Abstract
To identify clinical characteristics and mutation spectra in Chinese patients with renal angiomyolipoma (AML) associated with the tuberous sclerosis complex (TSC, TSC-AML), examined the efficacy and safety of short-term everolimus therapy (12 weeks). We analyzed the frequency distribution of each TSC-related clinical feature and investigated gene mutations by genetic testing. Some subjects received everolimus for 12 weeks at a dose of 10 mg/day, and the efficacy and safety of short-term everolimus therapy were examined. Finally, 82 TSC-AML patients were enrolled for analysis in this study. Of the 47 patients who underwent genetic testing, 22 patients (46.81%) had at least one detectable mutation in the TSC1 or TSC2 gene: 7 were TSC1 gene mutations, 13 were TSC2 gene mutations, and 2 were found in both TSC1 and TSC2. Everolimus treatment had a statistically significant effect on the renal AML volume reduction during follow-up (P < 0.05), and the mean reduction rate of volume for all cases was 56.47 ± 23.32% over 12 weeks. However, 7 patients (7/25; 28.00%) experienced an increase in renal AML tumor volume within 12 weeks after discontinuation of the everolimus treatment. Although most patients (27/30, 90.00%) experienced some adverse events during the treatment period, all such events were mild, and no patients discontinued or needed dose reduction because of adverse events. Overall, in this study, the mutation rate of TSC-AML patients is much lower than other reports. Short-term everolimus treatment for TSC-AML is effective and safe, but the stability is much lower than long-term therapy.
Similar content being viewed by others
Introduction
Tuberous sclerosis complex (TSC) is a systemic autosomal dominant genetic disorder that affects approximately 1–2 million individuals worldwide1. It is usually caused by mutations in either the hamartin gene (TSC1) or tuberin gene (TSC2)2,3, leading to the growth of nonmalignant hamartomas in various organs throughout the body, including the kidney, brain, lung, skin, and heart4,5,6.
Statistical studies show that approximately 80% of patients with TSC develop renal angiomyolipoma (AML), which is rich in fat, muscle, and blood vessels7. Unlike sporadic renal AML, most TSC-related renal AML (TSC-AML) patients often develop multiple bilateral lesions and experience a significant tumor burden on the kidneys8. Furthermore, TSC-AML tend to be larger, grow faster and at higher risk of bleeding9. In addition, AML can increase in size over time and may cause hypertension, renal failure or life-threatening hemorrhage, which causes the largest proportion of adult deaths from the disease10.
The TSC1 or TSC2 gene mutation occurs in approximately 80% of patients with TSC2,3. The TSC1 gene, located on chromosome 9q34, consists of 23 exons and codes for hamartin, a novel 130-kDa protein3. The TSC2 gene, located on chromosome 16p13.3, contains 41 exons and codes for tuberin, a 200-kDa protein2. Hamartin and tuberin form a tumor suppressor complex that regulates the activity of rapamycin complex 1 (mTORC1), a critical regulator of cell growth and proliferation11,12. Mutations in TSC1 or TSC2 result in the loss of the hamartin/tuberin complex, which leads to constitutive activation of mTORC1. Increased mTORC1 signaling results in the production of hamartomatous lesions of TSC12.
Growing knowledge about the molecular relationship between TSC and mTOR has led to the investigation of mTORC1 inhibition as a treatment approach in TSC6,13,14. Moreover, the International Tuberous Sclerosis Complex Consensus Conference (ITSCCC) held in 2012 has recommended mTOR inhibitors as the first-line treatment for TSC-AML when enlarged to 3 cm or more15. Everolimus is a derivative of rapamycin that suppresses the enlargement of tumors and promotes their regression by inhibiting mTORC116. Clinically, a randomized, double-blind, placebo-controlled EXIST-2 trial and extension studies have demonstrated the efficacy and manageable safety of everolimus for TSC-AML17,18.
In China, everolimus was included in national basic medical insurance, work injury insurance and the maternity insurance drug list in 2017. However, clinical information of TSC-AML patients in China and the effects of short-term everolimus therapy on TSC-AML are still limited. Thus, this study aimed to analyze the clinical characteristics and mutation spectra of patients with TSC-AML in Northwest China and to investigate the efficacy and safety of short-term everolimus therapy in patients with TSC-AML.
Methods
This was an open single-center clinical study conducted at Xi Jing Hospital and was reviewed and approved by the Fourth Military Medical University ethics committee (ethics committee’s number: KY20151536-1). The study was performed in accordance with the principles of the Declaration of Helsinki and all local regulations. Written informed consent was obtained from all participants.
Patient selection
From September 2015 to August 2018, a total of 167 patients with renal AML were screened at Xi Jing Hospital, 82 of whom were enrolled in this study. All 82 patients had been clinically (definitively or potentially) diagnosed with TSC per ITSCCC diagnostic criteria15. All patients underwent a systematic evaluation that covered 11 major criteria and 6 minor criteria of TSC before any interventions. Of these 82 patients, 47 patients (including 10 possible TSC-AML patients) were selected for the gene sequencing test, and 30 patients were recruited in the everolimus treatment trial.
