Abstract
Cassava (Manihot esculenta) is a major staple food, animal feed and energy crop in the tropics and subtropics. It is one of the most drought-tolerant crops, however, the mechanisms of cassava drought tolerance remain unclear. Abscisic acid (ABA)-responsive element (ABRE)-binding factors (ABFs) are transcription factors that regulate expression of target genes involved in plant tolerance to drought, high salinity, and osmotic stress by binding ABRE cis-elements in the promoter regions of these genes. However, there is little information about ABF genes in cassava. A comprehensive analysis of Manihot esculenta ABFs (MeABFs) described the phylogeny, genome location, cis-acting elements, expression profiles, and regulatory relationship between these factors and Manihot esculenta betaine aldehyde dehydrogenase genes (MeBADHs). Here we conducted genome-wide searches and subsequent molecular cloning to identify seven MeABFs that are distributed unevenly across six chromosomes in cassava. These MeABFs can be clustered into three groups according to their phylogenetic relationships to their Arabidopsis (Arabidopsis thaliana) counterparts. Analysis of the 5′-upstream region of MeABFs revealed putative cis-acting elements related to hormone signaling, stress, light, and circadian clock. MeABF expression profiles displayed clear differences among leaf, stem, root, and tuberous root tissues under non-stress and drought, osmotic, or salt stress conditions. Drought stress in cassava leaves and roots, osmotic stress in tuberous roots, and salt stress in stems induced expression of the highest number of MeABFs showing significantly elevated expression. The glycine betaine (GB) content of cassava leaves also was elevated after drought, osmotic, or salt stress treatments. BADH1 is involved in GB synthesis. We show that MeBADH1 promoter sequences contained ABREs and that MeBADH1 expression correlated with MeABF expression profiles in cassava leaves after the three stress treatments. Taken together, these results suggest that in response to various dehydration stresses, MeABFs in cassava may activate transcriptional expression of MeBADH1 by binding the MeBADH1 promoter that in turn promotes GB biosynthesis and accumulation via an increase in MeBADH1 gene expression levels and MeBADH1 enzymatic activity. These responses protect cells against dehydration stresses by preserving an osmotic balance that enhances cassava tolerance to dehydration stresses.
Similar content being viewed by others
Introduction
Cassava (Manihot esculenta) is a perennial euphorbiaceous shrub grown mainly for its starchy tubers, which are used as food, animal feed, and in non-food products1,2,3. With its inherent high drought and poor nutrients tolerance, as well as high starch content, cassava is a critical resource2,3,4,5,6,7. However, the mechanisms underlying the stress tolerance of cassava remain unclear. Drought is a major abiotic stress that can reduce crop output8. In some areas that experience unpredictable water shortages, enhancement of plant resistance or tolerance to drought is important for minimizing crop losses8. Various strategies to combat drought stress include maintaining water status through rapid stomatal closure and changes in growth and development patterns9. Both rapid stomatal movement and integrated growth plasticity involve long distance communication between different organs that is primarily mediated by the stress-related phytohormone abscisic acid (ABA)9.
ABA is indispensable for plant growth and development10. ABA regulates a variety of plant developmental processes such as leaf senescence, seed maturation and dormancy, bud dormancy, and adaptive responses to abiotic and biotic stresses, in particular drought and salinity, by controlling stomata closure, osmotic potential, and/or wax deposition11,12. In one verified model for ABA signaling, the presence of ABA is sensed by the pyrabactin resistance (PYR)/PYR-like (PYL)/regulatory components of the ABA receptor (RCAR)13,14,15. ABA ligation to the RCAR receptor induces a conformational change in members of the PYR/PYL/RCAR receptor family, rendering them able to bind and inhibit type 2C protein phosphatases (PP2C) and in turn release inhibition of sucrose non-fermenting 1-related protein kinase 2 (SnRK2)13,14,15. ABA-activated SnRK2 then phosphorylates ABA-responsive element (ABRE)-binding factors (ABFs) such as ABF2 to induce upregulation of ABA-responsive gene expression13,14,15.
ABF is a family of ABA-dependent bZIP transcription factors that interact with the ABRE (PyACGTGG/TC), a conserved cis-acting regulatory sequence found in the 5′ flanking regions of many ABA-responsive genes in plants16,17,18. The ABF family genes ABF1, ABF2, ABF3, and ABF4 are mainly expressed in vegetative tissues of Arabidopsis exposed to abiotic stress conditions19. Whereas ABF1 expression is significantly induced by cold but not osmotic stress20, expression of the other three ABF genes (ABF2, ABF3, and ABF4) is induced both by ABA and dehydration stresses such as drought and high salinity. These three factors have been shown to be key regulators of ABA signaling in response to osmotic stress20, as was found in Gossypium hirsutum in which overexpression of exogenous and endogenous ABFs substantially increase drought tolerance, primarily through stomatal regulation that reduces transpiration and photosynthetic productivity21.
Although ABFs play a significant role in responses to abiotic stresses in plants, little is known about the exact role ABFs play in cassava. To explore the role of Manihot esculenta ABFs (MeABFs) in drought tolerance of cassava, we identified and characterized candidate MeABFs. Using bioinformatic methods, these genes were localized in the cassava genome, constructed as phylogenetic trees, and analyzed for stress-responsive cis-elements in the promoter regions. We then investigated the expressional patterns of MeABFs in different cassava tissues after exposure to drought, PEG, or high salt. Drought, osmotic, or salt stresses in one or two cassava tissues induced significantly elevated expression of MeABFs.
Glycine betaine (GB), a bipolar quaternary ammonium compound that acts as an osmoprotectant, accumulates in many plant species in response to various abiotic stresses22. GB protects plants from abiotic stresses by maintaining the osmotic balance under various abiotic stresses and by stabilizing the quaternary structure of complex proteins23. We observed increases in GB content in cassava following exposure to drought, osmotic, or salt stress treatments. Betaine aldehyde dehydrogenase (BADH) converts betaine aldehyde into GB in plants24. As such, we speculated that MeABF and Manihot esculenta BADH (MeBADH) levels might be correlated. We further found that the promoter regions of MeBADH1 contain ABRE cis-acting element that could interact with ABFs. Indeed, MeBADH expression profiles in cassava exposed to drought, osmotic, or salt stress tended to be similar to those for MeABF genes. These results suggest that MeABFs may affect GB content by regulating MeBADH gene expression that in turn confers drought tolerance to cassava. In this study, we provide a theoretical foundation for further explanation of molecular mechanisms involved in cassava drought tolerance, and provide a basis for cultivation of novel drought-tolerance cassava varieties and breeding resources.
