Abstract
Soil aggregates are the basic units of soil structure, and their composition and carbon (C) stability directly affect soil fertility. As cementing agents, humic substances play an important role in the formation and stability of soil aggregates. Long-term fertilization not only changes the structure of humic acid (HA), but also affects the content and stability of organic C in soil aggregates. In this study, based on a long-term fertilization experiment, the relationship between the molecular structure of HA and the stability of organic C in the aggregates was examined. Compared with the non-fertilization control (CK), both the application of organic manure alone (M) and organic manure combined with inorganic fertilizer application (MNPK) increased organic C content in the bulk soil and in HA. In addition, the application of organic manure (M, MNPK) favored the formation of macroaggregates (>0.25 mm) and showed a higher organic C contents of soil aggregates with different sizes than the CK. Moreover, the content of aliphatic C, the ratios of aliphatic C/aromatic C and alkyl C/O-alkyl C was increased with the application of organic fertilizer. A significant negative correlation was observed between aromatic C and organic C content of the aggregates with sizes of >2 mm, 2–0.25 mm, and 0.25–0.053 mm (P < 0.05). The findings indicated that organic fertilization treatments (M and MNPK) increased the aliphatic C content of HA, which favored the increase in the organic C content and stability of the aggregates.
Similar content being viewed by others
Introduction
Soil aggregates are the structural units of soils formed by agglomeration and cementation of soil particles. Their quality and quantity not only affect soil fertility, but also maintain soil organic matter (SOM), and are important in carbon (C) sequestration. Humic substances (HS) are the cementing agents forming stable aggregates and are also the main substances involved in C sequestration1. The persistence of HS is reliant on being confined within the aggregates2. Once the aggregates are destroyed, HS will be exposed and decomposed by microorganisms3. Humic substances can maintain the stability of soil aggregates, and the interaction between HS and soil aggregates directly determines soil C sequestration4. However, the relations between HS and soil aggregates are poorly understood.
Many studies have suggested that microaggregates, especially those in the macroaggregates, play an important role in the formation and protection of C5,6,7. The organic matter in large aggregates mainly plays a role in physical protection, and is mostly derived from plant residues and sensitive to the management measures, with a short cycle7. The macroaggregates contain more C than the microaggregates8. The organic matter in small aggregates plays a major role in chemical protection and decomposes slowly, which is beneficial for long-term preservation9,10. Tisdall et al.11 indicated that macroaggregates (>0.25 mm) were mainly formed by the cementation of soil roots and hyphae, whereas microaggregates (<0.25 mm) were mainly formed by polyvalent cationic bridges and polysaccharides. The stability of soil aggregates is affected by soil physical structure and SOM, which in turn, is beneficial to the formation and stability of soil structure12. Studies have shown that the aliphatic C in the SOM is an important component of the stable soil C pool and thus plays an important role in C stability13. The aliphatic components in SOM are mainly fat, wax, and resin, have a major impact on the decomposition of SOM, and thus affect the release of nutrients and plant growth. Alkanes and fatty acids are a special recalcitrant material in soil C pool14. The hydrophobicity of aliphatic compounds is important for the stability of organic matter and aggregates15. As an active component of soil HS, humic acid (HA) plays a critical role in soil fertility and nutrient supply capacity. The purpose of this study was to examine the relationship between the HA-C components and the change in the organic C content of the aggregates under different fertilizations.
As SOM is a continuum of progressively decomposing organic compounds16, SOM stability as an ecosystem property was predominantly dependent on environment rather than molecular structure alone or physical recalcitrance17. To explore the controls of chemical or physical properties on SOM stability, a long-term (32 years) fertilization experiment with the same environment background was selected from the Scientific Observation Station of Heilongjiang Arable Land Conservation and Agricultural Environment, Ministry of Agriculture and Rural Affairs of the People’s Republic of China. The molecular structure of soil HA under different long-term fertilization treatments on black soil was studied by using 13C nuclear magnetic resonance spectroscopy (13C-NMR). The size composition of soil aggregates was also assessed based on the changes in the composition of soil aggregates and organic C content under different fertilization. The objective of this study was to test the relationship between soil HA-C structure and the stability of organic C in soil aggregates.