Of the 30 patients who were selected for everolimus treatment, all had been definitively diagnosed with TSC, and patients with possible TSC were excluded. All of these patients were 18 years or older and had either multiple or bilateral AML that were 3-cm or larger at their largest diameter.
Mutational analysis
Peripheral blood (10 mL) samples were collected from each patient and isolated from peripheral white blood cells. Then, DNA was extracted from peripheral blood leukocytes using standard methods. The mutational analysis of TSC genes was performed by next-generation sequencing (NGS), including the preparation of DNA libraries, the hybridization capture of target area and single-ended sequencing. Then, the data were analyzed, and all variation results were validated by the PCR-SSCP method (all genetic tests were performed by KANGSO Medical Institute, Beijing China). Finally, all of the mutations were compared with the Tuberous Sclerosis Database (www.lovd.nl/TSC1; www.lovd.nl/TSC2).
Everolimus treatment
Everolimus was administered orally at a dose of 10 mg/day, and the levels of everolimus in blood were measured at 2 weeks to ensure blood everolimus concentrations were between 5 and 15 ng/ml. All patients received everolimus for 12 weeks, while 25 patients were followed for an additional 12 weeks after the therapy was stopped.
The primary endpoint of this study was the efficacy of short-term everolimus therapy, which was assessed according to the reduction in the volume of renal AML. The volume of renal AML was evaluated using kidney magnetic resonance imaging or CT scanning, and all scans were assessed by central radiological review. The volume of renal AML before initiation of everolimus treatment was defined as “baseline”. Follow-up visits were performed at week 4 and 12 after the start of everolimus treatment and at week 12 after the treatment stopped. Treatment efficacy was defined as the volume reduction rate compared to the baseline, which was calculated with the formula: (VPre-Therapy − VPost-Therapy)/VPre-Therapy × 100%. (VPre-Therapy represents pre-everolimus therapy volume; VPost-Therapy represents post-everolimus therapy volume).
The secondary endpoint of this study was the frequency and severity of adverse events (AE). AEs were evaluated using a structured questionnaire covering the side effects reported during EXIST 2 or extension studies and were coded using the Medical Dictionary for Regulatory Activities (MedDRA) preferred term17,19. Furthermore, adverse events were continuously monitored in every follow-up visit and graded I-V according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute version 4.03 via investigator assessment20. To assess safety, a complete blood count, urinalysis and the levels of electrolytes (blood urea nitrogen, creatinine, glucose, hepatic enzymes, bilirubin, and serum lipids) were also tested in every patient. All laboratory tests were performed by the Department of Laboratory, Xi Jing Hospital, the Fourth Military Medical University.
Statistical analysis
Clinical characteristics were collected from all patients screened for TSC-AML during the experiment period, and the results were presented as a frequency distribution (absolute frequencies and valid percentages; n, %). The mutation spectra were the statistical result of the 47 patients who agreed to participate in the genetic testing, and the results were expressed in terms of frequency distribution. Efficacy and safety analyses were performed on all patients who received at least 12 weeks of everolimus treatment and had at least one follow-up assessment; missing data were not considered in the analyses. Descriptive analyses of patient characteristics were conducted, including central tendency and dispersion (mean ± standard deviation [SD]), or median and frequency distribution. The outcomes were analyzed using a t-test or χ2 test, and a P-value < 0.05 was considered statistically significant. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS, SPSS Inc, Chicago, USA) version 17.0.
Results
Clinical characteristics of patients with TSC-AML
A total of 82 Chinese patients with TSC-AML, including 65 definitively diagnosed patients and 17 patients with possible TSC-AML were enrolled in this study since 2015. The patients were 35 ± 12 years old (range 18–68 years), with a median age of 36 years. Among all patients, 28 were males (34.15%), and 54 were females (65.85%) (Table 1).
All patients underwent a comprehensive assessment before any interventions, including both physical and radiographic aspects. In addition, all clinical features were collected using a standardized clinical instrument that covers 11 major criteria and 6 minor criteria of TSC15. As shown in Table 1, all patients had at least two AMLs, with the largest diameter (≥3 cm). Among them, there were 44 (53.66%) patients with AMLs with the largest diameter (<10 cm), 38 (46.34%) patients with AMLs with the largest diameter (≥10 cm), and the average AMLs with the largest diameter of patients was 11.23 ± 6.47 cm (range 3.31–28.34 cm). Of these patients, all except 9 patients (89.02%) had angiofibromas or fibrous cephalic plaques, 38 patients (46.34%) exhibited hypomelanotic macules, and 33 patients (40.24%) had at least two ungual fibromas. Furthermore, 21 patients (25.61%) presented with shagreen patches, 3 patients (3.66%) had multiple retinal hamartomas, and 18 patients (21.95%) exhibited cortical dysplasias. In addition, subependymal nodules were present in 26 patients (31.71%), and subependymal giant cell astrocytoma was found in 2 patients (2.44%). In the 54 female patients, 19 patients (35.19%) were identified as having lymphangioleiomyomatosis (Table 1).