Results
MeABF identification and phylogenetic analysis
Seven members of the MeABF family were identified in the cassava cultivar South China 5 (SC5) by BLAST searches of the Phyzotome database and NCBI database. The resulting genes, termed MeABF1, MeABF2, MeABF3, MeABF4, MeABF5, MeABF6, and MeABF7, were then cloned and sequenced, and their sequences were aligned with the counterparts of the cassava cultivar AM560-2.
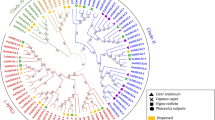
To examine phylogenetic relationships among ABFs, a phylogenetic tree was constructed using the amino acid sequences of ABFs from Arabidopsis and cassava (Supplementary Fig. S1 and Table S1). The phylogenetic tree revealed that the seven MeABFs could be classified into three groups (A, B, and C). Group A MeABFs underwent a significant expansion, as two MeABFs (MeABF2 and MeABF3) existed in cassava, whereas one corresponding Arabidopsis ortholog (Arabidopsis thaliana ABF2, AtABF2) were present. The size of Group B MeABFs decreased as evidenced by a single MeABF1 and three AtABFs (AtABF1, AtABF3, and AtABF4). Surprisingly, Group C included four MeABFs (MeABF4, MeABF5, MeABF6, and MeABF7) that were exclusive to cassava. Furthermore, MeABF2 and MeABF3, and MeABF5 and MeABF6 showed high sequence similarity with each of the other among seven MeABFs.
MeABF chromosomal distribution and cis-acting element analysis
To determine the chromosomal distribution of the identified MeABFs, we subjected the genomic sequences of the seven MeABFs to BLAST analysis against the cassava genome database. The location image shows that the seven MeABFs could be successfully mapped to six of the 18 chromosomes present in the cassava genome (Fig. 1). MeABF2 and MeABF4 were distributed on chromosome 18, and MeABF1, MeABF3, MeABF5, MeABF6, and MeABF7 were present on chromosomes 10, 5, 8, 11, and 2, respectively.
Distribution of MeABFs on cassava chromosomes. The chromosome numbers and size are indicated at the top and bottom of each bar, respectively. The numbers on the right side of the bars designated the approximate physical positions of the first exon of corresponding MeABFs on cassava genome. The triangles next to gene names show the transcription directions.
To further explore transcriptional regulation of MeABFs, the promoter sequence cis-elements of these genes were predicted using PlantCare software (Supplementary Table S2). Previous studies showed that promoters for most MeABFs contained one or more cis-elements of genes that act in hormone signaling pathways involving ABA (ABRE), jasmonic acid (CGTCA-motif and TGACG-motif), gibberellic acid (GARE-motif, P-box, and TATC-box), salicylic acid (TCA-element), and auxin (TGA-element). In this study, we found that the upstream regions of most of the identified MeABFs contained one or more cis-elements related to stress responses. These elements include Box-W1 (fungal elicitor responsive element), HSE (heat stress response element), LTR (low-temperature response element), MBS (MYB binding site involved in drought-inducibility), TC-rich repeats (cis-acting element involved in defense and stress responsiveness), W box (WRKY binding site involved in abiotic stress responsiveness), and box S (elicitor-responsive element involved in the wounding and pathogen responsiveness). Interestingly, the promoter regions of most MeABFs contain one element involved in circadian clock and additional elements involved in light responsiveness. The cis-regulatory elements present in the 5′ upstream regions of the MeABFs identified here suggested that they play an important mediating role in plant growth and development, as well as in responses to various stresses.
MeABF expression levels in cassava leaves under dehydration stresses
All of the measured MeABF genes of cassava leaf tissue showed differential expression profiles after drought, osmotic, or salt stress treatments (Fig. 2), except for MeABF5 and MeABF6, whose expression levels were too low to detect. This phenomenon also occurred in stems, roots and tuberous roots (Figs 3–5).
Expression profiles of MeABFs in cassava leaves under three stress conditions. Relative expression values for the target genes were calculated according to the 2−ΔΔCt method. The heatmap was generated based on the log2 of relative expression values using MeV software. Red, green and white indicate up-regulated, down-regulated and unchanged expression, respectively. Asterisk on the right corner of the value indicates a statistically significant difference at P -value < 0.05 and the absolute value of log2 relative expression values >1. D, drought stress; O, osmotic stress; S, salt stress.
Expression profiles of MeABFs in cassava stems under three stress conditions. Relative expression values for the target genes were calculated according to the 2−ΔΔCt method. The heatmap was generated based on the log2 of relative expression values using MeV software. Red, green and white indicate up-regulated, down-regulated and unchanged expression, respectively. Asterisk on the right corner of the value indicates a statistically significant difference at P -value < 0.05 and the absolute value of log2 relative expression values >1. D, drought stress; O, osmotic stress; S, salt stress.
Expression profiles of MeABFs in cassava roots under three stress conditions. Relative expression values for the target genes were calculated according to the 2−ΔΔCt method. The heatmap was generated based on the log2 of relative expression values using MeV software. Red, green and white indicate up-regulated, down-regulated and unchanged expression, respectively. Asterisk on the right corner of the value indicates a statistically significant difference at P -value < 0.05 and the absolute value of log2 relative expression values >1. D, drought stress; O, osmotic stress; S, salt stress.
Expression profiles of MeABFs in cassava tuberous roots under three stress conditions. Relative expression values for the target genes were calculated according to the 2−ΔΔCt method. The heatmap was generated based on the log2 of relative expression values using MeV software. Red, green and white indicate up-regulated, down-regulated and unchanged expression, respectively. Asterisk on the right corner of the value indicates a statistically significant difference at P -value < 0.05 and the absolute value of log2 relative expression values > 1. D, drought stress; O, osmotic stress; S, salt stress.
The transcript levels of the MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 of cassava leaves were significantly elevated or remained at a similar level as the control at all points in time in response to drought stress (Fig. 2, Supplementary Tables S3 and S4), with significant up-regulation by 33.33%, 83.33%, 100.00%, 33.33%, and 50.00%, respectively (Supplementary Table S5).
