Abstract
The present study investigates the hydrothermal liquefaction (HTL) of harmful green macroalgal blooms at a temperature of 270 °C with, and without a catalyst with a holding time of 45 min. The effect of different catalysts on the HTL product yield was also studied. Two separation methods were used for recovering the biocrude oil yield from the solid phase. On comparision with other catalyst, Na2CO3 was found to produce higher yiled of bio-oil. The total bio-oil yield was 20.10% with Na2CO3, 18.74% with TiO2, 17.37% with CaO, and 14.6% without a catalyst. The aqueous phase was analyzed for TOC, COD, TN, and TP to determine the nutrient enrichment of water phase for microalgae cultivation. Growth of four microalgae strains viz., Chlorella Minutissima, Chlorella sorokiniana UUIND6, Chlorella singularis UUIND5 and Scenedesmus abundans in the aqueous phase were studied, and compared with a standard growth medium. The results indicate that harmful macroalgal blooms are a suitable feedstock for HTL, and its aqueous phase offers a promising nutrient source for microalgae.
Similar content being viewed by others
Introduction
The appearance of a dense mat of macroalgae on water bodies is a widespread phenomenon. An algal bloom is a result of accumulating algal biomass in the slow-moving lake, pond or river. Macroalgae blooms are largely filamentous, unattached forms, and are mainly green algal species found in nutrient-rich, and temperate waters. Increase of nitrogen and other micropollutants into water bodies are linked to increases in macroalgal blooms worldwide1.
Accumulation of macroalgae in rivers and ponds leads to microbial decomposition that may reduce dissolved oxygen in the water bodies due to algal respiration2. Low dissolved oxygen content in water bodies leads to a change in biodiversity and species composition3. Macroalgal blooms lead to a decline in the growth of non-blooming algae and also affects the diversity of plankton and zooplankton in water bodies4. These algal blooms affect the ecosystem by changing the quality of water, light penetration, and alteration of the food chain, and food web5. Various initiatives have been undertaken to deracinate the problem related to algal blooms contaminate freshwater as well as marine water5. Many studies have been reported on producing biofuels from microalgae6. However, a limited number of studies are related to the use of macroalgae for biofuel production. Toxic algal blooms can be used as a cheap raw material for microalgae cultivation because its density has extensively increased worldwide in recent years due to wastewater being discharged into water bodies7.
Hydrothermal liquefaction (HTL) is a process in which wet algal feedstocks at 200–400 °C temperature and 10–15 MPa pressure gets converted into four products (1) Bio-crude oil (2) Gas (3) Solid phase and (4) Aqueous phase rich in organics and nutrients8. During the HTL process, along with lipids, proteins and carbohydrates also get converted into biocrude oil. Therefore, the yield of oil is higher using HTL8,9. Thus, HTL is well suited to a variety of biomass, including bacteria, wastewater sludge, and algal biomass, which is fast-growing but have low-lipid contents. This process provides the energetic advantages by the use of wet algal biomass and the efficient separation of products over alternative techniques such as lipid isolation and transesterification, pyrolysis etc9. HTL is ideal for conversion of high-moisture biomass into biocrude oil because water acts as a reaction medium and thus avoids the costly phase of biomass drying9.
The use of a catalyst was found more to be suitable for HTL of macroalgae as compared to microalgae because it increases the conversion of carbohydrates into biocrude oil10. Previous studies have shown that most of the catalysts lead to a significant increase in biocrude oil. HTL process will not be economically viable if these catalysts cannot be recycled properly11,12. Na2CO3 increases the HTL biocrude oil yield of terrestrial plants, and microalgae by promoting hydrolysis12.