Results
Effects of fertilization on the contents of SOC and HA
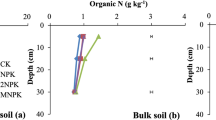
Compared with CK, the MNPK, M, and NPK treatments significantly increased SOC (P < 0.05) by 19.4%, 16.7% and 15.9%, respectively, and the SOC content of the MNPK treatment was significantly higher than those of M and NPK (Fig. 1). Compared with CK and NPK treatments, the content of soil HA-C was significantly increased by the organic fertilizer treatments (MNPK and M) (P < 0.05).
Effects of different fertilization treatments on carbon contents in HA and bulk soil. CK, no fertilizer treatment; NPK, chemical NPK fertilizers; M, organic manure treatment; MNPK, treatment of organic manure combined with chemical NPK fertilizers. Different lowercase letters for each parameter indicate significant difference among different treatments at P < 0.05.
Effects of fertilization on soil water-stable aggregate composition and organic C content
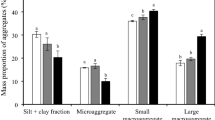
soil aggregates in the 2–0.25 mm and 0.25–0.053 mm were the dominant soil aggregate size fractions, and the average contents of these fractions across treatments were 46.5% and 39.6%, respectively (Fig. 2). Compared with CK, organic fertilization treatments (M and MNPK) significantly increased the proportions of aggregates in the >2 mm and 2–0.25 mm fractions, and NPK treatment increased the proportions of the aggregates in the 0.25–0.053 mm and <0.053 mm fractions. These results showed that the application of organic manure facilitates the formation of macroaggregates (>0.25 mm).
Generally, the highest organic C content was found in the 0.25–0.053 mm aggregate size fraction, followed by the >2 mm and 2–0.25 mm fractions, and the lowest organic C content was found in the <0.053 mm fraction (P < 0.05, Table 1). The increase in organic C content of the aggregates with MNPK treatment ranged from 9.8 (<0.053 mm) to 25.4% (0.25–0.053 mm) relative to the CK treatment. The SOC contents of aggregates under M treatment increased by 14.5–29.0% relative to that of the aggregates in CK treatment, and the largest increase was found in the >2 mm aggregate size fraction. The SOC contents of aggregates with different size fractions under NPK treatment increased by 10.8–18.0% relative to that of the aggregates in CK treatment, and the largest increase was also found in the >2 mm aggregate size fraction.
Solid-state 13C NMR Spectra and C distribution of HA
The solid 13C CPMAS NMR spectra of HA could be divided into four main resonance regions: alkyl C region (0–50 ppm), alkoxyl C (50–110 ppm), aromatic C (110–160 ppm), and carbonyl C (160–200 ppm)18. In this study, the main absorption peaks in the resonant regions were near 30, 55, 70, 129, and 170 ppm (Fig. 3). The absorption peaks at 30 ppm is usually assigned to the amorphous (CH2)n long chain C, which belongs to the category of alkyl C. The absorptions in the range of 55 to 60 ppm are associated with the Cs in the polypeptide and the methoxy group, and the absorption around 70 ppm is caused by the carbohydrate C19. The absorptions in the range of 128 to 132 ppm are mainly designated to the aromatic C with the substitutions of carboxyl group or the carboxyl group and the aromatic group C that is attached to H and is located in the ortho position to the O and N substituted groups. The absorptions of 170–172 ppm correspond to the absorptions of carboxylic acid, ester and amide C20.
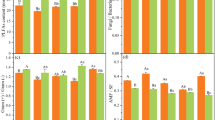
Compared with the CK treatment, the alkyl C contents of HA in the NPK, M, and MNPK treatments increased by 6.8%, 10.5%, and 14.1%, respectively (Table 2). The alkoxyl C contents were decreased in the NPK and MNPK treatments (Table 2). The aliphatic C (alkyl C + alkoxyl C) contents in the M and MNPK treatments were increased by 5.9% and 1.3%, respectively, relative to CK. The aromatic C contents in the NPK, M, and MNPK treatments decreased by 3.2%, 4.3%, and 5.0%, respectively, relative to CK. The carbonyl C contents in the NPK and MNPK treatments increased relative to CK. The application of organic fertilizers without or with chemical fertilizers (M and MNPK) significantly increased the ratios of aliphatic C/aromatic C and alkyl C/O-alkyl C.