In minor features aspects, “confetti” skin lesions were the most common, affecting 42 patients (51.22%), followed by dental enamel pits (23 patients; 28.04%), intraoral fibromas (19 patients; 23.17%) and retinal achromatic patches (5 patients; 6.10%). In addition, 3 patients (3.66%) presented with multiple renal cysts, and 1 patient (1.22%) had nonrenal hamartomas (Table 1).
Mutation spectra and novel sequence variants
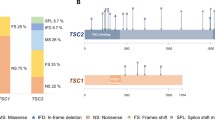
Out of 47 Chinese TSC-AML cases, TSC gene mutations were found in 22 patients (46.81%). Among 22 TSC gene mutations, 7 (14.89%) were TSC1 gene mutations, 13 (27.66%) were TSC2 gene mutations and 2 (4.26%) were both TSC1 and TSC2 gene mutations. Of these 22 patients, a total of 26 mutations were detected, and 3 patients had more than 1 type of mutation. Of all mutations, a total of 21 different kinds of mutations were identified, 5 in TSC1 and 16 in TSC2 (Table 2).
As shown in Table 2, the mutations in TSC1 and TSC2 can be distributed over the entire TSC1 and TSC2 gene regions; most of them were in exons (24/26; 92.31%), and only 2 of them were in introns (7.69%). Among TSC1 mutations, c.965 T > C was the most common mutation, which can be found in 4 cases. The c.965 T > C mutation is a type of missense mutation located in exon 10 of the TSC1 gene, and this mutation results in a p.Met322Thr amino acid change. Among TSC2 mutations, c.2032 G > A was the most common mutation and was involved in 2 cases. c.2032 G > A is also a type of missense mutation located in exon 19 of the TSC2 gene, and this mutation results in a p.Ala678Thr amino acid change. Among all mutation types, missense mutations were the most common, accounting for 57.69% (15/26), followed by frameshift mutations (4/26), deletions (3/26, including 2 large deletions), nonsense mutations (2/26) and splicing mutations (2/26) (Table 2). In addition, eleven novel nucleotide alterations that have not been registered in the LOVD database were identified in the present study, 10 of which are in the TSC2 gene: (c.1–34 G > C, c.1–35 G > A, c.3179 G > A, c.1830delC, c.2106_2107insT, c.1911C > T, c.1659_1660insTCGG, c.5227_5244del and exon 1-6del) and 1 in the TSC1 gene (c.1921C > T) (Table 2).
Among these 22 patients with TSC1 or TSC2 mutations, 7 of these patients’ families also underwent genetic tests and found that two of the mutations were familial, including c.826-827del, located in exon 9 of the TSC2 gene and c.2083 C > T, located in exon19 of the TSC2 gene (Fig. 1). The c.826-827del mutation was a kind of frameshift mutation, and this mutation results in p.Met276ValfsTer61 amino acid change. A total of 9 family members underwent TSC genetic testing, of which 3 exhibited the same mutation, namely, the patient, the mother and the younger brother. The c.2083 C > T mutation was a type of nonsense mutation resulting in a p.Gln695Ter amino acid change. For this case, the TSC genetic tests were performed in 8 family members, and 4 of them carried the same mutation, namely, the patient, the mother and two sons (Fig. 1).
Sequencing results and mutant pedigree map of familial mutations. (A) Sequencing results of two familial mutations. The red arrow indicates the point of mutation. (a) Sequencing results of mutation c.826_827del; (b) Sequencing results of mutation c.2083 C > T. (B) Mutant pedigree map of familial mutations. The arrow refers to the proband; square represents the male; circle represents the female; (a) Mutant pedigree map of mutation c.826_827del; b: Mutant pedigree map of mutation c.2083 C > T.
Comparison between TSC2 and non-TSC2 mutation populations
Within the group of patients who underwent a gene sequencing test, we compared the clinical features of patients with TSC2 mutations with patient populations with non-TSC2 mutations (TSC1 mutation or no mutation). As shown in Table 1, patients with TSC2 mutations had a significantly larger average AML largest diameter compared with patient populations with non-TSC2 mutations (16.37 ± 9.61 cm vs 9.14 ± 6.84 cm, P = 0.042). Ten patients (66.67%) with TSC2 mutations had a AML largest diameter of ≥10 cm, compared to only 11 patients (34.38%) in the non-TSC2 mutation group (P = 0.038). In addition, hypomelanotic maculesoccurred more frequently in the TSC2 mutation group (P = 0.007). Although the difference was not statistically significant between these two groups for the other clinical features, the TSC2 mutation group was younger and had a higher frequency of other major or minor features (Table 1).