Quality of the biocrude oil is dependent on the properties of the algal biomass. High carbon, hydrogen content and low ash, nitrogen, sulfur, and oxygen content containing biomass are considered as ideal for HTL13. HTL bio-crude ids dark in color, highly viscous liquid which is 10–10,000 times higher than that of conventional fuel and have a smoke-like smell14. High nitrogen content of algal biomass results in higher nitrogen content in HTL biocrude oil. This causes emission of toxic NOx which can be removed by the refining process. The high carbon content of algal biomass resulted in high biocrude yield by HTL process10. Composition of biocrude oil is mainly carbon content (71–73%), hydrogen content (7–8%), oxygen content (10–11%), nitrogen content (6–7%) and sulfur content (0–1%) which leads to less greenhouse gas emissions as compared to conventional biofuel and bioethanol10. Biocrude can be upgraded for the production of gasoline, jet fuel, and other fuel by using a suitable catalyst which can remove oxygen, nitrogen and double bonds15. However, HTL shows a negative energy balance, which is the principal disadvantage of this process. Fast heating and cooling also increase the yield of biocrude oil because of better conversion of protein, lipid, and carbohydrates into liquid fuel rather than solid or gaseous fuel10.
According to the energy-efficiency ratio, the use of HTL aqueous phase for microalgae cultivation shows a positive energy balance for biofuel production16. Recycling nutrients from wastewater could potentially fulfil the nutrients requirement for microalgae cultivation and scope to integrate the biofuel production and wastewater treatment17. Post-hydrothermal liquefaction aqueous phase can accumulate approximately 80% of nutrients and some organics, this provides an excellent opportunity for nutrient and carbon recycling8. The nutrients and carbon recycling have been investigated in some recent studies using aqueous phase of HTL18. These studies show that nutrients and carbon in the aqueous phase from hydrothermal liquefaction can be used for microalgae cultivation at different dilution factors (50–500 times)19. Several studies have been reported in the literature on nutrient cycling of HTL wastewater for microalgae cultivation, but the effect of a different catalyst on the growth of microalgae has not been reported yet. In a study, Jain et al.7 reported that freshwater toxic algal blooms are a promising feedstock for microalgae cultivation.
The present study investigates explicitly a novel integrated method of using harmful algal blooms as biomass for energy production that synergistically combines algal blooms biofuel production using the HTL process as given in Fig. 1. This study aims to experimentally confirm the feasibility of the harmful green algal blooms for biocrude production completed in four steps. Four steps includes (1) utilization of harmful macroalgal blooms for the HTL process. (2) Increase the yield of biocrude oil using different separation methods. (3) Study the effect of different catalysts on biocrude oil yield. (4) Use of aqueous phase of HTL processes with a catalyst for the cultivation of microalgae.
Results
Analysis of macroalgal blooms
The proximate analyses of macroalgal blooms (Supplementary Figs 1–3) are respectively listed in Table 1, 87.23% moisture, 62.01% volatile substantial, 19.02% ash content and 31.19% C, 8.42% H, 4.22% N and 54.61% O. FTIR spectrum reflects (Supplementary Fig. 4) three central regions, lipid band (around 1630 cm−1), amide band (1401 cm−1) and the carbohydrate region (1100–874 cm−1).
Product yields by HTL
In this study yields of bio-crude oil by the two separation methods were investigated. In the first separation method (without soxhlet), 10.03% of biocrude oil was obtained. The results indicated that oil yield was high (14.06%) in second separation method (with soxhlet). The Soxhlet extraction method was selected and then further evaluated for their bio-crude oil yield with different catalysts for 45 min at 270 °C as given in Fig. 1. The total bio-oil was 20.10% with Na2CO3, 18.74% with TiO2, 17.37% with CaO, and 14.6% without a catalyst. The biochar yield was 30.12%, 30.05%, 32.09% and 35.84% for Na2CO3, TiO2, CaO, and without catalyst respectively. The total biocrude oil yield was measured by the mixing of bio-oil-1 and bio-oil-2. The significant portion of biocrude oil one was obtained from the acetone extraction of the solid phase.