Compared to the control, the expression of MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 was significantly increased or unchanged in contrast to the control at any point in time in cassava leaves treated by osmotic stress, with the exception of MeABF2 (2 h and 8 h) and MeABF4 (2 h), which were suppressed at one or two points in time (Fig. 2, Supplementary Tables S3 and S4). The observed significant increases relative to control were 50.00%, 33.33%, 50.00%, 33.33%, and 50.00% for MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7, respectively (Supplementary Table S5).
The expression levels of MeABF1, MeABF2, and MeABF3 in cassava leaves were significantly increased only at 12 h, whereas the levels for MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 were significantly reduced or sustained at a similar level as the control at most time points by salt stress (Fig. 2, Supplementary Tables S3 and S4). The percentage change relative to control was 16.67%, 16.67%, 16.67%, 0%, and 0% for MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7, respectively, significantly induced by salt stress (Supplementary Table S5).
In cassava leaves, drought stress significantly elevated the expression of the highest number of MeABFs (60.00%), followed by osmotic stress (43.33%) and then salt stress (10.00%, Supplementary Table S5).
MeABF expression levels in cassava stems under dehydration stresses
In cassava stems, MeABF1 and MeABF3 expression levels were significantly up-regulated or sustained at a level similar to that of the control throughout the drought treatment period, except at 15 d when MeABF3 levels were significantly reduced. MeABF2, MeABF4, and MeABF7 expression was significantly decreased or unchanged at any point in time under drought stress (Fig. 3, Supplementary Tables S6 and S7). In addition, MeABF1 and MeABF3 expression was significantly upregulated by 83.33% and 33.33%, respectively, relative to control (Supplementary Table S8).
After osmotic stress treatment, MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 expression in cassava stems was significantly increased or unchanged relative to the control at all points in time, with the exception of MeABF7 levels at 2 h, which were significantly reduced (Fig. 3, Supplementary Tables S6 and S7). Expression of these five MeABFs was significantly induced relative to control by 66.67%, 50.00%, 83.33%, 16.67%, and 16.67%, respectively (Supplementary Table S8).
For salt stress treatment, MeABF1, MeABF2, MeABF3, and MeABF7 expression in cassava stems was significantly elevated or remained at a similar level as the control, whereas MeABF4 expression was significantly suppressed at any point in time except for at 1 h, when expression was significantly up-regulated (Fig. 3, Supplementary Tables S6 and S7). MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 expression was significantly elevated by 83.33%, 33.33%, 100.00%, 16.67%, and 16.67%, respectively, relative to the control (Supplementary Table S8).
Salt stress significantly elevated the expression of the highest number of MeABFs (50.00%) in cassava stems, followed by osmotic stress (46.67%) and then drought stress (23.33%, Supplementary Table S8).
MeABF expression levels in cassava roots under dehydration stresses
In cassava roots, transcript levels of MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 were significantly increased or sustained at a level similar to that of the control by drought stress, with the exception of MeABF2 and MeABF4, which were significantly reduced at only one point in time (9 d and 1 d, respectively; Fig. 4 and Supplementary Tables S9 and S10). The expression of these five was significantly induced by 66.67%, 66.67%, 33.33%, 50.00%, and 66.67%, respectively, relative to the control (Supplementary Table S11).
MeABF3 and MeABF7 expression in cassava roots was significantly up-regulated or unchanged in contrast to the control in response to osmotic stress. The expression levels of MeABF1, MeABF2, and MeABF4 were significantly decreased or sustained at a level similar to that of the control at any point in time under osmotic stress (Fig. 4, Supplementary Tables S9 and S10). The rate of significant up-regulation for MeABF3 and MeABF7 was 66.67% and 16.67%, respectively (Supplementary Table S11).
Under salt stress, MeABF3 expression was significantly induced (66.67%) or remained at a level similar to that of the control in cassava roots, except at 24 h, when the level was significantly suppressed. The expression levels of MeABF1, MeABF2, MeABF4, and MeABF7 in cassava roots were significantly reduced or unchanged at any point in time by salt stress (Fig. 4, Supplementary Tables S9–11).
In cassava roots, drought stress significantly elevated the expression of the highest number of MeABFs (56.67%), followed by osmotic stress (16.67%) and then salt stress (13.33%, Supplementary Table S11).
MeABF expression levels in tuberous roots under dehydration stresses
The transcript levels of MeABF1, MeABF2, MeABF3, and MeABF7 in cassava tuberous roots were significantly increased or sustained at a level similar to that of the control at all points in time by drought stress. MeABF4 expression levels in tuberous roots were significantly reduced or unchanged after drought stress treatment (Fig. 5, Supplementary Tables S12 and S13). The induction rate for MeABF1, MeABF2, MeABF3, and MeABF7 was 100.00%, 66.67%, 83.33% and 16.67%, respectively (Supplementary Table S14).
MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 expression in cassava tuberous roots was significantly up-regulated or unchanged relative to the control at any point in time by osmotic stress treatment, with the exception of MeABF2 (1 h), MeABF3 (1 h), and MeABF7 (1 h and 4 h), which were significantly down-regulated at one or two points in time (Fig. 5, Supplementary Tables S12 and S13). The rate of significant induction of expression was 100.00%, 16.67%, 66.67%, 50.00%, and 66.67%, respectively (Supplementary Table S14).
After salt stress treatment, MeABF1, MeABF2, MeABF3, MeABF4, and MeABF7 expression levels in tuberous roots were significantly reduced at any point in time (Fig. 5, Supplementary Tables S12 and S13).
Osmotic stress significantly elevated the expression of the highest number of MeABFs (60.00%) in cassava tuberous roots, followed by drought stress (53.33%) and then salt stress (0.00%; Supplementary Table S14).
Measurement of GB content in cassava
We also measured GB content in the fresh leaves of cassava that had been treated by drought, osmotic, or salt stress (Fig. 6, Supplementary Tables S15 and S16). The GB content was significantly altered by the three stresses and similar patterns manifested as an initial increase, a decrease, and then an increase and a decrease. Furthermore, GB levels increased and then decreased during the final stages under drought stress conditions. GB exhibited a maximum accumulation at 4 h after osmotic and salt stress treatments and at 15 d after drought stress treatment. Overall, GB content was elevated under drought, osmotic, and salt stress conditions in cassava leaves (Supplementary Table S17).