Treatment efficacy
A total of 30 patients who had been definitively diagnosed with TSC-AML were enrolled in the everolimus treatment study. Of the 30 patients, the median age was 35 (range 18–52) years, 17 were ≤ 35 years old (56.67%) and 13 were >35 years old (43.33%). Overall, the majority of patients (63.33%; 19/30) were female, and 11 patients (36.67%) were male. Twelve patients (40.00%) had a primitive volume (>200 cm3) of the largest renal AML at baseline. Genetic testing was performed in 19 patients, 5 with mutations to TSC1 or TSC2 (1 with mutations in TSC1, 4 with mutations in TSC2) and 14 with no mutations (Table 3).
All of the patients were given everolimus orally at doses of 10 mg per day. The duration of administration was 12 weeks. After 4 and 12 weeks of therapy, the mean renal AML volume decreased 36.51 ± 23.94% (range: 4.23%-84.47%; P < 0.05 for the change from the baseline value) and 56.47 ± 23.32% (range: 4.88%-97.36%; P < 0.05 for the change from the baseline value), respectively. In addition, we found that male patients or those with primitive volume (<200 cm3) experienced a better efficacy of treatment when they took everolimus for 12 weeks. However, there was no significant difference in the efficacy of treatment with respect to age and mutation type (mutation group vs no mutation group) (Table 4).
The reduction rate of volume from the baseline in each case is shown in Fig. 2A. All except 1 patient responded to everolimus (96.67%; 29/30), with a statistically significant volume reduction (>10% compared with baseline) after 12 weeks of treatment, and the largest rate of volume reduction from the baseline was 97.36% (882.47 cm3 to 23.26 cm3) after 12 weeks of therapy. However, the renal volume of the nonresponder only experienced a 4.88% (773.31 cm3 to 735.61 cm3) reduction after 12 weeks of treatment (Fig. 2A). The proportion of patients who achieved ≥30% reduction from baseline in renal AML volume increased from 53.33% (16/30) after 4 weeks of treatment to 86.67% (26/30) at week 12, and the proportion of patients with ≥50% reduction increased from 30.00% (9/30) after 4 weeks of treatment to 66.67% (20/30) after 12 weeks (Fig. 2B).
As shown in Fig. 3, in most patients, the AML volume decreased rapidly within 4 weeks after treatment initiation, but the rate of decrease significantly slowed down for the next 8 weeks. However, 7 of these patients (7/25; 28.00%) experienced an increase in renal AML volume within 12 weeks after discontinuation of the everolimus treatment (an increase larger than 10% compared with baseline). In addition, among these 7 progression patients, 2 patients experienced renal AML lesions increased over baseline levels, and the volume had increased to 160.41% (781.68 cm3 to 1253.93 cm3) and 172.19% (80.09 cm3 to 137.93 cm3) of the baseline, respectively. Despite this progression, all except one nonresponder and two progressive patients exhibited at least a 10% reduction in volume compared with baseline by the cut-off day.
Adverse events
The AEs were monitored throughout the treatment period and and no patients took other special drugs during this time. As shown in Table 5, a wide variety of everolimus treatment-related AEs occurred, and they were consistent with the known safety profile of everolimus17,18, which most commonly included stomatitis, headache, acne, cough and menstrual disorders.
In our study, all AEs noted were very mild, with no grade 3 or 4 AEs detected, and no patients were hospitalized with serious AEs. Overall, 27 patients (90.00%) experienced grade 1 or 2 AEs during the study period, and they all could be managed successfully through symptomatic treatment; no one needed dose reduction or temporary interruption of treatment. Notably, stomatitis was the most common AE, and it was reported in 27 patients, of whom 26 were grade 1 and 1 was grade 2. By corresponding oral care, all cases were improved in 4 weeks. Headache was reported in 11 patients (36.67%) and was the second most common AE. Upper respiratory infections in 4 cases (13.33%) of grade 1 and 1 case (3.33%) of grade 2 were detected, but no urinary tract infection was reported. In addition, 9 patients (30.00%) developed digestive system AEs, including nausea (4/30, 13.33%) and vomiting (5/30, 16.67%). Regarding severity, the most common grade 2 AEs were menstrual disorders. In nonmenopausal female patients (17patients), a total of 4 (23.53%) patients experienced menstrual disorders, all of which resolved in 8 weeks without any hormone treatment.
During the treatment period, everolimus treatment-related laboratory tests were also monitored, including blood, urine, liver and kidney functions. No other abnormalities were observed, except for 4 cases (13.33%) of hyperlipidemia (grade 1) and cytopenia (3 cases of grade 1 and 1 case of grade 2).
Discussion
TSC was initially described in 1862 by von Recklinghausen. It is an extremely variable disease that can affect virtually any organ in the body1. In the late nineteenth century, it was identified by simple clinical observations, such as epilepsy, cognitive retardation, and facial angiofibroma. Then, with other manifestations discovered and its pathogenesis revealed, the diagnostic criteria for TSC have also improved over time21. In 2012, the International Tuberous Sclerosis Complex Consensus Group holds the ITSCCC and update TSC diagnostic criteria15. The new diagnostic criteria includes new genetic testing results, reduced diagnostic classes from three (possible, probable, and definite) to two (possible, definite) and some minor changes to specific criteria15. However, the clinical features of TSC continue to be a principal means of diagnosis.