Analysis of the biocrude oil obtained by HTL
The biocrude oil obtained from the macroalgal blooms have been analyzed by Gas Chromatography (GC), and NIST library was used for the identification of compounds (Table 2). Nine main compounds were identified based on retention area % >1 during direct HTL without catalysts at 270 °C and 45 min of reaction time. The compounds such as amides derivatives, palmitic acid, phenolic compounds, and ketones derivatives, alkanes and alkenes derivatives and some furans were considered as central components of biocrude oil obtained by HTL of algal biomass20. Most of the components identified in biocrude oil extracted using macroalgal blooms were somewhat similar to those obtained from microalgae. However, differences in some of the compounds (Pyrrolo [1,2-a]pyrazine-1,4-dione hexahydro-3-(2-methylpropyl and Phytol) were also recorded during the study. This may be due to differences in algal species, composition of the macroalgal blooms and also different GC-MS analysis procedure implemented.
HHV and ER of algal blooms biocrude oil
The primary elements present in biocrude oils obtained by the catalytic and non-catalytic reaction is given in Table 3. The biocrude oil extracted in the absence of catalyst displayed higher carbon content (70.31%) rather than the macroalgal blooms.
Element enrichment percentage was higher in biocrude oil obtained using Na2CO3 as a catalyst. In the biocrude oil, the nitrogen concentration was less as compared to the green algae. This may be a result of nitrogenous compounds that are obtained in the liquid phase of the HTL process. The amount of nitrogen in the biomass of macroalgal blooms is higher than that of bio-crude oil as a result of generation of nitrogenous compounds during the HTL process in aqueous phase21. In contrast to our study, Ross et al.12 reported that using Na2CO3 there is an increase in nitrogen content, whereas Jena et al.20 observed a decrease in nitrogen content of biocrude oil with Na2CO3 catalyst. The sulfur content was recorded below one wt. % for Na2CO3 and TiO2 based reaction, whereas for petroleum crude oil, it varies between 0 and three wt.%22.
HHV of the biocrude oil was obtained with Na2CO3 (25.59MJ kg−1) followed by TiO2 (25.37MJ kg−1) and CaO (23.80MJ kg−1).These values are higher as compared to HHV of macroalgal blooms (9.45MJ kg−1).
Analysis of HTL water phase
Water phase obtained by the HTL of harmful macroalgal blooms had a very foul smell and is dark brown. The pH of catalytic reactions (7.8–8.3) was found to be higher than non-catalytic reaction (7.6) (Table 4). The same pattern of pH was also observed in the aqueous phase of other algal biomass after HTL when Na2CO3 was used as catalyst12,22.
The high amount of TOC present in the liquid phase of HTL is due to organic matter of feedstock dissolved in water. The nitrogen content in the HTL liquid phase increases in catalyzed reaction because algal proteins get converted into water-soluble amino acids and ammonia23.
Effect of catalysts stress on microalgal growth, biomass and lipid productivity
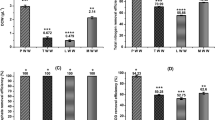
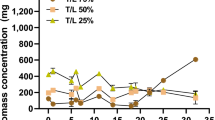
Microalgal growth in the aqueous phase of each catalyst is different; it may be due to removal and detoxification of catalyst by adsorption of catalysts on to the cell surface or intracellular metabolism of microalgae in response to catalysts. In the aqueous phase of Na2CO3 and TiO2 log phase lasted from day 6 to day 24. While in the aqueous phase of the CaO log phase lasted from day 5 to day 20 (Fig. 2). Overall microalgae, biomass productivity (g/l) after the stationary phase in the control medium (BBM) was found to decrease as compared to the HTL aqueous phase. As Fig. 2 shown that microalgae cultivated in HTL grew with the slow rate but the linear fashion after four days. Each catalyst affects the biomass and lipid productivity differently. Highest lipid yield and productivity of 32 ± 6.1% and 147 ± 1.3 mg L−1d−1 were recorded in Scenedesmus abundansgrown in aqueous phase containing catalyst TiO2 followed by CaO (Chorella minutissima 26.4 ± 1.7%; 157 ± 1.2 mg L−1d−1), Na2CO3 (Scenedesmus abundans 24.2 ± 2.1%; 110 ± 1.5 mg L−1d−1), and control (Chorella minutissima 22.16 ± 1.6%; 134 ± 2.1 mg L−1d−1) (Tables 5 and 6).
