with a caspase-8-dependent apoptotic cascade in glioma cells
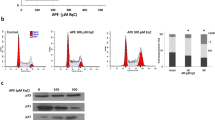
Next, to validate the above screening results, we performed kinetic experiments to evaluate the synergistic induction of cell death using a nontoxic concentration (0.5 μM) of pinoresinol. TRAIL-mediated cytotoxicity began to appear from 9 h after pinoresinol co-treatment, and rapidly increased up to 24 h (Fig. 2A). In addition, pinoresinol had a similar synergistic efficacy against TRAIL-mediated cytotoxicity in three other glioblastoma cell lines (U87MG, LNZ308, and U251). By contrast, normal astrocytes were not sensitive to TRAIL-induced killing when treated with pinoresinol (Fig. 2B). These results suggest that the TRAIL-sensitizing effects of pinoresinol might apply to a wide spectrum of glioblastoma cell lines that could be limited to cancer cells. Although TRAIL-induced cancer cell death was mainly apoptotic, it might also induce non-apoptotic cell death via non-canonical TRAIL signaling depending on the cellular context19,20,21. Thus, next, we examined whether cell death caused by pinoresinol plus TRAIL was associated with caspase-dependent apoptosis. As expected, pretreatment of LN428 cells with pancaspase and the irreversible caspase 8-inhibitors z-VAD-FMK and z-IETD-fmk completely abrogated the cytotoxicity induced by pinoresinol plus TRAIL (Fig. 2C,D). However, necrostain-1, an inhibitor of programmed necrosis, failed to protect against cell death, indicating that pinoresinol predominantly triggered apoptotic, rather than necrotic, cell death. To confirm the mode of TRAIL-mediated cell death sensitized by pinoresinol, cell death was analyzed by Annexin V and propidium iodide (PI) staining followed by flow cytometry. Consistently, treatment of pinoresinol plus TRAIL drastically increased the population of an early phase of apoptosis (Annexin V+), whereas very few cells were stained exclusively with PI, and such increased apoptotic population was prevented by co-treatment with z-VAD-FMK (Fig. 2E). To get more insights into the mechanisms underlying TRAIL-sensitized apoptosis, we sequentially analyzed the activation of processes of caspase signaling cascade, including those of initiator caspase (caspase-8) and as executor caspases (caspase-3 or −9) and the resultant PARP cleavage. In the kinetic analysis, we found that the treatment of pinoresinol plus TRAIL caused an activation of caspase-8 and −3, and PARP cleavage from 6 h onwards (Fig. 2F). Furthermore, pretreatment with z-IETD-fmk completely inhibited the activation of caspase cascade induced by pinoresinol plus TRAIL treatment. These results clearly indicate that casapse-8 activation is essential in the sensitization of pinoresinol-induced apoptosis in TRAIL-resistant glioblastoma cells.
Sensitization of TRAIL-induced apoptosis by non-toxic dose of pinoresinol requires caspase-8 activation in various glioblastoma cells, but not in normal astrocytes. (A) LN428 cells were treated with PINO (0.5 μM), TRAIL (50 ng/ml) and TRAIL (50 ng/ml) plus PINO (0.5 μM) for the indicated times. (B) TRAIL resistant glioblastoma cells (U87MG, LNZ308, LN428, and U251) and normal primary astrocytes were treated with PINO, TRAIL and TRAIL plus PINO for 24 h. Cell death was quantified as in Fig. 1A. Data were normalized to the rate of spontaneous cell death occurring in untreated cells. Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with the TRAIL only-treated group. (C,D) LN428 cells were treated with PINO, TRAIL and TRAIL plus PINO for 24 h in the absence or presence of caspase or necroptosis inhibitor z-VAD-fmk (20 μM)/z-IETD-fmk (50 μM) or Nec-1 (30 μM). (C) Cell death was quantified as in A. Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with the PINO/TRAIL-treated group. (D) Cells were visualized using an inverted microscope. (E) LN428 cells were treated with PINO, TRAIL and TRAIL plus PINO for 24 h in the absence or presence of z-VAD-fmk. Cells were subjected to Annexin V/PI staining, and then analyzed by flow cytometry. (F) LN428 cells were treated with PINO (0.5 μM), TRAIL (50 ng/ml) and TRAIL plus PINO for indicated times. Whole cell lysates were subjected to immunoblotting with the indicated antibodies (left) and densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with the TRAIL only-treated group.
Sensitizing efficacy of pinoresinol on TRAIL-mediated apoptosis is not associated with either NF-κB or p53
Pinoresinol exhibits anti-inflammatory properties via blockade of the NF-κB pathway in several immune and cancer cells22,23,24. Given the well-established ability of NF-κB to regulate TRAIL resistance through induction of its anti-apoptotic genes25, it was hypothesized that the anti-NF-κB effects of pinoresinol might contribute to sensitization against TRAIL-induced apoptosis. Consistent with previous studies22,23,24, pretreatment of LN428 cells with pinoresinol significantly decreased the transcriptional activity of NF-κB induced by either TNF or TRAIL, while pinoresinol alone did not affect the basal level of NF-κB activity (Fig. 3A). However, unexpectedly, LN428 cells with prevention of NF-κB activation by overexpression of the IκBα super-repressor (SR-IκBα), which could not be phosphorylated due to substitutions of serine 32 and serine 36 by alanine, were found to have a similar extent of cell death after TRAIL or pinoresinol plus TRAIL treatment, although TNF-induced cell death was drastically enhanced (Fig. 3B). In addition, pretreatment with the NF-κB inhibitor TPCA failed to affect cell death upon TRAIL or pinoresinol plus TRAIL treatment (Fig. 3C). These results suggest that the TRAIL-sensitizing efficacy conferred by pinoresinol was unlikely to be a result of NF-κB inhibition. Although p53 activation plays an important role in TRAIL sensitization1,26, the involvement of p53 in pinoresinol-induced TRAIL sensitization was excluded because LN428 cells retained mutant-p5327. Consistent with this possibility, we found that pinoresinol treatment also sensitized TRAIL-induced cell death in p53 null HCT116 cells to a similar extent as it did with wild type (WT) cells, despite the remarkable differences in cytotoxicity after camptothecin treatment between these two cell types (Fig. 3D). Moreover, no detectable induction of p53 and its target p21 upon pinoresinol alone or pinoresinol plus TRAIL treatment in WT-HCT116 cells, despite the fact that the substantial amounts of p53 and p21 were induced by a DNA damaging agent, camptothecin in WT-HCT116 cells but not in p53- null HCT116 cells (Fig. 3E). Such findings thus indicate that pinoresinol’s effect on TRAIL-mediated cell death is independent of p53.