The clinical manifestations of TSC are quite diverse and distinctive, including lesions of the brain, skin, heart, lungs, retina and kidneys. All these manifestations are divided into 11 major features and 6 minor features15. In this study, we reported the clinical features of 82 patients with TSC-AML in northwestern China. In addition to renal AML, angiofibromas (≥3) or fibrous cephalic plaques were the most common major features in TSC-AML patients and were identified in 89.02% (73/82) of patients. In minor features aspects, confetti skin lesions were the most common in all patients. Similar results were reported by Yi Cai22.
In addition to diagnosis by clinical diagnostic criteria, the genetic analysis was also an independent diagnostic criterion for TSC15. As the molecular biology and genetics that underly the pathophysiologic process of TSC were elucidated in the late years, comprehensive and reliable screens for TSC gene mutations are well established, and more pathogenic mutations have been identified21. TSC1 and TSC2 genes are both pathogenic mutations of TSC, and approximately 80% of patients with a clinical diagnosis of TSC have exhibited gene mutations2,3.TSC2 mutations are more common than TSC1 mutations, and TSC2 has been shown to be mutated in approximately 70% of TSC patients, whereas TSC1 mutations can only be found in 20% of TSC cases2,3. Furthermore, TSC2 mutations are associated with a more severe phenotype and a higher frequency and larger renal AML size compared with non-TSC2 mutations22,23,24. Our study showed similar results: patients with TSC2 mutations had larger AMLs and a higher frequency of other clinical features compared with patient populations with non-TSC2 mutations.
In this experiment, we examined mutations of theTSC1 and TSC2 genes in 47 TSC-AML patients. Of these patients, gene mutations were found in 22 patients: 7 were TSC1 gene mutations, 13 were TSC2 gene mutations and 2 were TSC1 and TSC2 gene mutations. In comparing our study with previous reports, the mutation rates are lower than those of other regions (other parts of China)22,23,25,26,27,28,29. The low detection mutation rate found in this study could be due to: (1) clinical possibly TSC patients may had lower gene mutation rates (there were 10 clinical possibly patients also underwent genetic testing); (2) mutations in intronic and promoter regions, which might disrupt gene expression, and be missed by most mutation screening methods; (3) difficulty of detecting mutations by any conventional method in patients with diagnostic features of tuberous sclerosis and low rate of mosaicism for either TSC1 or TSC2 mutations30,31; (4) additional causative loci that could account for a few patients with tuberous sclerosis and we could not detect it using conventional screening methods32,33; (5) the specificity of the population in Northwest China. However, due to the limited sample size, this conclusion needs to be deeply verified. For all these reasons, it is still difficult to assert that the genetic mutation rate of TSC patients in Northwest China is lower than other regions. Therefore, more accurate researches are needed to further verify the findings in this study.
NGS is a powerful technology that allows for a more accurate, more depth and less time-consuming genetic analysis that was never before possible32. To date, 928 unique DNA variants of TSC1 (update to September 03, 2018) and 2689 unique DNA variants of TSC2 (update to September 24, 2018) have been reported and listed on the TSC Variation Database site (www.lovd.nl/TSC1; www.lovd.nl/TSC2). In this study, a total of 21 different types of mutations were identified, 11 (52.38%) of which were newly discovered. Such a high rate of novel mutations may be due to racial specificity, which may also be another reason for the low mutation rate. Similarly, the literature shows that the TSC mutation spectra and rates vary from country to country21,22,33,34,35,36,37. A previous study showed that alterations in TSC1 and TSC2 can be distributed over the entire TSC1 and TSC2 gene regions. Our study showed similar results. Of these 21 mutations, 5 were located in TSC1, and 16 were in TSC2. These mutations were distributed in the exon or intron regions of the TSC1 and TSC2 genes, but the most common regions in the TSC1 and TSC2 genes were exon 10 and exon 19, respectively. Moreover, in TSC2 mutations, there were 2 nucleotide alterations that were identified as familial mutations; three of the 9 family members (c.826_827del) and four of 8 family members (c.2083 C > T) showed the same mutation. Although there are many advantages of NGS, and it can definite diagnosis TSC, the high cost of sequencing makes it impossible for many poor areas to diagnose all patients suspected of TSC by gene sequencing32.