Discussion
FTIR spectrum of harmful macroalgal blooms reflects three central regions, lipid band (around 1630 cm−1), amide band (1401 cm−1) and the carbohydrate region (1100–874 cm−1). Peaks between 2516–1401 cm−1 presents the lipid and phenolic content of algal blooms24. Higher phenolic content was reported in marine macroalgae25. The sharp peak at 1639 cm−1 is C=O amide stretching of proteins present in algae26.
In the present study, for the first time harmful macroalgal blooms were used in HTL process for biocrude oil production. High-temperature (300–500 °C with holding time of 30–60 min) based HTL process for the conversion of biomass to bio-crude oil have been reported in previous studies27,28. However, the low-temperature (250–290) HTL process is still in the trial stage. In this study maximum, 20.1 ± 0.3% oil was obtained in the presence of catalyst Na2CO3 at 270 °C for 45 min.
The presence of catalysts showed the increase in oil yield and decrease in the biochar formation29. Catalysts increase the quality of biocrude oil by two ways (a) Introduction of catalysts at the time of the HTL process. (b) Upgrade the bio-oil quality after HTL. Catalysts from renewable resources are getting attention because they reduce the production cost of biofuels30. In this study, eggshell were used to produced CaO catalyst. Use of Na2CO3 as a catalyst in HTL leads to increases in the yield of biocrude oil from algal blooms. The bio-oil produced with Na2CO3 also had high heating value. Yeh et al.31 reported that Na2CO3 increases the yield ofbiocrude oil during the HTL of algal biomass containing a high content of carbohydrates by converting it into the oil.
In this study maximum yield of crude oil (20.10%) was reported with catalyst Na2CO. The presence of Na2CO3 catalyst has mainly suited the conversion of carbohydrates into biocrude oil in macroalgae and plant-based biomass12,23. However, Na2CO3 is inimical to the conversion of algal lipids to biocrude oil and not suitable for algal species contain high lipid contents23. Shakya et al.22 reported that higher amount of carbohydrates containing microalgae species led to increasing in bio-oil yield when Na2CO3 is used as a catalyst. In this study, HHV and ER value of algal blooms biocrude oil with catalyst Na2CO3 were 25.59MJ/Kg and 27.50% respectively. At similar pressure, Alhassan et al.32 reported the 21.15 ± 0.82 MJ/kg and ER 41.48% of biocrude oil of Jatropha curcas cake under 250 °C temperature. The energy efficiency of HTL biocrude oil depends on the HHV33.
Many catalysts such as KOH, NaOH, CH3COOH, and H2SO4 have been used by various researchers in HTL of microalgae, and these catalysts can also be reused as growth media for the growth of microalgae along with aqueous phase12,21. Wang et al.27 reported that by using TiO2 in HTL of microalgae at 300 °C leads to the highest bio-oil yield and the maximum liquefaction conversion.
The higher organic carbon content of macroalgae was responsible for the higher yield of bio oil34. The crude oil obtained from this study was 14–20% dw which is more similar to as reported in different green macroalgal species, Enteromorpha prolifera 23.0%16, Oedogonium 26.2% and Ulva 18.7% of dry weight34.
Algal biomass protein first to gets converted into amino acids and finally into amines and amides by HTL process12. Ketones and phenols produced during HTL were obtained from carbohydrates by hydrolysis and dehydration process. The lipids were responsible for the generation of alkenes16. Change in nitrogen concentration of aqueous phase of HTL depends on the temperature, type of catalyst, and algae strain12,22. High nitrogen content was reported in this study with Na2CO3 as a catalyst. Similar results were observed by Shakya et al.22, when Na2CO3 used as a catalyst in HTL of Isochrysis and Pavlova at low temperature, as Na2CO3 increased hydrolysis of protein into water-soluble compounds.