Identification of MeBADHs, cis-acting elements, and expression analysis
Two members of the MeBADH family, termed MeBADH1 and MeBADH2, were identified in cassava using the same methods as those used to identify MeABFs. Cis-acting element analysis in the promoter sequence of these two genes showed that the upstream region of MeBADH1 contained three types of cis-elements that act in hormone signaling pathways involving ABA (ABRE), gibberellin (GARE-motif), and auxin (TGA-element), whereas the MeBADH2 promoter contained only the ERE cis-element (ethylene-responsive element) (Supplementary Table S18). The MeBADH1 and MeBADH2 promoter regions both contained two or more kinds of cis-elements related to stress responses, and seven or more kinds of cis-elements involved in light responsiveness. The MeBADH1 promoter also contained a circadian cis-element. MeBADH1 and MeBADH2 showed differential expression patterns in cassava leaf tissue after drought, osmotic, and salt stress treatments (Fig. 7; Supplementary Tables S19 and S20). MeBADH1 expression was significantly induced by 50%, 16.67%, and 0% under drought, osmotic, and salt stresses, respectively. Meanwhile, induction of MeBADH2 expression was minimal in response to all three stresses (Supplementary Table S21).
Expression profiles of MeBADHs in cassava leaves under three stress conditions. Relative expression values for the target genes were calculated according to the 2−ΔΔCt method. The heatmap was generated based on the log2 of relative expression values using MeV software. Red, green and white indicate up-regulated, down-regulated and unchanged expression, respectively. Asterisk on the right corner of the value indicates a statistically significant difference at P -value < 0.05 and the absolute value of log2 relative expression values > 1. D, drought stress; O, osmotic stress; S, salt stress.
Discussion
Effect of dehydration stresses on MeABF transcription levels in cassava
Abiotic stresses, such as drought or salinity, cause intensive losses to agricultural production worldwide25. Plants have evolved complex molecular, cellular, and physiological mechanisms to respond to these stresses in order to survive adverse conditions21. These mechanisms include signaling pathways, such as those activated by ABA, and other stress-responsive gene families21. ABA acts as a growth regulator in response or tolerance to abiotic stressors such as drought, salinity, cold, and heat26,27,28,29. The ABF family of bZIP transcription factors functions in ABA signaling pathways and plays an important role in plant responses to stresses18,21. Here we found that in various cassava organs, MeABFs exhibited differential expression patterns after drought, osmotic, and salt stress treatments(Figs 2–5), which was consistent with trends we observed in cassava in our previous study that identified genes involved in ethylene signaling in response to dehydration stresses8. Our results of this study showed that drought stress in cassava leaves and roots, osmotic stress in cassava tuberous roots, and salt stress in cassava stems induced expression of the highest number of MeABFs in terms of significantly elevated expression (Supplementary Tables S5, S8, S11 and S14). Although many of the MeABFs overlap with AtABF homologs in Arabidopsis in that their expression is induced by similar abiotic stresses and their target genes overlap, differences in their temporal and spatial expression patterns indicate that each has a unique function16,19,30,31,32,33.
The main negative effect of drought on plants is a shortage of water available to tissues. To reduce the amount of water lost to transpiration under drought stress, in cassava plants, leaf stomata partially close and leaf area is reduced via ABA signaling pathway, which restricts the formation of new leaves, results in leaves having a smaller size and that droop, and promotes leaf loss34,35,36. Leaf formation is regarded as an important indicator used to assess the drought tolerance of various cassava varieties37.
NaCl can negatively affect the energetic, hydric, and nutritional equilibria of plants38. Plants grown under salt stress are first affected by water stress and then by sodium and chloride ion-mediated toxicity and nutritive stresses39. Salinity can reduce the biomass, leaf area, and photosynthetic rate of cassava plants40. Therefore, NaCl not only causes the dehydration of cassava plants, similar to drought stress, but also disturbs other physiological processes and metabolic pathways related to ion homeostasis.
Unlike NaCl, which readily enters the cells to cause toxicity, PEG, a nonabsorbable, non-metabolizable and non-toxic osmotic agent, is often used because of its high molecular weight, which precludes its entry into plant cells via the cell wall41,42. Our results indicated that PEG affect plants through a different mechanism than that involved drought and salt stresses; this mechanism awaits further elucidation.
Other environmental factors that affect MeABF transcription in cassava
Under dehydration stress, circadian clock and light conditions also appear to regulate ABA- mediated gene expression, likely conferring versatile tolerance and repressing growth under stress conditions20. Analysis of cis-acting elements in MeABF promoter sequences showed that MeABF transcription can be regulated by circadian clock, light, and hormones, except for by biotic and abiotic stresses (Supplementary Table S2).
Circadian clock is an endogenous and cell-autonomous biological timekeeper that generates roughly 24 h rhythms and provides an adaptive advantage by synchronizing physiological and metabolic processes of the plant to the external environment43. Plants are usually exposed to drought stress during the day, and water deficit crises would be at maximum levels near the end of the day44. Consequently, ABA-dependent drought responses are usually gated primarily around dusk45. The rhythmic expression of drought-responsive genes confers rhythmic modulation of drought responses throughout the day, as seen in Arabidopsis and poplar46,47. The ABA-inducible MYB96 transcription factor activates TIMING OF CAB EXPRESSION 1 (TOC1) expression by binding directly to its gene promoter48. This regulation may be direct, as PSEUDO-RESPONSE REGULATOR 7 directly binds to the promoter region of ABA DEFICIENT 1, which encodes a zeaxanthin epoxidase involved in ABA biosynthesis49. The pervasive TOC1 also interacts with ABA INSENSITIVE 3 (ABI3), which has roles in ABA signaling and drought tolerance50. Bidirectional interactions between circadian oscillator and output pathways have also been observed in ABA-related physiological processes51.