In patients with TSC, renal problems (renal failure or tumoral complications, retroperitoneal hemorrhage, and metastases of renal cell carcinoma) is the second leading cause of death or disability, second only to severe intellectual disability38,39. The cause of death in TSC-AML patients is associated with progressive increases in size, which in turn lead to renal failure or spontaneous hemorrhage40,41. Thus, current guidelines recommend that the key goal of treatment for TSC-AML is to prevent renal AML progressive enlargement, thereby ameliorating the risk of future bleeding, impaired renal function and mortality15. Hence, the 2012 ITSCCC recommended mTOR inhibitors as the first-line therapy for asymptomatic renal AML measuring larger than 3 cm in diameter15. Two mTOR inhibitors, sirolimus and everolimus, have been evaluated for their efficacy and safety in the treatment of various manifestations associated with TSC42,43. Everolimus is an oral mTOR inhibitor derived from sirolimus, which had been approved by FDA in the treatment of patients with TSC-AML and adult patients with TSC-subependymal giant cell astrocytoma44, and the improved pharmacokinetic profile of everolimus over sirolimus makes it an attractive, noninvasive option for patients. Its efficacy and safety in the treatment of TSC-AML had been investigated in EXIST-2 and extension studies recently; the clinical trial found 54.93% of patients (39/71) showed 50% shrinkage of the tumors after they were administered everolimus for 6 months, and a pronounced benefit was demonstrated with continued use of everolimus17,18. In the present study, we tested the efficacy and safety of short-term everolimus therapy for TSC-AML. The results revealed that the volume of AML decreased 50% or more in 63.33% (19/30) of patients after 12 weeks of everolimus therapy, which showed a similar tumor size-reducing effect with prior studies45,46,47. Furthermore, the reduction rate of tumor size will gradually slow with the extension of treatment time, and these results are consistent with data reported by the EXIST-2 and extension trials17,18. However, our research shows that 7 patients (7/25; 28.00%) who were primarily sensitive to everolimus treatment, experienced an increase in renal AML tumor volume after the treatment stopped. Although similar results have been reported in other studies48,49,50, the AML progression rate was much higher in this study than in other long-term everolimus treatment studies. This may be due to a number of reasons, but the main reason is that the treatment time is short and possibly related to the sensitivity of the Chinese race to everolimus treatment. Overall, compared with long-term (≥6 months) everolimus treatment, the reduction rate of renal AML volume in short-term use of everolimus was similar, but with a higher risk of developing renal AML progression after discontinuation. This finding potentially implies that long-term use of everolimus in the treatment of TSC-AML results in more stable treatment efficacy than shorter-term use.
In addition to the treatment efficacy of everolimus for TSC-AML, a wide variety of everolimus treatment-related adverse events have also been reported17,18,51,52,53. Reported adverse events of everolimus include stomatitis, headache, infections, vomiting, hypercholesterolaemia, and irregular menstruation17,18. In the EXIST-2 extension study, nasopharyngitis (43%), stomatitis (43%) and headache (30%) were the most common adverse events with everolimus therapy18. In previous observations, most of the adverse events of everolimus treatment were grade 1–2, and few patients discontinued treatment for adverse events18. Our study showed similar results; although most patients presented with side effects, no patients discontinued or needed dose reduction because of adverse events, all of which were grade 1 or 2 and reversible. Menstrual disorders were also reported in this study, which occurred at a high rate and was the most common grade 2 adverse event in the nonmenopausal female group (4/17; 23.52%). Although the cause of menstrual disorders is still not very clear, some researchers think it may be caused by mTOR inhibitors disturbed hormone levels53,54,55. Because many TSC-AML patients are young, reduction of gonadal function should be explained to patients before the initiation of everolimus treatment. Overall, treatment with everolimus was safe during the observation period, and all adverse events were mild and manageable in this study.
Several limitations should be emphasized in the present study. Because of the relatively small sample size and because only descriptive statistics were available from a single center, a large multicenter study is needed in the future. A further limitation was the short observation time. In the present study, we only observed 12 weeks after the treatment stopped, and a comprehensive assessment of the long-term effects of short-term use of everolimus in the treatment of TSC-AML could not be performed.
Conclusions
In summary, this open single-center clinical study explored the clinical features and gene mutation information of TSC-AML patients in Northwest China and observed the efficacy and safety of short-term use of everolimus in patients with TSC-AML. Overall, the TSC gene mutation rate of TSC-AML patients in this study is much lower than in other reports. The short-term everolimus treatment for TSC-AML is effective and safe, but the stability is much lower than long-term therapy.
References
Curatolo, P., Bombardieri, R. & Jozwiak, S. Tuberous sclerosis. Lancet 372, 657–668, https://doi.org/10.1016/S0140-6736(08)61279-9 (2008).
European Chromosome 16 Tuberous Sclerosis, C. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75, 1305–1315 (1993).
van Slegtenhorst, M. et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277, 805–808 (1997).
Crino, P. B., Nathanson, K. L. & Henske, E. P. The tuberous sclerosis complex. The New England journal of medicine 355, 1345–1356, https://doi.org/10.1056/NEJMra055323 (2006).
Franz, D. N., Bissler, J. J. & McCormack, F. X. Tuberous sclerosis complex: neurological, renal and pulmonary manifestations. Neuropediatrics 41, 199–208, https://doi.org/10.1055/s-0030-1269906 (2010).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. The New England journal of medicine 358, 140–151, https://doi.org/10.1056/NEJMoa063564 (2008).