The aqueous phase of HTL had a high amount of soluble organic compound, carbon, and nitrogen. Kumar et al.35 reported that CaCl2 increases microalgal biomass productivity. High lipid content was reported in catalysts containing aqueous phase as compared to control. These results were in congruence with other studies in which an increase in lipid content on exposure to heavy metals was reported36. Oxidative stress results in degradation of the photosynthetic machinery, while the protective mechanism of algal cells leads to the accumulation of unsaturated fatty acids37.
This experimental work confirmed that harmful macroalgal blooms are a suitable feedstock for HTL and aqueous phase can be reuse as a nutrient source for cultivation of microalgal biomass.
Material and Methods
Materials
Microalgae strains Chorella minutissima (MCC-27), Scenedesmus abundans (NCIM 2897), Chlorella singulari UUIND,and Chlorella sorokiniana UUIND6 were used in present study. All the chemicals, including TiO2 and Na2CO3 and solvents used, were HPLC grade and acquired from Himedia, India.
Proximate analysis of green macroalgal blooms
Green macroalgal blooms were locally collected during the winter season (December-February 2017) from water pond, and freshwater river nearby Uttaranchal University, Uttarakhand, India. The ash content of algal blooms biomass was determined according to the NREL Analytical Procedure38. Elemental compositions of algal blooms were determined by the elemental analyzer (Thermo Fisher, USA). The proximate analysis of the sample was carried out using the standard methods given by the Association of Official Analytical Chemists (AOAC). FTIR analysis (FTIR 6700, NICOLET) of algal blooms biomass frequency range 4000–450 cm−1 were used.
Experimental procedure of hydrothermal liquefaction (HTL)
The Hydrothermal liquefaction (HTL) of harmful macroalgal blooms was carried out in a 100 ml high-pressure autoclave (Parr reactor) operated in a batch mode. Different types of catalysts in different concentrations were used in HTL process reported in the literature, but 10:1 (feedstock: catalyst) ratio has been reported to give the maximum conversion rate of feedstock to crude oil by HTL27,39. The reactor was loaded with wet algal blooms with/without catalyst in 10:1 ratio. Three types of catalysts were used in this study viz; TiO2, Na2CO3,and CaO. The CaO catalyst was prepared from eggshells according to the protocol given by Niju et al.40. The reactor was heated up to 270 °C and pressure 4.5 MPa with He gas, a heating rate of 5 °C/min for holding time of 45 min. After the completion of reaction, the reactor was immediately cooled and opened; gas was vented off, water phase and solid mixture were separated from each other by vacuum filtration. The filtrate was marked as an aqueous phase which consists of dissolved organic compounds. Two separation methods were used for recovering these products.
In the first separation method, the solid phase was treated with acetone three times to recover the oil phase. Acetone was evaporated at 60 °C, and the resulting oil phase was weighed and marked as Biocrude oil 1. The water phase was treated with diethyl ether. Aspirating out upper phase and diethyl ether was evaporated in a rotary evaporator. The total biocrude oil obtained was measured gravimetrically and marked as biocrude oil 2.
In the second separation, Karagoz et al.29 method were used with some modificationa, briefly the solid phase was acidified to pH 1–2 with H2SO4 (1.3 M) overnight and dried next day. Biocrude oil from the solid mixture was extracted using soxhlet extraction apparatus with acetone as solvent until the solvent in the thimble becomes colorless. Allowed to stand for three h, upper pahse was separated out. The lower phase (pallets) contain catalyst or residues of catalyst. Acetone was recovered from the upper phase at 60 °C. The extracted oil phase was weighed and marked as biocrude oil-1. Diethyl ether was added to liquid phase and aspirating out upper phase and evaporated diethyl ether in a rotary evaporator and remaining fraction was measured gravimetrically and marked as biocrude oil- 2.
Biocrude oil- 1 was mixed with biocrude oil- 2 for the calculation of %wt of total biocrude oil. Analysis of biocrude samples was doen using an elemental analyzer (Thermo Fisher, USA).