Correlation between HA-C structure and the organic C content in soil aggregates
The aromatic C content had a significant negative correlation with the aggregates of >2 mm, 2–0.25 mm, and 0.25–0.053 mm (P < 0.05; Table 3). The organic C content in the >2 mm aggregate had a significant positive correlation with that in the 2–0.25 mm (P < 0.05) and a significant negative correlation with that of the <0.053 mm aggregate (P < 0.05). The organic C content in the 2–0.25 mm aggregate size fraction had a significant negative correlation with that in the 0.25–0.053 mm aggregate size fraction (P < 0.05).
Discussion
Characteristics of the changes in Soil HA molecular structure
The present study showed that the HA-C structure in the humus of black soil included waxy substance, carbohydrates, carboxyl-substituted aromatic C, carboxylic acids, amide C, some peptides, and methoxy C. The ratio of aliphatic C to aromatic C is usually considered as an index of the humification degree of HA structure29. The higher percentages of aliphatic C relative to aromatic C in HA in the treatments M and MNPK (Table 2) indicated that long-term organic fertilizers simplified the structure of HA and decreased the aromaticity. Alkyl C is derived from plant biopolymers (cutin and wax) and microbial metabolites, and is a stable organic C component that is difficult to degrade. In contrast, alkoxyl C is relatively easily decomposed21, so the alkyl C/O-alkyl C ratio is usually used as an indicator for the degree of decomposition of organic C22. In the current study, the fertilization treatments increased the percentage of alkyl C in HA compared with the CK. The applications of organic manure and inorganic fertilizers allowed more crop residues to enter soils, which likely improved soil microbial activity, and further increased the use of alkoxyl C, causing alkyl C accumulation. In addition, the percentage of alkoxyl C was decreased with the treatment of NPK and MNPK compared to the CK. As a result, the alkyl C/alkoxyl C ratios in all the fertilization treatments were increased relative to the CK, which was beneficial for improving the stability of SOC. The hydrophobic C/hydrophilic C ratio can reflect the degree of hydrophobicity of HS, and is closely related to the stability of the SOC and aggregates. In general, the higher the ratio, the higher the stability of the SOC and the agglomerates. This is because the hydrophobic organic matter containing numerous functional groups, such as –COOH and –OH, can form microaggregates with the charges on the soil mineral surface via hydrogen bonds or polyvalent cation bridges, and the aggregates formed by this cementation have a high stability22,23. Giovannini et al.24 found that hydrophobic organic compounds improved the stability of aggregates and Capriel et al.25 showed a good correlation between aliphatic organic compounds and the stability of aggregates. The present study showed a slight increasing trend of the hydrophobic C/hydrophilic C ratio in HA with organic fertilization, but there was no statistically significant differences, which deserves further study.
Relationship between Soil HA-C structure and the Organic C in aggregates
Long-term fertilization has a direct or indirect effect on the formation and stability of soil aggregates, leading to a change in the distribution of soil nutrients in aggregates26. As an important component of humus, HA is involved in the distribution of organic C in aggregates, and its structural changes will inevitably affect the distributions of organic C in aggregates. Huang et al.27 suggested that long chain aliphatic C was a stable C structure in soil, and a higher hydrophobic C/hydrophilic C ratio indicated a higher stability of SOC and aggregates. In the present study, the negative correlation between HA aliphatic C and aromatic C, and that between aromatic C and C content in the >2 mm and 2–0.25 mm and 0.25–0.053 mm aggregates (Table 3) indicated that the decrease of aromatic C content and the increase of aliphatic C content in HA increased the organic C in the agglomerates. Therefore, the increase in aliphatic C content in HA with organic fertilization treatment could help to increase the content and stability of organic C in aggregates. The alkyl C/alkoxyl C and hydrophobic C/hydrophilic C ratios had a positive correlation with organic C contents in aggregates of all size fractions, indicating that all the fertilizations could increase the proportion of organic C that was difficult to decompose in the aggregates and improve the stability of soil C. The above results demonstrated that soil HA structure was correlated to the content of organic C in the aggregates. The organic fertilization treatment could significantly increase SOC and HA contents and increase the proportion of macroaggregates (>0.25 mm). Moreover, the content and stability of organic C in microaggregates (<0.25 mm) were increased because of the increase in the aliphatic C content. Since HA is an important component of humus and is involved in the distribution of organic C in the aggregates, the hydrophobicity of the aliphatic C increases the stability of C and allows microaggregates to contain more organic C in the form of chemical protection, thus improving soil structure and increasing soil C pool.