Castagnetti, M., Vezzu, B., Laverda, A., Zampieri, S. & Rigamonti, W. Urological counseling and followup in pediatric tuberous sclerosis complex. The Journal of urology 178, 2155–2159, https://doi.org/10.1016/j.juro.2007.07.058 (2007).
O’Callaghan, F. J., Noakes, M. J., Martyn, C. N. & Osborne, J. P. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int 94, 853–857, https://doi.org/10.1111/j.1464-410X.2004.05046.x (2004).
Nelson, C. P. & Sanda, M. G. Contemporary diagnosis and management of renal angiomyolipoma. The Journal of urology 168, 1315–1325, https://doi.org/10.1097/01.ju.0000028200.86216.b2 (2002).
Bissler, J. J. & Kingswood, J. C. Renal angiomyolipomata. Kidney Int 66, 924–934, https://doi.org/10.1111/j.1523-1755.2004.00838.x (2004).
Dibble, C. C. et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 47, 535–546, https://doi.org/10.1016/j.molcel.2012.06.009 (2012).
Huang, J. & Manning, B. D. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412, 179–190, https://doi.org/10.1042/BJ20080281 (2008).
Wienecke, R. et al. Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. Am J Kidney Dis 48, e27–29, https://doi.org/10.1053/j.ajkd.2006.05.018 (2006).
Franz, D. N. et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59, 490–498, https://doi.org/10.1002/ana.20784 (2006).
Krueger, D. A., Northrup, H. & International Tuberous Sclerosis Complex Consensus, G. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric neurology 49, 255–265, https://doi.org/10.1016/j.pediatrneurol.2013.08.002 (2013).
Tabernero, J. et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26, 1603–1610, https://doi.org/10.1200/JCO.2007.14.5482 (2008).
Bissler, J. J. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381, 817–824, https://doi.org/10.1016/S0140-6736(12)61767-X (2013).
Bissler, J. J. et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant 31, 111–119, https://doi.org/10.1093/ndt/gfv249 (2016).
Franz, D. N. et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381, 125–132, https://doi.org/10.1016/S0140-6736(12)61134-9 (2013).
Chevli, C. et al. Effect of pretreatment prostate volume on urinary quality of life following intensity-modulated radiation therapy for localized prostate cancer. Res Rep Urol 5, 29–37, https://doi.org/10.2147/RRU.S38093 (2013).
Lam, H. C., Nijmeh, J. & Henske, E. P. New developments in the genetics and pathogenesis of tumours in tuberous sclerosis complex. J Pathol 241, 219–225, https://doi.org/10.1002/path.4827 (2017).
Cai, Y., Li, H. & Zhang, Y. Assessment of Tuberous Sclerosis Complex Associated With Renal Lesions by Targeted Next-generation Sequencing in Mainland China. Urology 101, 170 e171–170 e177, https://doi.org/10.1016/j.urology.2016.10.056 (2017).
Dabora, S. L. et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68, 64–80, https://doi.org/10.1086/316951 (2001).
Zeng, L. H. et al. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet 20, 445–454, https://doi.org/10.1093/hmg/ddq491 (2011).
Jones, A. C. et al. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum Mol Genet 6, 2155–2161 (1997).
Yamashita, Y. et al. Analysis of all exons of TSC1 and TSC2 genes for germline mutations in Japanese patients with tuberous sclerosis: report of 10 mutations. Am J Med Genet 90, 123–126 (2000).
Jones, A. C. et al. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 64, 1305–1315, https://doi.org/10.1086/302381 (1999).
Yang, H. M. et al. The analysis of mutations and exon deletions at TSC2 gene in angiomyolipomas associated with tuberous sclerosis complex. Exp Mol Pathol 97, 440–444, https://doi.org/10.1016/j.yexmp.2014.09.013 (2014).
Yang, G. et al. Phenotypic and genotypic characterization of Chinese children diagnosed with tuberous sclerosis complex. Clinical genetics 91, 764–768, https://doi.org/10.1111/cge.12920 (2017).
Kwiatkowska, J., Wigowska-Sowinska, J., Napierala, D., Slomski, R. & Kwiatkowski, D. J. Mosaicism in tuberous sclerosis as a potential cause of the failure of molecular diagnosis. The New England journal of medicine 340, 703–707, https://doi.org/10.1056/NEJM199903043400905 (1999).
Peron, A., Au, K. S. & Northrup, H. Genetics, genomics, and genotype-phenotype correlations of TSC: Insights for clinical practice. American journal of medical genetics. Part C, Seminars in medical genetics 178, 281–290, https://doi.org/10.1002/ajmg.c.31651 (2018).
Nellist, M. et al. Targeted Next Generation Sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Med Genet 16, 10, https://doi.org/10.1186/s12881-015-0155-4 (2015).
Zheng, L. Y. et al. Novel mutations in Chinese Han patients with tuberous sclerosis complex: Case series and review of the published work. J Dermatol 45, 867–870, https://doi.org/10.1111/1346-8138.14349 (2018).