Sensitizing effect of pinoresinol against TRAIL-mediated apoptosis is not associated with either NF-κB or p53 signaling pathway. (A) LN428 cells were transfected with an NF-κB-responsive reporter plasmid (p2xNF-B-Luc) and pRSV-β-gal. After 24 h, cells were treated with PINO (0.5 μM) alone for 6 h, or pretreated with PINO (0.5 μM) for 30 min, followed by TNF (30 ng/ml) or TRAIL (50 ng/ml) for additional 6 h. The luciferase assays were performed as describe in Methods, and the activity of each sample was normalized according to β-galactosidase activity. Each column shows the mean ± SE of three independent experiments. *p < 0.05, compared with the TNF or TRAIL only-treated group. (B) LN428 cells were transfected with mock or IκBα super-repressor (SR-IκBα) plasmid. After 24 h, cells were treated with PINO, TNF, TRAIL or TRAIL plus PINO for additional 24 h. (C) LN428 cells were pretreated with IKKα/β inhibitor TPCA-1 (0.5 μM) for 30 min, followed by PINO, TRAIL or TRAIL plus PINO for additional 24 h. (D) Wild-type and p53 null HCT116 cells were treated with PINO, TRAIL plus PINO and campthothecin (Cpt, 100 μM) for 24 h. (B–D) Cell death was quantified as in Fig. 1A. Data were normalized to the rate of spontaneous cell death occurring in untreated cells. Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with the mock-transfected group. #p < 0.05, compared with the wild-type HCT116 cells. (E) Wild-type and p53 null HCT116 cells were treated with PINO and campthothecin for 24 h. Whole cell lysates were subjected to immunoblotting with the indicated antibodies (left) and densitometry analysis of the bands from the relevant proteins was performed (right). *p < 0.05, compared with the none-treated group.
Pinoresinol accelerates DISC formation by down-regulating cFLIPL expression
To characterize the underlying mechanism involved in pinoresinol-induced sensitization of glioma cells against TRAIL-mediated apoptosis, we determined the expression levels of several apoptosis-related proteins in the death receptor signaling pathway after exposure to pinoresinol for different times in LN428 cells. Notably, the protein expression levels of the long isoform of cellular FLIP (cFLIPL) and survivin were drastically decreased in a time-dependent manner in LN428 cells with pinoresinol (Fig. 4A, panels 6,7). Reductions of cFLIPL and survivin were accompanied by increased levels of cleaved-RIP1 and truncated-Bid (t-Bid) in cells upon pinoresinol plus TRAIL treatment (Fig. 4A, panels 4,11). Furthermore, we observed that pinoresinol was also able to down-regulate cFLIPS expression with a similar kinetics with cFLIPL in HT-29 cells, though the expression level of cFLIPS was extremely low or nondetectable in glioblastoma cells including LN428 cells and LNZ308 cells (Fig. 4B). These results suggest that expression of cFLIP isoforms is highly cell type-specific and pinoresinol-induced cFLIP downregulation, especially in cFLIPL, may play a predominant role in sensitizing TRAIL-mediated apoptosis in glioblastoma cells. By contrast, the protein levels of signaling components of TRAIL including death receptors, adaptor proteins, other inhibitor of apoptosis proteins, and Bcl-2 family proteins were not affected or only modestly affected in cells after pinoresinol treatment. The expression of cFLIPL and survivin proteins are regulated by either transcriptional or post-translational modifications such as ubiquitin-mediated proteasomal degradation28,29,30,31. In contrast to the observed down-regulation of cFLIPL and survivin protein levels, treatment with pinoresinol did not change their mRNA levels at any of the time points examined (Fig. 4C). However, pretreatment with the proteasome inhibitor MG132 sufficiently prevented the down-regulation of cFLIPL and survivin expression by pinoresinol (Fig. 4D), suggesting that pinoresinol might reduce the protein levels of cFLIPL and survivin via proteasome-mediated degradation rather than through transcriptional control.
Pinoresinol down-regulates the expression of cFLIPL and survivin at the post-translational levels via proteasome-mediated degradation. (A) LN428 cells were treated with PINO (0.5 μM) in the absence or presence of TRAIL (50 ng/ml) for the indicated times. (B) LN428, LNZ308 and HT29 cells were treated with PINO (0.5 μM) for the indicated times. Whole cell extracts were subjected to immunoblotting with the indicated antibodies (left). Densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with none-treated group. (C) Total RNA was prepared at the indicated times after PINO (0.5 μM) treatment in LN428 cells, and RT-PCR was performed with the primers specific to human cFLIPL, survivin and GAPDH. After PCR amplification, the products were separated by agarose gel electrophoresis and visualized using ethidium bromide staining. (D) LN428 cells were treated with PINO (0.5 μM) for the indicated times in the absence or presence of proteasome inhibitor MG-132 (10 μM). Whole cell extracts were subjected to immunoblotting with the indicated antibodies (left), and densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with none-treated group. #p < 0.05, compared with PINO only-treated group.