ELONGATED HYPOCOTYL 5 (HY5), a constitutively-nuclear bZIP protein, was the first transcription factor that was found to promote photomorphogenesis and has been extensively studied52,53. HY5 physically interacts with B-box 21 (BBX21), BBX22, BBX24 and BBX25, and the bZIP domain of HY5 and the B-box domains of these BBX proteins mediate their interactions54,55,56,57,58,59. HY5 also promotes BBX22 expression by directly binding to its promoter54, whereas BBX24 and BBX25 repress BBX22 expression by interfering with HY5 transcriptional activity58. BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter60. In addition, ABI5 can directly activate its own expression, whereas BBX21 negatively regulates this activity through interactions with ABI5. These results indicate that BBX21 coordinates with HY5 and ABI5 on the ABI5 promoter and that these transcriptional regulators work in concert to integrate light and ABA signaling in Arabidopsis60. The influence of ABA on phototropin expression is negligible at the mRNA level, but prominent at the protein level, and ABA appears to enhance plant sensitivity to light and promote the chloroplast avoidance response61. An influence of ABA on light-mediated chloroplast positioning has also been implicated in succulent plants exposed to water-deficit stress62.
Collectively, these observations may support the view that the circadian clock and light mediate the ability of a plant to adapt to daily changes in water status by controlling endogenous MeABF levels and subsequently MeABF gene expression. Interestingly, the cis-acting element ABRE is present in MeABF promoter sequences (Supplementary Table S2). Therefore, MeABFs could likely bind their own promoter to activate their expression as was demonstrated in studies on ABI560. Furthermore, expression of two genes encoding 9-cis-epoxycarotenoid dioxygenase, a key enzyme in ABA biosynthesis, was up-regulated in response to water deficits63 and might be responsive to ABA itself64. These findings indicated that a positive feedback loop controls ABA biosynthesis and ABA signaling during water deficit stress. A more recent study found that ABFs played a role in the negative feedback regulation of ABA signaling by mediating rapid ABA-mediated induction of group A PP2C gene expression65.
Effect of dehydration stresses on GB content in cassava leaves
To cope with harsh habitats such as high salt, drought, heat, and cold, plants have evolved various types of tolerance mechanisms, among which the accumulation of compatible solutes plays a key role in balancing the intracellular osmotic potential of plants24. Under conditions of water deficit or salinity stress, plant ABA levels increase dramatically, restricting water loss by stimulating stomatal closure and protecting cellular machinery against dehydration damage by promoting the accumulation of osmo-compatible solutes44. GB is regarded as one of the most effective compatible solutes66. GB can protect cells from stresses by maintaining an osmotic balance with the surrounding environment and by stabilizing the quaternary structures of complex proteins, such as antioxidant enzymes and the oxygen-evolving PSII complex67.
Taken together, we found that cassava leaves had increased GB concentrations in response to drought, osmotic, and salt stresses (Fig. 6, Supplementary Table S17). Furthermore, osmotic stress had the largest impact on the levels of GB content followed by drought stress and then salt stress (Supplementary Table S17). These results suggest that cassava plants may induce expression of BADH genes via the ABA signaling pathway to increase the GB content and adapt to harsh habitats.
MeABF regulation of MeBADH transcription
In higher plants, BADH catalyzes the key step of GB biosynthesis23. In Ammopiptanthus nanus, endogenous expression of AnBADH is strongly induced by exposure to high salt, drought, ABA, heat, or cold24. Heterologous expression of AnBADH in Arabidopsis enhanced its tolerance to high salt and drought stresses, suggesting that BADH could play a critical role in plant abiotic tolerance through ABA signaling pathways24. An incremental increase in BADH activity induced by ABA treatment of maize also promoted increases in GB content under drought stress68. Activated SnRK2s phosphorylate and activate BADH, as do scavenging reactive oxygen species and many other proteins related to abiotic tolerance69,70,71. Therefore, we speculate that ABFs may activate BADH production in plants after ABFs are phosphorylated and activated by SnRK2s via the ABA signaling pathway.
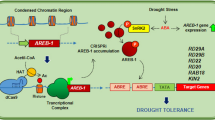
To examine this possibility, in this study we assessed transcriptional expression of MeBADH1 and MeBADH2 in cassava (Fig. 7; Supplementary Tables S19 and S20). MeBADH1 expression was induced in cassava leaves in response to drought and osmotic stresses, whereas MeBADH2 levels were not affected. A similar pattern was observed in Arabidopsis, wherein one BADH family member was targeted to leucoplasts and involved in tolerance to high salt and drought, while another family member was targeted to peroxisomes and did not confer abiotic tolerance72,73,74. MeBADH1 promoter sequences contained an ABRE, a cis-acting regulatory element that interacts with ABFs (Supplementary Table S18). Furthermore, in cassava leaves exposed to drought, osmotic, or salt stress, the MeBADH1 expression pattern was concomitant with expression profiles of MeABFs (Figs 2 and 7; Supplementary Tables S5 and 21). Therefore, after various dehydration stress treatments, the expression of MeABFs in cassava plants may activate MeBADH1 transcription by binding to the MeBADH1 promoter in response to ABA signaling, and promote GB biosynthesis and accumulation by mediating increases in MeBADH1 gene expression and MeBADH1 enzymatic activity. These responses could protect cassava plant cells from dehydration stresses by preserving the osmotic balance, thereby improving tolerance of cassava plants to dehydration stresses (Fig. 8).
Proposed mechanism by which ABF promotes plant tolerance to dehydration stress65. In response to dehydration stress, ABA is ligated to PYR/PYL/RCAR proteins that interact with PP2Cs and inhibit their activity, in turn activating SnRK2, which phosphorylates ABF. ABF gene and protein expression is promoted by ABF themselves during ABA signaling. ABF promotes rapid BADH protein synthesis and triggers generation and accumulation of GB, which maintains osmotic balance to increase cassava tolerance to dehydration stresses.
Conclusions
In summary, increasing evidence supports that ABF is an ABA-dependent transcription factor that regulates expression of downstream ABA-responsive genes in response to a variety of abiotic stresses in plants. In this study, we identified seven MeABFs that were distributed unevenly across six chromosomes, and clustered into three groups according to their phylogenetic relationships to Arabidopsis counterparts. Analysis of the 5′-upstream region of MeABFs revealed putative cis-acting elements related to hormone signaling, stress, and light responses, as well as circadian clock. Expression profiles of MeABF genes displayed clear differences among leaf, stem, root, and tuberous root tissues under normal and stress conditions, including drought, osmotic, or salt stress. Drought stress in cassava leaves and roots, osmotic stress in tuberous roots, and salt stress in stems induced expression of the highest number of MeABFs that showed significantly elevated expression. The GB content increased under drought, osmotic, or salt stress conditions in cassava leaves. BADH1 is involved in GB synthesis and the promoter sequence of MeBADH1 contains an ABRE cis-acting element that would interact with ABFs. Furthermore, MeBADH1 expression profiles were consistent with those for MeABFs in cassava leaves after the three stress treatments. These findings suggest that MeABFs activate MeBADH1 transcription by binding to the MeBADH1 promoter to induce MeBADH1 production that in turn promotes GB biosynthesis that can preserve the osmotic balance of cassava cells to provide tolerance to dehydration stresses.