HHV and energy recovery (ER)
The essential properties of HTL biocrude oil such as biocrude oil yield percentage, HHV, element enrichment %, and ER were calculated using the empirical formulas given below27,41.
Aqueous phase water quality analysis
Aqueous phase quality analysis was done to estimate (TN), i.e., Total Nitrogen (NO3−, NO2−& NH4+) and (TP) Total phosphorus (PO4−). HACH DR 5000 analyzer was used to measure Total Chemical Oxygen (COD) demand. The Total Organic Carbon (TOC) was analyzed by a TOC analyzer (Shimadzu TOC-V).
Cultivation of microalgae on the aqueous phase
Four microalgal strains Chlorella Minutissima, Chlorella sorokiniana UUIND6, Chlorella singularis UUIND5, and Scenedesmus abundans were used in this study.
Scenedesmus abundans wasprocured from NCIM, Pune, India. Chlorella Minutissima was procured from IARI, New Delhi, India. Chlorella singularis UUIND5 and Chlorella sorokiniana UUIND6 were earlier isolated by our group.
Microalgal strains were cultivated in 1 L shake flasks containing different dilutions of the aqueous phase of catalytic and non-catalytic reaction and Bold’s Basal Medium (BBM) as a control for 14 days with 16 h light: 8 h dark photoperiod and irradiated with LED tubes (200 μmol m−2 s−1). BBM was prepared according to the protocol developed by Guarnieri et al.42.
Three concentrations 200×, 400× and 600× of the aqueous phase of non-catalytic reaction were prepared by the dilution of distilled water (Table 1). The effect on the growth rate of microalgae strains was observed by taking absorbance at 750 nm using UV–Vis spectrophotometer (Shimadzu 133 model no. 1700). Dilution 400× was capable of growing the maximum biomass and was further evaluated for their efficiency in biomass production with 1% BBM and 400× dilution of the aqueous phase of the catalytic reaction.
Determination of total lipid content (%, w/w) and lipid productivity of the cultivated microalgae
For extraction of lipids, first of all, samples were dried from the % ml culture broth. Further, the microalgal cells were broken down using liquid nitrogen with the help of a mortar and pestle. The fine powder was obtained from which the lipids were extracted using chloroform:methanol (2:1) kept overnight at room temperature, with constant shaking35. The extract obtained was treated with 0.034% MgCl2, centrifuged at 3500 rpm for 5 min. The supernatant was washed two-three times with 1 ml of 2 N KCl/methanol (4:1 v/v). 5 ml of chloroform/methanol/water (3:48:47, v/v/v) was added to it. The bottom chloroform layer was transferred to a new test tube, and lipids yield was measured gravimetrically. Lipid production and percentage of lipid were calculated by the following equations35:
Statistical analysis
All the experiments were done in triplicates (n = 3) and are presented in mean value ± SD. A GraphPad Prism software (version7:0) with p < 0.05 was used in this study.
References
Valiela, I. et al. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography. 42, 1105–1118 (1997).
Sfriso, A., Marcomini, A. & Pavoni, B. Relationships between macroalgal biomass and nutrient concentrations in a hypertrophic area of the Venice Lagoon Italy. Marine Environmental Research. 22, 297–312 (1987).
Edgar, G. J., Barrett, N. S., Graddon, D. J. & Last, P. R. The conservation significance of estuaries: A classification of Tasmanian estuaries using ecological, physical, and demographic attributes as a case study. Biological Conservation. 92, 383–397 (2000).
Jones, M. & Pinn, E. The impact of a macroalgal mat on benthic biodiversity in Poole Harbour. Mar Poll Bull. 53(1–4), 63–71 (2006).
Van Dolah, F. M., Fire, S. E., Leighfield, T. A., Mikulski, T. A. & Doucette, G. L. Determination of paralytic shellfish toxins in shellfish by receptor binding assay: a collaborative study. Journal of AOAC International. 95, 795–812 (2012).
Brennan, L. & Owende, P. Biofuels from microalgae; A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable Sustainable Energy Rev. 14, 557–577 (2010).