Next, we examined whether downregulation of cFLIPL and survivin by pinoresinol affected the facilitated TRAIL-mediated cytotoxicity by overexpressing these genes in LN428 cells. Consistent with its critical function to antagonize caspase-8, overexpression of WT cFLIPL resulted in a significant decrease in cell death and caspase cascade activation induced by pinoresinol plus TRAIL treatment (Fig. 5A,B). We also found that a cFLIPL mutant (cFLIPL-K167/195R containing modified major ubiquitin acceptor sites) more profoundly abrogated pinoresinol plus TRAIL-induced caspase-dependent apoptosis, compared to that of WT cFLIPL. However, the cell death induced by pinoresinol plus TRAIL was not affected by the overexpression of survivin, suggesting that downregulation of upstream anti-apoptotic protein anti-cFLIPL, rather than survivin, contributed to an important mechanism involving pinoresinol sensitization of TRAIL-induced cell death.
Pinoresinol-mediated down-regulation of cFLIPL accelerates TRAIL DISC formation. (A,B) LN428 cells were transfected with plasmids expressing flag-tagged FLIPL (wild type; WT or K167/195 R) and WT-survivn plasmids for 24 h, and followed by TRAIL (50 ng/ml) plus PINO (1 μM) for 12 h. (A) Cell death was quantified as in Fig. 1A. Data were normalized to the rate of spontaneous cell death occurring in untreated cells. Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with the mock-transfected group. #p < 0.05, compared with the wild-type FLIPL-transfected group. (B) Whole cell extracts were subjected to immunoblotting with the indicated antibodies (left), and densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with mock-transfected group. (C) LN428 cells were treated with TRAIL (50 ng/ml) in the absence or presence of PINO (0.5 μM) for the indicated times. Cell extracts from each sample were subjected to immunoprecipitation with anti-caspase-8 antibody. Immunoprecipitants were analyzed by immunoblotting with indicated antibodies. A total of 1% of the cell extract volume from each sample was used as input control (left). Densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with none-treated group.
The cFLIP isoforms compete directly with procaspase-8 for binding to FADD in a TRAIL-dependent fashion, thus inhibiting procaspase-8/-10 recruitment to form the DISC31,32. It is therefore possible that downregulation of cFLIPL by pinoresinol might directly affect the formation of the DISC, an early signaling event in TRAIL-induced apoptosis. An immunoprecipitation assay using an anti-caspase-8 antibody revealed that treatment of LN428 cells with TRAIL in the absence of pinoresinol led to efficient recruitment of cleaved cFLIPL to the isolated DISC, whereas DISC-bound FADD and caspase-8/-10 were only weakly detected (Fig. 5C, left panel, rows 1–3). These results suggest that in LN428 cells, the TRAIL-induced recruitment of cFLIPL into the DISC was an important step before caspase-8 activation to exhibit resistance against TRAIL cytotoxicity. However, pretreatment with pinoresinol promoted an increase in TRAIL-mediated DISC formation and procaspase-8/10 processing, concomitant with decreasing amounts of DISC-bound cFLIPL (Fig. 5C, left panel, rows 4–6). More importantly, in pinoresinol-pretreated cells, activation of procaspase-8 processing within the DISC following TRAIL treatment proceeded to completion, as shown by the appearance of the active p18 subunit of mature caspase-8. Taken together, these results strongly suggest that a reduced amount of DISC-bound cFLIPL played a major role in TRAIL sensitization by pinoresinol.
Pinoresinol-mediated down-regulation of cFLIPL is mediated via de novo protein synthesis inhibition
Next, we identified the underlying mechanism by which pinoresinol directly controls ubiquitin-mediated degradation of cFLIPL. As expected, co-immunoprecipitation analyses showed that treatment of cells with MG132 led to an increase in polyubiquitinated cFLIPL with concomitant enhanced protein levels (Fig. 6A). However, we unexpectedly detected lower levels of ubiquitinated cFLIPL in cells treated with pinoresinol plus MG132, compared to cells exposed to MG132 cells, indicating that the accelerated proteasomal degradation of cFLIPL by pinoresinol was not achieved through direct activation of the ubiquitination process. Given that cFLIPL and survivin are unstable proteins with a rapid turnover29,33, we addressed whether the reduced protein levels by pinoresinol were associated with de novo protein synthesis of cFLIPL and survivin. Treatment with either pinoresinol or cycloheximide (CHX) did not influence the cellular amounts of DRs and adaptor proteins, including DR4/5, FADD, RIP1, and TRAF2 (Fig. 6B). By contrast, pinoresinol was able to down-regulate the expression levels of cFLIPL and survivin with similar kinetics to that of CHX. Furthermore, the down-regulating effect by either pinoresinol or CHX was not accelerated by the combined treatment of pinoresinol and CHX. These results suggest that in a similar manner to CHX, pinoresinol inhibited de novo synthesis of proteins with a rapid turnover cFLIP and survivin.