Methods
Plant materials
Samples of cassava cultivar SC5 were obtained from the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, China. To investigate the MeABF and MeBADH expression patterns and GB content in plantlet tissues in response to various treatments, 3-month-old cassava plants grown in plastic flower pots were used. For drought stress treatment, irrigation was withheld until no leaves remained on the plants. Since the plants lived for 18 days after drought treatment, we collected materials at 0 d, 3 d, 6 d, 9 d, 12 d, 15 d and 18 d after drought treatment. For osmotic or salt stress treatments, samples were collected at 0, 1, 2, 4, 8, 12, and 24 h after treatment with 400 mM NaCl or 30% PEG-60008. For the control, cassava plants were watered on intervals of every three days. Experiments were carried out on three independent biological replicates.
After the experimental treatments, cassava plants were removed carefully from the plastic pots to allow collection of leaf, stem, root, and tuberous root tissues. All samples were frozen by immersion in liquid nitrogen immediately after collection and stored at −80 °C until analyzed.
Isolation and identification of MeABFs and MeBADHs
To isolate MeABF and MeBADH genes, the nucleotide and amino acid sequences of AtABFs and Arabidopsis thaliana BADHs (AtBADHs) were downloaded from the database of TAIR (http://www.arabidopsis.org). The nucleotide and amino acid sequences of MeABF and MeBADH family members were searched using BLAST server and AtABF and AtBADH sequences as the query at Phyzotome (http://phytozome.jgi.doe.gov/pz/portal.html) database and NCBI (http://www.ncbi.nlm.nih.gov/) database8.
The cDNA sequences of likely MeABF and MeBADH genes in the cassava cultivar SC5 were cloned, sequenced, and then aligned with their counterparts from the cassava cultivar AM560-2. If these gene sequences showed more than 95% homology to those of the AM560-2, then identification was considered positive. Identified MeABF and MeBADH genes were reannotated and named.
In silico analysis of MeABFs and MeBADHs
Phylogenetic and molecular evolutionary analyses of amino acid sequences of ABFs from cassava and Arabidopsis were performed using the MEGA 6.0 software75 by the neighbor-joining method76 with 1,000 bootstrap replicates.
Chromosomal locations of MeABFs were obtained using the BLAST program in Phyzotome websit (http://phytozome.jgi.doe.gov/pz/portal.html) and MapInspect software (http://mapinspect.software.informer.com) was subsequently used to draw the location images of MeABFs8.
To investigate the cis-elements in the promoter sequences of MeABFs and MeBADHs, the 1.5 kb genomic DNA sequences located the upstream of initiation codon (ATG) representing the core promoter regions of these genes were retrieved from the database of Phyzotome (http://phytozome.jgi.doe.gov/pz/portal.html). These sequences were converted to FASTA formatted sequences, and saved to pure text files. PlantCare software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html) was used to identify the putative cis-acting regulatory elements located within these sequnces. Firstly, click the ‘Search for CARE’ button, and a new window will appear. Then, click the ‘Select file’ button, and submit the FASTA formatted sequences. Simultaneously, fill in the email address and the sequence name or ID. Finally, click the ‘Search’ button, and the predicted results will be obtained.
Expression analysis of MeABFs and MeBADHs
Total RNA was extracted from the various tissues of cassava plants using the cetyltrimethyl ammonium bromide method8. Contaminating DNA in total RNA samples was eliminated by treatment with DNase I (RNase-free; Thermo Scientific, USA). First-strand cDNA was synthesized from 1.0 µg of total RNA using the first-strand cDNA synthesis kit according to the manufacturer’s instructions (Thermo Scientific, USA).
Gene-specific primer pairs (Supplementary Table S22) for quantitative real-time PCR (qRT-PCR) were designed using Primer Express Software v3.0 (Thermo Scientific, USA). The PCR mix for qRT-PCR contained 1.0 µl of diluted cDNA, 10 µl of 2× SYBR Green PCR Master Mix (Thermo Scientific, USA), and 200 nM of each gene-specific primer in a final volume of 20 µl. To determine the expression profiles of MeABFs and MeBADHs in cassava from stress-treated and control plants, qRT-PCR was performed using a Stratagene Mx3005P thermal cycler (Agilent Technologies, USA). Three independent biological replicates were performed for each time point of three stress treatments, with three technical replicates per qRT-PCR. The cassava Actin gene (Supplementary Tables S1 and S22) was used as reference gene for the normalization of RNA steady-state level, and the 2−ΔΔCt method was used to calculate the relative expression values for the target genes8.
The log2 relative expression values were then visualized as heatmaps using the Multiple Experiment Viewer (MeV) software8. The absolute value of log2 relative expression values > 1.0 and P -value < 0.05 (determined by two-tailed Student’s t -test) was used as the threshold to assess the significant change in gene expression.
Quantitation analysis of GB
GB content was measured using the commercially available plant GB assay kit (Cat. No. ml036338; Shanghai Enzyme-linked Biotechnology Co., Ltd., China). The GB content of fresh leaves from stress-treated and control cassava plants was measured using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions. Statistical analysis was carried out using Student’s t -test and assigned P -value < 0.05, which was considered statistically significant.
References
Olsen, K. M. & Schaal, B. A. Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am. J. Bot. 88, 131–142 (2001).
Nassar, N. M., Hashimoto, D. Y. & Fernandes, S. D. Wild Manihot species: botanical aspects, geographic distribution and economic value. Genet. Mol. Res. 7, 16–28 (2008).
Laban, T. F., Kizito, E. B., Baguma, Y. & Osiru, D. Evaluation of Ugandan cassava germplasm for drought tolerance. Intl. J. Agri. Crop Sci. 5, 212–226 (2013).