Lee, J. S. et al. Mutational analysis of paediatric patients with tuberous sclerosis complex in Korea: genotype and epilepsy. Epileptic Disord 16, 449–455, https://doi.org/10.1684/epd.2014.0712 (2014).
Niida, Y. et al. Mutational analysis of TSC1 and TSC2 in Japanese patients with tuberous sclerosis complex revealed higher incidence of TSC1 patients than previously reported. J Hum Genet 58, 216–225, https://doi.org/10.1038/jhg.2013.3 (2013).
Papadopoulou, A. et al. Screening for TSC1 and TSC2 mutations using NGS in Greek children with tuberous sclerosis syndrome. Eur J Paediatr Neurol 22, 419–426, https://doi.org/10.1016/j.ejpn.2018.01.026 (2018).
Avgeris, S. et al. Mutational analysis of TSC1 and TSC2 genes in Tuberous Sclerosis Complex patients from Greece. Sci Rep 7, 16697, https://doi.org/10.1038/s41598-017-16988-w (2017).
Amin, S. et al. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol 59, 612–617, https://doi.org/10.1111/dmcn.13352 (2017).
Shepherd, C. W., Gomez, M. R., Lie, J. T. & Crowson, C. S. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66, 792–796 (1991).
Prando, A. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Int Braz J Urol 28, 578–579 (2002).
Yamakado, K. et al. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology 225, 78–82, https://doi.org/10.1148/radiol.2251011477 (2002).
Micozkadioglu, H., Koc, Z., Ozelsancak, R. & Yildiz, I. Rapamycin therapy for renal, brain, and skin lesions in a tuberous sclerosis patient. Ren Fail 32, 1233–1236, https://doi.org/10.3109/0886022X.2010.517345 (2010).
Curatolo, P. et al. The Role of mTOR Inhibitors in the Treatment of Patients with Tuberous Sclerosis Complex: Evidence-based and Expert Opinions. Drugs 76, 551–565, https://doi.org/10.1007/s40265-016-0552-9 (2016).
Coombs, E. J. Role of mTOR inhibition in the treatment of patients with renal angiomyolipomas. J Am Assoc Nurse Pract 25, 588–596, https://doi.org/10.1002/2327-6924.12081 (2013).
Miller, J. M. et al. The effects of everolimus on tuberous sclerosis-associated lesions can be dramatic but may be impermanent. Pediatr Nephrol 30, 173–177, https://doi.org/10.1007/s00467-014-2949-6 (2015).
Dabora, S. L. et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One 6, e23379, https://doi.org/10.1371/journal.pone.0023379 (2011).
Davies, D. M. et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 17, 4071–4081, https://doi.org/10.1158/1078-0432.CCR-11-0445 (2011).
Bissler, J. J. et al. Angiomyolipoma rebound tumor growth after discontinuation of everolimus in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. PloS one 13, e0201005, https://doi.org/10.1371/journal.pone.0201005 (2018).
Cai, Y. et al. Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: a two years trial. Orphanet J Rare Dis 13, 43, https://doi.org/10.1186/s13023-018-0781-y (2018).
Brakemeier, S. et al. Treatment effect of mTOR-inhibition on tissue composition of renal angiomyolipomas in tuberous sclerosis complex (TSC). PLoS One 12, e0189132, https://doi.org/10.1371/journal.pone.0189132 (2017).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456, https://doi.org/10.1016/S0140-6736(08)61039-9 (2008).
Eisen, H. J. et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. The New England journal of medicine 349, 847–858, https://doi.org/10.1056/NEJMoa022171 (2003).
Braun, M. et al. Ovarian toxicity from sirolimus. The New England journal of medicine 366, 1062–1064, https://doi.org/10.1056/NEJMc1113145 (2012).
Fritsche, L. et al. Testosterone concentrations and sirolimus in male renal transplant patients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 4, 130–131 (2004).
Huyghe, E. et al. Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: an overview. Transpl Int 20, 305–311, https://doi.org/10.1111/j.1432-2277.2006.00423.x (2007).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81272812) and from the Forth Military Medical University of China (Technology Development Fund; No. 2018XC005).
Author information
Authors and Affiliations
Contributions
Jianxin Ni, Chunlin Hao, Teng Wang and Pengfei Liu, collected the clinical data; Fengqi Yan, Weijun Qin, Lei Yu, Geng Zhang, Fei Liu, Xiaojian Yang and Bo Yang analyzed the data; Fengqi Yan, Guojun Wu and Weijun Qin prepared the figures; Fengqi Yan and Guojun Wu wrote the main manuscript; Jianlin Yuan and Guojun Wu designed the experiments. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ni, J., Yan, F., Qin, W. et al. Mutational analysis of renal angiomyolipoma associated with tuberous sclerosis complex and the outcome of short-term everolimus therapy. Sci Rep 9, 14337 (2019). https://doi.org/10.1038/s41598-019-49814-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49814-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.