Pinoresinol-induced down-regulation of cFLIPL is mediated via de novo protein synthesis inhibition. (A) LN428 cells were co-transfected with plasmids expressing flag-tagged cFLIPL and HA-tagged ubiquitin plasmids for 24 h. The cells were then treated with PINO (0.5 μM) for 4 h in the absence or presence of MG-132 (10 μM). Cell extracts from each sample were subjected to immunoprecipitation with anti-flag antibody followed by immunoblotting with anti-HA antibody for detection of ubiquitinated cFLIPL. A total of 1% of the cell extract volume from each sample was used as input control (left). Densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with none-treated group. (B) LN428 cells were treated with PINO (0.5 μM), cycloheximide (CHX, 10 μM) and PINO (0.5 μM) plus CHX (10 μM) for indicated times. Whole cell extracts were subjected to immunoblotting with the indicated antibodies (left). Densitometry analysis of the bands from the relevant proteins was performed (right). Data represents the mean ± SE of three independent experiments. *p < 0.05, compared with none-treated group. (C) The in vitro coupled transcription and translation assay was performed using 1-Step Human Coupled IVT Kit as described in Methods. pCFE-GFP plasmid as template in HeLa cell lysate supplemented with indicated concentrations of CHX and PINO. (D) In vitro translation inhibition assay with increased amount of PINO. In vitro transcribed mRNA encoding EGFP was purified as described in Methods. 3 µg of EGFP mRNA each in HeLa cell lysate was incubated for 6 h with indicated concentrations of CHX and PINO. The in vitro translation yield of EGFP protein was monitored by immunoblotting with anti-Turbo GFP or anti-GFP antibody, respectively and quantified using a densitometry from the relevant proteins (top). Percent inhibition of protein synthesis was calculated by dividing the band intensities from each concentration of PINO over control DMSO-treated samples (bottom).
To directly assess whether the down-regulation of protein expression by pinoresinol is due to the impairment of the general translational machinery, we conducted a cell-free in vitro transcription and translation assay. As shown in Fig. 6C, pinoresinol suppressed the production of green fluorescent protein (GFP), similar to a well-known protein translation inhibitor CHX, in a dosage dependent manner. To rule out the possibility that pinoresinol-dependent suppression of protein synthesis is caused by hampering transcriptional processes, subsequent in vitro translation response was assessed by using in vitro synthesized EGFP mRNA. Consistently, incubation with 1 μM pinoresinol completely interfered the EGFP protein production with a similar efficacy of 10 μM CHX (Fig. 6D). Taken together, these data indicate that pinoresinol directly interferes a de novo protein synthesis without affecting transcriptional machinery.
Discussion
Glioblastoma is a heterogenous group of invasive malignant primary brain tumors with high mortality34. Although all populations of cancer cells contribute in their own way to drive tumor growth, the molecular changes disrupting the apoptotic pathway are considered a pathological hallmark of glioblastoma. Emerging evidence suggests that cells within the glioblastoma exhibit abnormalities of the cell death pathway such as overexpression of antiapoptotic proteins or silencing of key death effectors35,36. Importantly, genomic analyses of human glioblastomas have shown that caspase-8, an essential component of the DISC, is frequently inactivated by either gene mutations or promotor methylation7,37,38,39. Thus, resistance to DR-mediated cytotoxicty in glioblastoma cells might occur as a step of DISC assembly. Consistent with this possibility, we found that in a series of glioblastoma cells including LN428 cells treated with TRAIL, complete activation of caspase-8 and functional DISC formation were blocked. However, pinoresinol treatment resulted in cells resistant to apoptosis with an increased recruitment of both procaspase-8 and FADD to the TRAIL DISC, and complete activation of caspase-8. It is therefore possible that pinoresinol-induced TRAIL sensitization was conducted at the level of the DISC/caspase-8 axis.
Accumulating evidence presently suggests that cFLIP is a key player in the DR-mediated apoptotic pathway that retains the sublethal activation of caspase-8 at the DISC31,32,40. Consequently, elevated levels of cFLIP in tumor tissues from patients with a variety of cancers including lung cancer, Burkitt’s lymphoma, cervical caricinoma and colorectal carcinoma are correlated with poor clinical outcomes41,42,43,44, implicating the existence of a strong association between suppression of DISC-mediated apoptosis by cFLIP and tumorigenesis. An important finding from the present study is that protein levels of cFLIPL were significantly reduced by pinoresinol treatment, and the ectopic overexpression of cFLIPL, significantly suppressed caspase-8 activation and reduced the susceptibility to cell death caused by pinoresinol/TRAIL treatment in LN428 cells. Changes in the expression levels of cFLIPL by pinoresinol therefore appear to be responsible for TRAIL sensitization in glioma cells. In this regard, it is important to determine the potential mechanism involved in the pinoresinol-induced downregulation of cFLIPL expression. It has previously been reported that cFLIPL expression is tightly regulated at the transcriptional level by a number of stimuli, including NF-κB transcription factor45,46, mitogen-activated protein kinase47 and protein kinase B/Akt48,49. Previous pharmacological and biochemical studies have reported that pinoresinol exhibits anti-inflammatory and anti-cancer effects, in part through the inhibition of NF-κB22,23,24. It is therefore possible that pinoresinol suppresses cFLIPL expression through NF-κB inhibition. In accordance with previous observations, we found that pinoresinol potently inhibited the NF-κB activity in LN428 cells in response to TNF and TRAIL. However, we did not observe a decrease in cFLIPL mRNA expression levels in cells treated with pinoresinol concentrations of 0.5–1 μM, which caused cFLIPL depletion and maximal TRAIL sensitization. Furthermore, the expressions of a subset of NF-κB-inducible genes, including TRAF2, cIAP1/2, XIAP, and Bcl-XL, were unaffected by pinoresinol treatment. These findings raise the possibility that down-regulatory effects of pinoresinol on cFLIPL expression are not associated with transcriptional regulation of NF-κB. These discrepancies of transcriptional regulation between cFLIPL expression and NF-κB may have resulted from different concentrations of pinoresinol. Indeed, pinoresinol must be used at a relatively high concentration (≥10 μM) to exhibit anti-NF-κB activity in several types of cells22,23,50, suggesting that other factors may be involved in the effects of cFLIPL depletion by low concentrations of pinoresinol.