El-Sharkawy, M. A. International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica. 44, 481–512 (2006).
Bakayoko, S. et al. Impact of water stress on fresh tuber yield and dry matter content of cassava (Manihot esculenta Crantz) in Côte d’Ivoire. Afr. J. Agric. Res. 4, 21–27 (2009).
Ogola, J. & Mathews, C. Adaptation of cassava (Manihot esculenta) to the dry environments of Limpopo, South Africa: growth, yield and yield components. Afr. J. Agric. Res. 6, 6082–6088 (2011).
Pérez, J. C. et al. Genetic variability of root peel thickness and its influence in extractable starch from cassava (Manihot esculenta Crantz) roots. Plant breeding. 130, 688–693 (2011).
Ren, M. Y. et al. Expression patterns of members of the ethylene signaling-related gene families in response to dehydration stresses in cassava. PLoS One. 12, e0177621, https://doi.org/10.1371/journal.pone.0177621 (2017).
Nir, I. et al. The tomato DELLA protein PROCERA acts in guard cells to promote stomatal closure. Plant Cell. 29, 3186–3197 (2017).
Raghavendra, A. S., Gonugunta, V. K., Christmann, A. & Grill, E. ABA perception and signalling. Trends Plant Sci. 15, 395–401 (2010).
Nambara, E. & Kuchitsu, K. Opening a new era of ABA research. J. Plant Res. 124, 431–435 (2011).
Sah, S. K., Reddy, K. R. & Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7, 571, https://doi.org/10.3389/fpls.2016.00571 (2016).
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R. & Abrams, S. R. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679 (2010).
Hubbard, K. E., Nishimura, N., Hitomi, K., Getzoff, E. D. & Schroeder, J. I. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes. Dev. 24, 1695–1708 (2010).
Klingler, J. P., Batelli, G. & Zhu, J. K. ABA receptors: the START of a new paradigm in phytohormone signalling. J. Exp. Bot. 61, 3199–3210 (2010).
Fujii, H. et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 462, 660–664 (2009).
Yoshida, T. et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 61, 672–685 (2010).
Yoshida, T., Mogami, J. & Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 21, 133–139 (2014).
Yoshida, T. et al. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 38, 35–49 (2015).
Fujita, Y., Fujita, M., Shinozaki, K. & Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 124, 509–525 (2011).
Kerr, T. C. C. et al. Ectopic expression of two AREB/ABF orthologs increases drought tolerance in cotton (Gossypium hirsutum). Plant Cell Environ. 41, 898–907 (2018).
Fan, W., Zhang, M., Zhang, H. & Zhang, P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One. 7, e37344, https://doi.org/10.1371/journal.pone.0037344 (2012).
Wang, F. W. et al. Cloning and characterization of a novel betaine aldehyde dehydrogenase gene from Suaeda corniculata. Genet. Mol. Res. 15, gmr7848, https://doi.org/10.4238/gmr.15027848 (2016).
Yu, H. Q. et al. A betaine aldehyde dehydrogenase gene from Ammopiptanthus nanus enhances tolerance of Arabidopsis to high salt and drought stresses. Plant Growth Regul. 83, 265–276 (2017).
You, J. et al. A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol. 166, 2100-2114 (2014).
Chinnusamy, V., Gong, Z. & Zhu, J. K. Abscisic acid-mediated epigenetic processes in plant development and stress responses. J. Integr. Plant Biol. 50, 1187–1195 (2008).
Wang, Y. et al. Comparative transcriptome analysis of tomato (Solanum lycopersicum) in response to exogenous abscisic acid. BMC Genomics. 14, 841, https://doi.org/10.1186/1471-2164-14-841 (2013).
Pilati, S. et al. Abscisic acid is a major regulator of grape berry ripening onset: new insights into ABA signaling network. Front. Plant Sci. 8, 1093, https://doi.org/10.3389/fpls.2017.01093 (2017).
Mine, A. et al. Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity. Proc. Natl. Acad. Sci. USA 114, 7456–7461 (2017).
Choi, H., Hong, J., Ha, J., Kang, J. & Kim, S. Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 275, 1723–1730 (2000).
Fujita, Y. et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 17, 3470–3488 (2005).
Furihata, T. et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. USA 103, 1988–1993 (2006).
Fujita, Y. et al. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 147, 15–27 (2013).
El-Sharkawy, M. A. & Cock, J. H. Response of cassava to water stress. Plant Soil. 100, 345–360 (1987).
Calatayud, P. A., Llovera, E., Bois, J. F. & Lamaze, T. Photosynthesis in drought-adapted cassava. Photosynthetica. 38, 97–104 (2000).
Alves, A. C. & Setter, T. L. Response of cassava leaf area expansion to water deficit: cell proliferation, cell expansion and delayed development. Ann. Bot. 94, 605–613 (2004).
Okogbenin, E. et al. Phenotypic approaches to drought in cassava: review. Front. Physiol. 4, 93, https://doi.org/10.3389/fphys.2013.00093 (2013).
Pasternak, D. Salt tolerance and crop production-a comprehensive approach. Annu. Rev. Phytopathol. 25, 271–291 (1987).
Carretero, C. L., Cantos, M., García, J. L., Azcón, R. & Troncoso, A. Arbuscular-mycorrhizal contributes to alleviation of salt damage in cassava clones. J. Plant Nutr. 31, 959–971 (2008).
Gleadow, R., Pegg, A. & Blomstedt, C. K. Resilience of cassava (Manihot esculenta Crantz) to salinity: implications for food security in low-lying regions. J. Exp. Bot. 67, 5403–5413 (2016).
Joshi, R., Shukla, A. & Sairam, R. K. In vitro screening of rice genotypes for drought tolerance using polyethylene glycol. Acta Physiol. Plant. 33, 2209–2217 (2011).
Hadi, F. et al. Comparative effect of polyethylene glycol and mannitol induced drought on growth (in vitro) of canola (Brassica napus), cauliflower (Brassica oleracea) and tomato (Lycopersicon esculentum) seedlings. Int. J. Biosci. 4, 34–41 (2014).
Greenham, K. & McClung, C. R. Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 16, 598–610 (2015).
Robertson, F. C., Skeffington, A. W., Gardner, M. J. & Webb, A. A. Interactions between circadian and hormonal signalling in plants. Plant Mol. Biol. 69, 419–427 (2009).