Conclusion
Results indicated that soil HA structure was correlated with organic C content in aggregates. M and MNPK treatments significantly increased SOC and HA content, increased the proportion of macroaggregates (>0.25 mm) and aliphatic C content, and then increased the content and stability of organic C in microaggregates (<0.25 mm). As an important component of humus, soil HA participated in the distribution of organic C in aggregates. The hydrophobicity of aliphatic C in HA improved the stability of C, formed chemical protection for soil microaggregates, and increased the content of organic C in microaggregates, thus improving soil structure and increasing soil C pool.
Methods
Study site and experiment design
The black soil fertility long-term monitoring station (Scientific Observation Station of Heilongjiang Arable Land Conservation and Agricultural Environment, Ministry of Agriculture), established in 1979 is located in Guangming village of Minzhu town at Harbin, Heilongjiang Province (E126°51′05″, N45°50′30″, 151 m a.s.l.). The station is on the Songhua River secondary stream terrace. The region has a temperate continental monsoon climate and a ≥ 10 °C annual average effective accumulated temperature of 2700 °C, annual precipitation of 533 mm, and a 135-day frost-free season. The soil was developed from loess-like parent material, with the following soil properties in 1979: 15.5 g kg−1organic C, 1.47 g·kg−1 total nitrogen (N), 1.07 g·kg−1 total phosphorus (P) as P2O5, 25.16 g·kg−1 total potassium (K) as K2O, 151 mg·kg−1 alkaline hydrolyzable N, 51 mg·kg−1 available P, 200 mg·kg−1 available K, and pH of 7.2.
The long-term experiment was conducted using a wheat (year of 1980)-soybean-maize rotation. Four treatments were arranged with completely random design with three replicates. The size of each plot area was 36 m2. The treatments include control (no fertilization, CK), organic manure (M), inorganic N, P, and K fertilizers (NPK), and combined organic manure, inorganic N, P, and K fertilizers (MNPK). The amounts of applied fertilizers in different crop years are shown in Table 4. The organic manure was pure horse manure, collected from the same horse farmers, and applied after the maize was harvested in each rotation at a pure N amount of 75 kg·ha−1 (about 18,600 kg·ha−1 of horse manure). The contents of organic C, N, phosphorus as P2O5, and potassium as K2O in manure were 163.6 g·kg−1, 5.8 g·kg−1, 6.5 g·kg−1, and 9.0 g·kg−1, respectively. The N, P, and K fertilizers were applied in the fall (during maize season, 50% N fertilizer was applied in the fall, and a top dressing of the remaining 50% was performed in the big trumpet period). The N, P, and K fertilizers were urea (N, 46%), tripe superphosphate (P2O5, 46%), and potassium sulfate (K2O, 50%). The detailed information was described by Zhang et al.28.
The tested soil samples were collected at a depth of 0–20 cm after the fall harvest in 2012 (maize) and S-type sampling was adopted, with a total of five sampling sites. After root fragments and stones were removed, a portion of the samples were air-dried and passed a sieve (<2 mm) for further analyses of HA and nutrients.
HA separation and purification
The detailed method of HA separation and purification was described by our previous study29. Briefly, 100 g air-dried soils and 1 mol L−1 HCl solution were added into a glass bottle at a ratio soil to solution of 1:1. The ratio was adjusted to 1:10 with HCl solution after standing for 1 h. The mixture was then shaken for 1 h and then centrifuged at a speed of 3500 r min−1 for 15 min. After that, the supernatant was discarded and the sediment was adjusted to pH of 7 with 1 mol L−1 NaOH solution. After standing for 1 h, the soil to solution ratio was adjusted to 1:10 by adding 0.1 mol L−1 NaOH solution. Each glass bottle was flushed with N2 for 1 min and then sealed. Under the N2 atmosphere, each bottle was shaken once an hour for 12 h, and then settled overnight. Samples were centrifuged at a low speed, acidified to pH 1.0 with 6 mol L−1 HCl solution, and then settled again for 12–16 h. Following three extractions and low-speed centrifugations, the sediment was HA. The HA precipitant was dissolved in a small amount of 0.1 mol L−1 KOH solution under an N2 atmosphere, and then solid KCl was added to achieve a concentration of K+ at 0.3 mol L−1. After 1 h settling and centrifugation at a high speed of 8000 r min−1 for 20 min, the supernatant was adjusted to pH 1.0 with 6 mol L−1 HCl solution, and then settled for 12–16 h. The upper clear solution was discarded. The HA was soaked in 0.1 mol L−1 HCl and 0.3 mol L−1 HF mixed acid to reduce the content of ash to less than 1%. The samples were then placed into an electrodialyzer until no Cl−1 was detected in distilled water by AgNO3. The liquid was concentrated by rotary evaporation and then freeze-dried for later use.