On the other hand, the expression levels of cFLIPL were regulated by the ubiquitin–proteasomal pathway with a short half-life29,51. In this respect, it is of particular interest that, in LN428 cells, pinoresinol induces proteasomal degradation of cFLIPL or ectopic expression of the cFLIPL mutant (cFLIPL-K167/195 R), which significantly abolishes sensitization by pinoresinol to TRAIL-induced cytotoxicity. Furthermore, the results of an in vitro translational assay showed that pinoresinol directly inhibited de novo protein synthesis, which has similar efficiency with the well-known protein synthesis inhibitor CHX. These results raise the possibility that pinoresinol disrupts de novo protein synthesis, particularly for fast turnover proteins such as cFLIPL, leading to proteasomal degradation with decreased stability. Nevertheless, it is currently unclear how pinoresinol inhibits de novo protein synthesis. Further studies to identify the ribosomal proteins that interact with pinoresinol in the translational machinery will be critical for a complete understanding of the mechanism of action, and for the development of novel TRAIL-based chemotherapies. Earlier, it has been reported that survivin plays an essential role in cell cycle progression52. In this study, we found that pinoresinol induced a G2/M arrest with an increase in the G2 population (Supplementary Fig. 34), suggesting that down regulation of survivin by pinoresinol might be relevant in limiting cell division, especially in G2-M phase rather than apoptosis. In addition, it has recently been reported that pinoresinol suppresses the efflux of chemotherapeutic drugs outside by interacting with P-glycoprotein (P-gp) encoded by multidrug resistant-1 (MDR-1) gene53,54. Since P-gp efflux function was shown to contribute to TRAIL resistance via controlling the endogenous level of TRAIL in certain types of cancer cells55,56, such an anti-Pgp activity of pinoresinol might constitute another mechanism in enhancing TRAIL efficacy in TRAIL-resistant cancers including glioblastoma. Thus, future bioavailability study of TRAIL and pinoresinol using in vivo preclinical models are required to further validate TRAIL and pinoresinol-based therapeutic development for glioblastoma.
Methods
Isolation of pinoresinol
Pinoresinol was isolated from our chemical study on Rubia philippinensis, as described previously17. Briefly, a methylene chloride (CH2Cl2)-soluble fraction (50 g) was prepared from solvent extraction of R. philippinensis extract (150 g) using CH2Cl2 and water. Vacuum liquid chromatography of CH2Cl2-soluble fraction on silica gel column (20 × 20 cm) eluting with n-hexanes-EtOAc (20:1, 10:1, 5:1, 3:1, 2:1) and CHCl3-MeOH (8:1) resulted in the preparation of six column fractions (D-1 → D-6). Fraction D-6 (10 g) was separated by reversed-phase C18 column chromatography [column: SNAP cartridge KP-C18-HS (400 g), mobile phase: MeOH-H2O (10:90 → 100:0, 7 L)] and 11 subfractions (D-6-1 → D-6-11) were obtained. Pinoresinol (tR 34.0 min, 40 mg) was purified from D-6-4 (360 mg) by preparative HPLC [column: Phenomenex C18 (250 × 21.2 mm), mobile phase: MeOH-H2O (50:50, 4 mL/min)]. Pinoresinol: brownish amorphous powder; ESIMS m/z 359.2 [M + H]+, 381.1 [M + Na]+, 357.1 [M-H]−, 393.1 [M + Cl]−;1H NMR (300 MHz, methanol-d4): 3.13 (2H, m, H-8, H-8′), 3.80 (2H, Ha-9, Ha-9′), 3.85 (6H, s, OCH3-3, OCH3-3′), 4.22 (2H, Hb-9, Hb-9′), 4.70 (2H, d, J = 2.7, H-7, H-7′), 6.77 (2H, overlapped, H-5, H-5′), 6.79 (2H, overlapped, H-6, H-6′), 6.95 (2H, H-2, H-2′); 13C NMR (75 MHz, methanol-d4): 133.8 (C-1, C-1′), 111.0 (C-2, C-2′), 149.1 (C-3, C-3′), 147.3 (C-4, C-4′), 116.1 (C-5, C-5′), 120.0 (C-6, C-6′), 87.5 (C-7, C-7′), 55.3 (C-8, C-8′), 72.6 (C-9, C-9′), 56.4 (OCH3-3, OCH3-3′).
Structure determination of pinoresinol
The molecular formula of purified compound was deduced as C20H22O6 based on the ESIMS protonated ion at m/z 359.2, the sodium-adduct ion at m/z 381.1, the deprotonated ion at m/z 357.1, and the chloride-adduct ion at m/z 393.1 (calcd. for C20H22O6, m/z 358.1). The 1H NMR data displayed a pair of symmetric benzene ring system at δH 6.77 (2H, overlapped, H-5, H-5′), 6.79 (2H, overlapped, H-6, H-6′), 6.95 (2H, H-2, H-2′). The proton signals at δH 3.13 (2H, m, H-8, H-8′), 3.80 (2H, Ha-9, Ha-9′), 4.22 (2H, Hb-9, Hb-9′), 4.70 (2 H, d, J = 2.7, H-7, H-7′) indicated two symmetrical tetrahydrofuran substructure of lignan. In the 13C NMR spectroscopic data, six benzene signals at δC 111.0~149.1, two oxygenated carbons at δC 87.5 and 72.6, one methine at δC 55.3, and a methoxy group at δC 56.4 supported the symmetric structure of tetrahydrofuran-type lignan. The chemical structure was identified as pinoresinol by NMR spectroscopic and LC-MS data analyses57.