Legnaioli, T., Cuevas, J. & Mas, P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 28, 3745–3757 (2009).
Wilkins, O., Bräutigam, K. & Campbell, M. M. Time of day shapes Arabidopsis drought transcriptomes. Plant J. 63, 715–727 (2010).
Wilkins, O., Waldron, L., Nahal, H., Provart, N. J. & Campbell, M. M. Genotype and time of day shape the Populus drought response. Plant J. 60, 703–715 (2009).
Lee, H. G., Mas, P. & Seo, P. J. MYB96 shapes the circadian gating of ABA signaling in Arabidopsis. Sci. Rep. 6, 17754, https://doi.org/10.1038/srep17754 (2016).
Liu, T., Carlsson, J., Takeuchi, T., Newton, L. & Farré, E. M. Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. Plant J. 76, 101–114 (2013).
Khandelwal, A. et al. Role of ABA and ABI3 in desiccation tolerance. Science. 327, 546, https://doi.org/10.1126/science.1183672 (2010).
Jeong, Y. Y. & Seo, P. J. Bidirectional regulation between circadian clock and ABA signaling. Commun. Integr. Biol. 10, e1296999, https://doi.org/10.1080/19420889.2017.1296999 (2017).
Osterlund, M. T., Hardtke, C. S., Wei, N. & Deng, X. W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 405, 462–466 (2000).
Ulm, R. et al. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101, 1397–1402 (2004).
Chang, C. S., Maloof, J. N. & Wu, S. H. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 156, 228–239 (2011).
Chang, C. S. et al. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J. 54, 205–219 (2008).
Datta, S., Hettiarachchi, C., Johansson, H. & Holm, M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell. 19, 3242–3255 (2007).
Datta, S. et al. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell. 20, 2324–2338 (2008).
Gangappa, S. N. et al. The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell. 25, 1243–1257 (2013).
Jiang, L. et al. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 22, 1046–1057 (2012).
Xu, D. et al. Convergence of Light and ABA signaling on the ABI5 promoter. PLoS Genet. 10, e1004197, https://doi.org/10.1371/journal.pgen.1004197 (2014).
Eckstein, A., Krzeszowiec, W., Banaś, A. K., Janowiak, F. & Gabryś, H. Abscisic acid and blue light signaling pathways in chloroplast movements in Arabidopsis mesophyll. Acta Biochim. Pol. 63, 449–458 (2016).
Kondo, A., Kaikawa, J., Funaguma, T. & Ueno, O. Clumping and dispersal of chloroplasts in succulent plants. Planta. 219, 500–506 (2004).
Schwartz, S. H., Tan, B. C., McCarty, D. R., Welch, W. & Zeevaart, J. A. Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochim. Biophys. Acta. 1619, 9–14 (2003).
Xiong, L., Lee, H., Ishitani, M. & Zhu, J. K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol. Chem. 277, 8588–8596 (2002).
Wang, X. et al. ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol. 221, 341–355 (2018).
Ahmad, R., Lim, C. J. & Kwon, S. Y. Glycine betaine: a versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol. Rep. 7, 49–57 (2013).
Tian, F. et al. Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J. 5, 73–82 (2017).
Zhang, L. et al. Modulation role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 13, 3189–3202 (2012).
Gorecka, M. et al. Abscisic acid signalling determines susceptibility of bundle sheath cells to photoinhibition in high light-exposed Arabidopsis leaves. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130234, https://doi.org/10.1098/rstb.2013.0234 (2014).
Phan, T. T. et al. Overexpression of sugarcane gene SoSnRK2.1 confers drought tolerance in transgenic tobacco. Plant Cell Rep. 35, 1891–1905 (2016).
Lv, D. W. et al. Proteomic and phosphoproteomic analysis reveals the response and defense mechanism in leaves of diploid wheat T. monococcum under salt stress and recovery. J. Proteomics. 143, 93–105 (2016).
Missihoun, T. D., Schmitz, J., Klug, R., Kirch, H. H. & Bartels, D. Betaine aldehyde dehydrogenase genes from Arabidopsis with different sub-cellular localization affect stress responses. Planta. 233, 369–382 (2011).
Missihoun, T. D. et al. Overexpression of ALDH10A8 and ALDH10A9 genes provides insight into their role in glycine betaine synthesis and affects primary metabolism in Arabidopsis thaliana. Plant Cell Physiol. 56, 1798–1807 (2015).
Reumann, S. et al. In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol. 150, 125–143 (2009).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Acknowledgements
This study was supported by The National Key Research and Development Program of China (No. 2017YFD0202105), The National Nature Science Foundation of China (No. 31201603), China Agriculture Research System (No. CARS-31), The Hainan Natural Science Foundation (No. 312051).
Author information
Authors and Affiliations
Contributions
R.J.F., M.Y.R. and L.F.L. analyzed the data and drafted the manuscript; P.L., Y.D.Z. and J.H.X. designed the study; M.Y.R., R.J.F. and L.F.L. performed the bioinformatic analysis; M.Y.R., L.F.L., T.Y.Y., K.L. and W.T. obtained the RNA from the tissue samples; M.Y.R., F.W., Y.F.C., D.Z. and Q.S. performed qRT-PCR; D.B.Z., M.Y.Z. and D.F.Q. carried out the measurement of physiological indicator; M.P., X.G., P.L., Y.D.Z. and J.H.X. coordinated the study and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, RJ., Ren, MY., Lu, LF. et al. Involvement of abscisic acid-responsive element-binding factors in cassava (Manihot esculenta) dehydration stress response. Sci Rep 9, 12661 (2019). https://doi.org/10.1038/s41598-019-49083-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49083-3
This article is cited by
-
Genome-wide identification and expression analysis of the WNK kinase gene family in soybean
Molecular Breeding (2024)
-
The Arabidopsis thaliana integrin-like gene AT14A improves drought tolerance in Solanum lycopersicum
Journal of Plant Research (2023)
-
Morphophysiological Responses and Tolerance Mechanisms in Cassava (Manihot esculenta Crantz) Under Drought Stress
Journal of Soil Science and Plant Nutrition (2023)
-
Significance of brassinosteroids and their derivatives in the development and protection of plants under abiotic stress
Biologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.