Jain, P., Arora, N., Mehtani, J., Pruthi, V. & Majumder, C. B. Pretreated algal bloom as a substantial nutrient source for microalgae cultivation for biodiesel production. Bioresource Technology. 242. 152–160 (2017).
Yu, G., Zhang, Y., Schideman, L., Funk, T. L. & Wang, Z. Hydrothermal liquefaction of low lipid content microalgae into bio-crude oil. Transactions of the ASABE. 54(1), 239–246 (2011).
Peterson, A., Vogel, F., Lachance, R., Froeling, M. & Antal, M. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 1, 32–65 (2008).
Vardon, D. R., Sharma, B. K., Blazina, G. V., Rajagopalan, K. & Strathmann, T. J. Thermo- the chemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol. 109, 178–87 (2012).
Guo, Y., Thomas, Y., Wenhan, S., Dongha, iX. & Shuzhong, W. A review of bio-oil production from hydrothermal liquefaction of algae. Renewable and Sustainable Energy Reviews. 48, 776–790 (2015).
Ross, A. B. et al. Hydrothermal processing of microalgae using alkali and organic acids. Fuel. 89, 2234–2243 (2010).
Cole, A. et al. From Macroalgae to Liquid Fuel via Waste-Water Remediation, Hydrothermal Upgrading, Carbon Dioxide Hydrogenation, and Hydrotreating. Energy Environ. Sci., https://doi.org/10.1039/C6EE00414H (2016).
Demirbaş, A. Thermochemical conversion of biomass to liquid products in the aqueous medium. Energy Source. 27, 1235–1243 (2005).
Kumar, K. et al. Recent developments on biofuels production from microalgae and macroalgae. Renew Sustain Energy Rev. 65, 235–49 (2016).
Zhou, D., Zhang, L., Zhang, S., Fu, H. & Chen, J. Hydrothermal liquefaction of macroalgae Enteromor phaprolifera to bio-oil. Energy Fuel. 24, 4054–4061 (2010).
Slade, R. & Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts, and prospects. Biomass and Bioenergy. 53, 29–38 (2013).
Arun, J., Varshini, P., Prithvinath, P. K., Priyadarshini, V. & Gopinath, K. P. Enrichment of bio-oil after hydrothermal liquefaction (HTL) of microalgae C. vulgaris grown in wastewater: Bio-char and post HTL wastewater utilization studies. Bioresource Technology. 261, 182–187 (2018).
Zhou, Y., Schideman, L., Yu, G. & Zhang, Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 6, 3765–3779 (2013).
Jena, U., Das, K. C. & Kastner, J. R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensisAppl. Energy. 98, 368–375 (2012).
Muppaneni, H. K. et al. Hydrothermal liquefaction of Cyanidioschyzonmerolae and the influence of catalysts on products. Bioresour. Technol. 223, 91–97 (2017).
Shakya, R. et al. Effect of temperature and Na2CO3 catalyst on hydrothermal liquefaction of algae. Algal Research. 12, 80–90 (2015).
Biller, P. & Ross, A. B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 102, 215–225 (2011).
Chingombe, P., Saha, B. & Wakeman, R. J. Surface modification and characterization of coal-based activated carbon. Carbon 43, 3132–43 (2005).
Alberto, M. R., Faryas, M. E. & Manca de Nadra, M. C. Effect of gallic acid, and catechin on Lactobacillus hilgardii growth and metabolism of organic compounds. J. Agric. Food Chem. 49, 4359–4363 (2001).
Mahapatra, D. M. & Ramachandra, T. V. Algal biofuel: bountiful lipid from Chlorococcum sp. proliferating in municipal wastewater. Curr Sci 105, 47–55 (2013).
Wang, W. et al. Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresource Technology. 250, 474–480 (2018).
Qian, L., Wang, S. & Savage, P. E. Hydrothermal liquefaction of sewage sludge under isothermal and fast Conditions. Bioresour. Technol. 232, 27–34 (2017).