Antibodies and chemicals
The antibodies and chemicals were obtained from the following resources; anti-PARP (#556362), anti-XIAP (#610716), anti-FADD (#610399), anti-RIP1 (#610459), anti-p53 (#554147) and anti-p21 (#556430) antibodies (BD Biosciences, San Diego, CA, USA); anti-caspase-3 (#9662), anti-caspase-8 (#9746), anti-caspase-9 (#9508) and anti-Bid (#2002) antibodies (Cell signalling Technology, Beverly, MA, USA); anti-caspase-10 (M059-3) antibody (MBL, WOBURN, MA, USA); anti-Bcl-XS/L (sc-271121), anti-survivin (sc-17779), anti-TRAF2 (sc-876), anti-GFP (sc-9996) and anti-HA (sc-805) antibodies (Santa Cruz, CA, USA); anti-c-FLIP (ALX-804-961) antibody (Enzo Life Sciences, Farmingdale, NY, USA); anti-cIAP1/2 (#07-759) antibody (Upstate Biotech, Waltham, MA, USA); anti-DR5 (#ab181846) antibody (Abcam, Cambridge, UK); anti-DR4 (NB100-56528) antibody (Novus, Centennial, CO, USA); anti-TurboGFP (PA5-22688) antibody (Thermo scientific, Waltham, Massachusetts, USA); anti-actin (A2066), anti-flag (F3165, 1:2,000 dilution) antibodies (Sigma-Aldrich, St. Louis, MO, USA). the pan caspase inhibitor Z-VAD-FMK, MG-132, TPCA-1 (Calbiochem, San Diego, CA, USA); recombinant TNF (R & D Systems, Minneapolis, MN, USA); recombinant human TRAIL/Apo2 ligand (Peprotech, Rocky Hill, NJ, USA).
Cell culture and primary astrocytes preparation
Human malignant glioblastoma cells (LN428, LNZ308, U87MG, and U251MG) were kindly provided by Dr. Yongwan Kim (Dongsung Cancer Center, Daegue, Korea). HCT 116, a human colorectal cancer cells and its p53-knockout derivates were kindly provided by Dr. Deug Y. Shin (Dankook University, Cheonan, Korea). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) including 10% fetal bovine serum, 2 mmol/L glutamine and 100 U/mL penicillin/streptomycin. The normal primary astrocytes were prepared from the neonatal rats, as described previously58, and cultured in Minimum Essential Media (MEM) with 10% FBS, 2 mmol/L glutamine and 100 U/mL penicillin/ streptomycin.
Plasmids, transfection and luciferase assay
The expression plasmids of survivn (pCA-flag-survivn) and cFLIPL (pCA-flag- cFLIPL) were gift from Dr. Taeg K. Kwon (Keimyung University, Daegu, Korea). The point-mutant of cFLIPL at lysine 167/195 (cFLIPL-K167/195 R) was generated by a QuickchangeTM Site-Directed Mutagenesis kit as previously described59. For luciferase assay, LN428 cells were transiently co-transfected with p2xNF-kB-Luc and pRSV-β-galactosidase into 1 × 106 cells per well in 6-well plates for 24 h using Lipofectamine reagent (Invitrogen). Cell were then treated with TNF (30 ng/ml) or TRAIL (50 ng/ml) for an additional 6 h, and the luciferase activities were measured using a luciferase assay kit (Promega, Madison, CA, USA) according to the manufacturer’s instructions. Luciferase activity obtained were normalized to β-galactosidase activity of each sample.
Cell death assessment
Cell death was determined using Cell Titer-glo Luminescent Cell Viability Assay kit (Promega Co., Fitchburg, WI, USA), according to the manufacturer’s instructions. Luminescent signals were measured by Tecan Infinite Plate reader (Tecan group Ltd., Männedorf, Switzerland), and Viability rates were calculated following the formula: viability rates = (1 − medicating/control) × 100%. Representative images were also taken by an inverted microscope.
Flow cytometry analysis
After LN428 cells were treated with pinoresinol, as described in the figure legends, the cells were harvested and examined for the mode of apoptotic and necrotic cell death by double staining with FITC-conjugated annexin V and propidium iodide (PI) in 10 mM HEPES buffer, pH 7.4, according to the manufacturer’s instructions (BD FITC Annexin V kit). For cell cycle analysis, cells were suspended with ice-cold phosphate buffered saline (PBS) and fixed in 70% ethanol. Cells were washed with PBS and added 100 μg/ml PI solution including 50 μg/ml RNase in PBS for 30 min at room temperature. The cells were analyzed with a FACScan flow cytometer (BD Biosciences).
Immnunoblotting and immunoprecipitation
Upon treatment, cells were lysed in ice-cold M2 buffer (20 mM Tris, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM PMSF, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, and 1 µg/ml leupeptin). For immunoblot analysis, cell lysates were resolved on 8–12% SDS-polyacrylamide gel (PAGE), transferred onto the PVDF membrane (GE Healthcare Life Sciences). Membranes were serially incubated with specific primary antibodies (1:1,000 dilution) and secondary antibody (1:2,000 dilution), and immunoblots were visualized by enhanced chemiluminescence (ECL) kit (EMD Millipore). For immunopreciptation assays, the cell lysates were incubated with anti-caspase-8 antibody and protein A-agarose beads at 4 °C overnight. The immunoprecipitants were washed three times with M2 buffer and analyzed by immunoblotting.
RT-PCR analysis
Total RNA was isolated using a ReliaPrep RNA Miniprep kit (Promega), and cDNA was prepared using a M-MLV reverse transcriptase (Promega), according to the manufacturer’s instructions. PCR amplification was carried out using the primer pairs specific to each human genes (cFLIPL, 5′-CTGGTTGCCCCAGATCAACT-3′ and 5′-CCCAGGGAAGTGAAGGTGTC-3′; Survivin, 5′-TGACGACCCCATAGAGGAACA-3′ and 5′-TCAATCCATGGCAGCCAGC-3′; GAPDH, 5′-CACCATCTTCCAGGAGCGAG-3′and 5′-GATGGCATGGACTGTGGTCA-3′).