Nuclear magnetic resonance spectroscopy (CPMAS13C-NMR) analysis
Humic substances are mainly divided into HA, fulvic acid, and humin. As an active component of soil HS, HA was selected to run the NMR analysis in the present study. The solid-state 13C-NMR spectra were collected by nuclear magnetic resonance spectrometer (BrukerAV400, Swiss). The cross-polarized magic angle spin (CPMAS) technique was adopted, the 13C resonant frequency was 400.18 MHz, and the magic angle was 8 kHz. The contact time was 2 ms, the recycle delay was 3 s, and the number of data points was 3000. The chemical shift was calibrated by external standard 2, 2-dimethyl-2-silapentane-5-sulfonate sodium salt, the integral area was automatically given by the instrument, and the relative content of each type of C was expressed by the percentage of an integral area of one chemical shift range over the total integral area.
Determination of aggregate size and organic C content
Aggregates were graded using the wet sieving method. The air-dried soil sample (100 g) was placed on the top layer of the three-stack (2 mm, 0.25 mm, and 0.053 mm) mechanical sieve shaker. After immersion in distilled water for 5 min at room temperature, the sample was shaken at a frequency of 0.5 Hz and an up-down motion amplitude of 3 cm for 2 min. After sieving, the aggregates on each sieve stack were rinsed into the beaker to obtain water-stable aggregates with particle size >2 mm, 2–0.25 mm and 0.25–0.053 mm, and the aggregate <0.053 mm was settled in the barrel for 48 h and then transferred to the beaker after the supernatant was discarded. The aggregates in the beaker were dried and weighed and the percentage of aggregates in each size fraction was calculated9. The SOC content was measured using a total organic C analyzer (Multi N/C 2100S, Analytik Jena, Germany).
Statistical analyses
The CPMAS13C-NMR spectra were analyzed by MestRe Nova (version 6.0.3, Mestrelabs Research, SL, Santiago de Compostela, Spain). After the source data were extracted and analyzed, Microsoft Office Excel 2010 and Origin 8.0 software were used for data processing and plotting, the data in “Available Data” were plotted by fitted curve stacking method in Origin, and SPSS 17.0 statistical analysis software was used to determine whether there were significant differences between groups (Duncan method) and to conduct correlation analyses.
References
Albert, U. I., Antonio, F. P. & Burrow, D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 125, 321–330 (2005).
Golchin, A., Baldock, J. A. & Oades, J. M. A model linking organic matter decomposition, chemistry, and aggregate dynamics/Lal, R., K., imb1e, J., Follett, R. et al. Soil Processes and the Carbon Cycle. Boca Raton, FL: CRC Press, 245–266 (1992).
Bongiovanni, M. D. & Lobartini, J. C. Particulate organic matter, carbohydrate, humis acid contents in soil macro-and microaggregates as affected by cultivation. Geoderma 136, 660–665 (2006).
Pan, G. X. et al. Core issues and research progresses of soil science of C sequestration. Acta Pedologica Sinica 44(2), 327–337 (2007).
Six, J., Bossuyt, H. & Degryze, S. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 79, 7–31 (2004).
Gale, W. J., Cambardella, C. A. & Bailey, T. B. Root-derived carbon and the formation and stabilization aggregates. Soil Sci. Soc. Am. J. 64, 201–207 (2000).
Puget, P., Balesdent, J. & Chenu, C. Dynamics of soil organic matter associated with primary particle size fractions of water-stable aggregates. Eur. J. Soil Bio. 51, 595–605 (2000).
Puget, P., Chenu, C. & Balesdent, J. Total young organic matter distributions in aggregate of silly cultivated soils. Eur. J. Soil Sci. 46, 449–459 (1995).
Cambardella, C. A. & Elliott, E. T. Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils. Soil Sci. Soc. Am. J. 57, 1071–1076 (1993).
Besnard, E., Chenu, C. & Balesdent, J. Fate of particulate organic matter in soil aggregates during cultivation. Eur. J. Soil Sci. 47, 495–503 (1996).
Tisdall, J. M. & Oades, J. M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 33, 141–163 (1982).