Karagoz, S., Bhaskar, T., Muto, A., Sakata, Y. & Uddin, M. A. Low-temperature hydrothermal treatment of biomass: effect of reaction parameters on products and boiling point distributions. Energy Fuels. 18(1), 234–241 (2004).
Abdullah, S. H. Y. S. et al. A review of biomass-derived heterogeneous catalyst for sustainable biodiesel production. Renew Sust Energy Rev 70, 1040–51 (2017).
Yeh, T. M. et al. Hydrothermal catalytic production of fuels and chemicals from aquatic biomass. J. Chem. Technol. Biotechnol. 88, 13–24 (2013).
Alhassan, Y., Kumar, N. & Bugaje, I. M. Hydrothermal liquefaction of de-oiled Jatropha curcas cake using Deep Eutectic Solvents (DESs) as catalysts and co-solvents. Bioresour Technol 199, 375–381 (2016).
Schaschke, C. A. Dictionary of Chemical Engineering; Oxford University Press: Oxford, UK (2014).
Neveux, N. et al. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresource Technology. 155, 334–341 (2014).
Kumar, V., Kumar, R., Rawat, D. & Nanda, M. Synergistic dynamics of light, photoperiod and chemical stimulants influence biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl Biol Chem. 61, 7–13 (2018).
Yang, J., Cao, J., Xing, G. & Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese, and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 175, 537–544 (2015).
Zhang, Y. M., Chen, H., He, C. L. & Wang, Q. Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS One 8, 1–12 (2013).
Sluiter, A. Determination of ash in biomass. NREL Biomass Analysis Technology Team Laboratory Analytical Procedure #005. NREL, Golden, CO; online at, http://www.nrel.gov/biomass/analytical_procedures.html#lap-005 (2005).
Zhu, Z., Rosendahl, L., Toor, S. S., Yu, D. & Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Applied Energy 137, 183–192 (2015).
Niju, S., Meera, K. M., Begum, S. & Anantharaman, N. Modification of eggshell and its application in biodiesel production. Journal of Saudi Chemical Society. 18, 702–706 (2014).
Channiwala, S. A. & Parikh, P. P. A unified correlation for estimating HHV of solid, liquid, and gaseous fuels. Fuel. 81(8), 1051–1063 (2002).
Guarnieri, M. T., Nag, A., Yang, S. & Pienkos, P. T. Proteomic analysis of Chlorella vulgaris: potential targets for enhanced lipid accumulation. J Proteome. 93, 245–253 (2013).
Author information
Authors and Affiliations
Contributions
Conception and design of the study by Vinod Kumar, Mikhail S. Vlaskin and Manisha Nanda. Drafting and Analysis, interpretation of the data by Sanjay Kumar, Monu Verma, P.K. Chauhan, Harish Chandra Joshi, Waseem Ahmad, Vivekanand Bahuguna, Poonam Negi, Nishesh Sharma, Bharti Ramola, Indra Rautela.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, V., Kumar, S., Chauhan, P.K. et al. Low-temperature catalyst based Hydrothermal liquefaction of harmful Macroalgal blooms, and aqueous phase nutrient recycling by microalgae. Sci Rep 9, 11384 (2019). https://doi.org/10.1038/s41598-019-47664-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47664-w
This article is cited by
-
Aqueous phase recycling: impact on microalgal lipid accumulation and biomass quality
Environmental Science and Pollution Research (2024)
-
Numerical optimization of hydrothermal liquefaction process for the production of bio-oil and bio-char from Citrus maxima peel waste and product characterization
Environmental Science and Pollution Research (2023)
-
Recent Advancements in Microalgal Mediated Valorisation of Wastewater from Hydrothermal Liquefaction of Biomass
BioEnergy Research (2023)
-
Photosynthetic microalgae–based carbon sequestration and generation of biomass in biorefinery approach for renewable biofuels for a cleaner environment
Biomass Conversion and Biorefinery (2023)
-
Microwave-assisted pretreatment of harmful algal blooms for microbial oil-centered biorefinery approach
Biomass Conversion and Biorefinery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.