In vitro translation analysis
In vitro coupled transcription and translation analysis was performed with the 1-Step Human Coupled IVT Kit ± DNA (ThermoFisher Scientific) following the manufacturer’s instructions. Briefly, the translation reaction was assembled with pre-incubation of HeLa cell lysates with 1 μg of circular DNA (pCFE-GFP) as a template. The reaction mixtures were incubated for 12 hours at 30 °C and were stopped with loading buffer for SDS-PAGE. The expression levels of EGFP proteins were examined by immunoblot analysis using anti-TurboGFP antibody. For in vitro translational analysis, EGFP mRNA was synthesized after digesting pcGlobin2-EGFP with XhoI, as described previously60. Synthetic EGFP mRNA was generated by using a mMESSAGE mMACHINE T7 Transcription kit (ThermoFisher Scientific) following manufacturer’s instructions. Each 3 µg of purified EGFP mRNA was incubated with HeLa cell lysates for 6 hours at 30 °C with indicated concentrations of PINO and CHX as described in figure legends. The expression level of EGFP was measured through immunoblotting with anti-GFP antibody and then quantified by densitometry using an image J software.
Statistical analysis
Data are expressed as the mean ± S.E. from at least three separate experiments performed triplicate. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Bonferroni t-test for multi-group comparison tests. Student’s t-test was used to compare the mean values from the two groups. P < 0.05 was considered as statistically significant.
References
Kretz, A. L. et al. TRAILblazing Strategies for Cancer Treatment. Cancers (Basel) 11, 456 (2019).
Sessler, T., Healy, S., Samali, A. & Szegezdi, E. Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling. Pharmacol Ther 140, 186–99 (2013).
Tummers, B. & Green, D. R. Caspase-8: regulating life and death. Immunol Rev 277, 76–89 (2017).
Feltham., R., Vince, J. E. & Lawlor, K. E. Caspase-8: not so silently deadly. Clin Transl Immunology 6, e124 (2017).
Bin, L. et al. Tumor-derived mutations in the TRAIL receptor DR5 inhibit TRAIL signaling through the DR4 receptor by competing for ligand binding. J Biol Chem 282, 28189–28194 (2007).
Yu, R. et al. DR4 specific TRAIL variants are more efficacious than wild-type TRAIL in pancreatic cancer. Cancer Biol Ther 15, 1658–1666 (2014).
Li, M. et al. Q482H mutation of procaspase-8 in acute myeloid leukemia abolishes caspase-8-mediated apoptosis by impairing procaspase-8 dimerization. Biochem Biophys Res Commun 495, 1376–1382 (2018).
Finnberg, N., Klein-Szanto, A. J. & El-Deiry, W. S. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest 118, 111–123 (2008).
Fulda, S. Safety and tolerability of TRAIL receptor agonists in cancer treatment. Eur J Clin Pharmacol 71, 525–527 (2015).
Wajant, H. Molecular Mode of Action of TRAIL Receptor Agonists-Common Principles and Their Translational Exploitation. Cancers (Basel) 11, 954 (2019).
von Karstedt, S., Montinaro, A. & Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer 17, 352–366 (2017).
Yuan, X. et al. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev 37, 733–748 (2018).
Xu, K. et al. Structural and bioactive studies of terpenes and cyclopeptides from the Genus Rubia. Chem Cent J 7, 81 (2013).
Do, M. T., Hwang, Y. P., Kim, H. G., Na, M. & Jeong, H. G. Mollugin inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. J Cell Physiol 228, 1087–1097 (2013).
Gong, X. P. et al. Anti-diarrheal and anti-inflammatory activities of aqueous extract of the aerial part of Rubia cordifolia. BMC Complement Altern Med 17, 20 (2017).
An, Y. et al. Activation of the p53 pathway with digiferrol isolated from Rubiaphilippinensis induces cell cycle arrest, apoptosis, and autophagy in colon cancer cells. Food ChemToxicol 118, 514–522 (2018).
Bajpai, V. K. et al. Cytotoxic properties of the anthraquinone derivatives isolated from the roots of Rubia philippinensis. BMC Complement Altern Med 18, 200 (2018).
Oh., J. et al. NMR-Based Investigation of Hydrogen Bonding in a Dihydroanthracen-1(4H)one from Rubia philippinensis and Its Soluble Epoxide Hydrolase Inhibitory Potential. J Nat Prod 81, 2429–2435 (2018).
He, W. et al. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 8, 1811-–1821 (2012).
Azijli, K., Weyhenmeyer, B., Peters, G. J., de Jong, S. & Kruyt, F. A. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ 20, 858–868 (2013).
Yang, M. et al. Poly-ADP-ribosylation of HMGB1 regulates TNFSF10/TRAIL resistance through autophagy. Autophagy 11, 214–224 (2015).
Jung, H. W. et al. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci Lett 480, 215–220 (2010).
During, A., Debouche, C., Raas, T. & Larondelle, Y. Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J Nutr 142, 1798–1805 (2012).
Lin, B. et al. Anti-inflammatory constituents from the root of Litsea cubeba in LPS-induced RAW 264.7 macrophages. Pharm Biol 54, 1741–1747 (2016).
Zhang, L. & Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12, 228–237 (2005).
Zhao, J., Lu, Y. & Shen, H. M. Targeting p53 as a therapeutic strategy in sensitizing TRAIL-induced apoptosis in cancer cells. Cancer Lett 314, 8–23 (2012).
Tabuchi, K., Fukuyama, K., Mineta, T., Oh-Uchida, M. & Hori, K. Altered structure and expression of the p53 gene in human neuroepithelial tumors. Neurol Med Chir 32, 725–732 (1992).
Martín-Pérez, R., Niwa, M. & López-Rivas, A. ER stress sensitizes cells to TRAIL through down-regulation of FLIP and Mcl-1 and PERK-dependent up-regulation of TRAIL-R2. Apoptosis 17, 349–363 (2012).
Poukkula, M. et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem 280, 27345–27355 (2005).