Peng, X. H., Zhang, B. & Zhao, Q. G. A review on relationship between soil organic carbon pools and soil structure stability. Acta Pedologica Sinica 41(4), 618–623 (2004).
Rumpel, C. et al. Effect of base hydrolysis on the chemical composition of organic matter of an acid forest soil. Org. Geochem. 36, 239–249 (2005).
Derenne, S. & Largeau, C. A review of some important families of refractory macromolecules: composition, origin, and fate in soils and sediments. Soil Sci. 166, 833–847 (2001).
Baldock, J. A. et al. Cycling and composition of organic matter in terrestrial and marine ecosystems. Mar. Chem. 92, 39–64 (2004).
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Zhang, J. J. et al. Effect of long-term application of organic fertilizer on structural characteristics of human in black soil-a solid-state 13CNMR study. Scientia Agricultural Sinica 42, 2223–2228 (2009).
Mylotte, R. et al. Insights into the composition of recalcitrant organic matter from estuarine sediments using NMR spectroscopy. Org. Geochem. 98, 155–165 (2016).
Dou, S., Zhang, J. J. & Li, K. Effect of organic matter applications on 13C-NMR spectra of humic acids of soil. Eur. J. Soil Sci. 59, 532–539 (2008).
Ussiri, D. A. N. & Johnson, C. E. Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma 111, 123–149 (2003).
Piccolo, A. & Mbagwu, J. S. C. Role of hydrophobic components of soil organic matter in soil aggregate stability. Soil Sci. Soc. Am. J. 63, 1801–1810 (1999).
Spaccini, R., Mbagwu, J. S. C., Conte, P. & Piccolo, A. Changes of humic substances characteristics from forested to cultivated soils in Ethiopia. Geoderma 132, 9–19 (2006).
Giovanini, G., Lucchesi, S. & Cervelli, S. Water -repellent substances and aggregate stability in hydrophobic soil. Soil Sci. 135, 110–113 (1983).
Capriel, P., Beck, T. & Harter, P. Relationship between soil aliphatic fraction extracted with supercritical hexane, soil microbial biomass and soil aggregate stability. Soil Sci. Soc. Am. J. 54, 415–420 (1990).
Liu, Z. L. et al. Effects of long-term fertilization on aggregate size distribution and nutrient content. Soils 43, 720–728 (2011).
Huang, Y., Baocai, L., Bryant, C., Bol, R. & Eglinton, G. Radiocarbon dating of aliphatic hydrocarbons: a now approach for dating passive-fraction carbon in soil horizons. Soil Sci. Soc. Am. J. 63, 1181–1187 (1999).
Zhang, J. et al. Effects of long-term fertilization on soil humic acid composition and structure in Black Soil. PLoS One 12, e0186918 (2017).
Zhang, J. et al. Evolution over years of structural characteristics of humic acids in Black Soil as a function of various fertilization treatments. J. Soils Sed. 19, 1959–1969 (2019).
Acknowledgements
The study was supported by the National Key R&D Program of China (2018YFD0200407, 2016YFD0300806-2), National Natural Science Foundation of China (41771284, 41571285), and the Key Fund of Heilongjiang Academy of Agricultural Sciences (2018KYJL011).
Author information
Authors and Affiliations
Contributions
J.Z. and D.W. conceived the project. J.Z., F.C., B.Z. designed the experiment, J.Z., S.C., Y.L. and E.K., L.S. performed the filed sampling and lab analyses, J.Z. and L.J.L. carried out the data analysis, J.Z. wrote the first draft and L.J.L. revised and comments on the manuscript. All co-authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Chi, F., Wei, D. et al. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci Rep 9, 11908 (2019). https://doi.org/10.1038/s41598-019-48406-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48406-8
This article is cited by
-
The active compound in Rheum officinale Baill, aloe-emodin promotes tomato seedling growth
Plant Growth Regulation (2024)
-
Anaerobic digestion of primary winery wastewater sludge and evaluation of the character of the digestate as a potential fertilizer
Biomass Conversion and Biorefinery (2023)
-
Application of organic and chemical fertilizers promoted the accumulation of soil organic carbon in farmland on the Loess Plateau
Plant and Soil (2023)
-
Effects of soil organic matter components and iron aluminum oxides on aggregate stability during vegetation succession in granite red soil eroded areas
Journal of Mountain Science (2022)
-
Response of the chemical structure of soil organic carbon to modes of maize straw return
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.