Pahlavan, Y. et al. Survivin modulatory role in autoimmune and autoinflammatory diseases. J Cell Physiol 234, 19440–19450 (2019).
Humphreys, L., Espona-Fiedler, M. & Longley, D. B. FLIP as a therapeutic target in cancer. FEBS J 285, 4104–4123 (2018).
Kang, K., Lee, S. R., Piao, X. & Hur, G. M. Post-translational modification of the death receptor complex as a potential therapeutic target in cancer. Arch Pharm Res 42, 76–87 (2019).
Nogueira-Ferreira, R., Vitorino, R., Ferreira-Pinto, M. J., Ferreira, R. & Henriques-Coelho, T. Exploring the role of post-translational modifications on protein-protein interactions with survivin. Arch Biochem Biophys 538, 64–70 (2013).
Cai, X. & Sughrue, M. E. Glioblastoma: new therapeutic strategies to address cellular and genomic complexity. Oncotarget 9, 9540–9554 (2017).
Eisele, G. & Weller, M. Targeting apoptosis pathways in glioblastoma. Cancer Lett 332, 335–345 (2013).
Fulda, S. Cell death-based treatment of glioblastoma. Cell Death Dis 9, 121 (2018).
Martinez, R. et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis 28, 1264–1268 (2007).
Hervouet, E., Vallette, F. M. & Cartron, P. F. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis 1, e8 (2010).
Liccardi, G. et al. RIPK1 and Caspase-8 Ensure Chromosome Stability Independently of Their Role in Cell Death and Inflammation. Mol Cell 73, 413–428 (2019).
Tsuchiya, Y., Nakabayashi, O. & Nakano, H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int J Mol Sci 16, 30321–30341 (2015).
Valnet-Rabier, M. B. et al. c-Flip protein expression in Burkitt’s lymphomas is associated with a poor clinical outcome. Br J Haematol 128, 767–773 (2005).
Yao, Q. et al. Prognostic significance of TRAIL signalling molecules in cervical squamous cell carcinoma. J Clin Pathol 69, 122–127 (2016).
Ullenhag, G. J. et al. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res 13, 5070–5075 (2007).
Hutchinson, R. A. et al. IHC-based subcellular quantification provides new insights into prognostic relevance of FLIP and procaspase-8 in non-small-cell lung cancer. Cell Death Discov 3, 17050 (2017).
Micheau, O., Lens, S., Gaide, O., Alevizopoulos, K. & Tschopp, J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21, 5299–5305 (2001).
Gordy, C., Liang, J., Pua, H. & He, Y. W. c-FLIP protects eosinophils from TNF-α-mediated cell death in vivo. PLoS One 9, e107724 (2014).
Marques-Fernandez, F. et al. TNFα induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis 4, e493 (2013).
Skurk, C. et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem 279, 1513–1525 (2004).
Wang, X. et al. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther 7, 1156–1163 (2008).
Mitsuhashi, S., Kishimoto, T., Uraki, Y., Okamoto, T. & Ubukata, M. Low molecular weight lignin suppresses activation of NF-kappaB and HIV-1 promoter. Bioorg Med Chem 16, 2645–2650 (2008).
Sayers, T. J. V. et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood 102, 303–310 (2003).
Mita, A. C., Mita, M. M., Nawrocki, S. T. & Giles, F. J. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14, 5000–5005 (2008).
González, M. L. et al. Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative. Front Pharmacol 8, 205 (2017).
Laiolo, J. et al. Analogues of the Lignan Pinoresinol as Novel Lead Compounds for P-glycoprotein (P-gp) Inhibitors. ACS Med Chem Lett 9, 1186–1192 (2018).
Galski, H. et al. P-glycoprotein-dependent resistance of cancer cells toward the extrinsic TRAIL apoptosis signaling pathway. Biochem Pharmacol 86, 584–596 (2013).
Souza, P. S. et al. Expression of the multidrug transporter P-glycoprotein is inversely related to that of apoptosis-associated endogenous TRAIL. Exp Cell Res 336, 318–328 (2015).
Xie, L. H., Akao, T., Hamasaki, K., Deyama, T. & Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem Pharm Bull (Tokyo) 51, 508–515 (2003).
Baek, H. et al. The anti-inflammatory role of extranuclear apurinic/apyrimidinic endonuclease 1/redox effector factor-1 in reactive astrocytes. Mol Brain 9, 99 (2016).
Won, M. et al. Novel anti-apoptotic mechanism of A20 through targeting ASK1 to suppress TNF-induced JNK activation. Cell Death Differ 17, 1830–1841 (2010).
Ro, H., Soun, K., Kim, E. J. & Rhee, M. Novel vector systems optimized for injecting in vitro-synthesized mRNA into zebrafish embryos. Mol Cells 17, 373–376 (2004).
Acknowledgements
This work as supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1A2A1A05001225; No. 2017R1A5A2015385; No. 2017R1A2A2A05001340). We thank Prof. Han-Ming Shen (National University of Singapore) for fruitful comments and assistance in the preparation of this article.
Author information
Authors and Affiliations
Contributions
S-R.L. participated in the design of the study, carried out bench experiments and analyzes data. H.S.B., K.K., X.P., E.J. helped carrying out bench experiments related to this study. H.R. helped the experiments for the in-vitro translation assay. K.Q., I.P., M.N. carried out the experiments for the purification of pinoresinol and LC-mass analysis. G.M.H. designed this study and wrote the manuscript with comments from the coauthors, and all authors collaborated on the work.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, SR., Quan, K.T., Byun, H.S. et al. Accelerated degradation of cFLIPL and sensitization of the TRAIL DISC-mediated apoptotic cascade by pinoresinol, a lignan isolated from Rubia philippinensis. Sci Rep 9, 13505 (2019). https://doi.org/10.1038/s41598-019-49909-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49909-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.