Abstract
Weight loss progresses with the progression of idiopathic pulmonary fibrosis (IPF), and acute exacerbation of IPF (AE-IPF) frequently occurs in its advanced stage. Adiponectin and leptin are adipokines produced from adipose tissue, and are related to thinness and obesity, respectively. Additionally, these adipokines are implicated in the regulation of inflammation and fibrosis centering on peroxisome proliferator-activated receptor γ (PPARγ). However, the relationship between adiponectin/leptin and AE-IPF remains poorly known. We conducted this study to evaluate levels of serum adiponectin/leptin, and to elucidate the clinical importance of adiponectin and leptin in patients with AE-IPF. Thirty-two patients (39 episodes) who were diagnosed with AE-IPF at our hospital from 1997 to 2016 were retrospectively studied. Serum adiponectin and leptin concentrations were measured with enzyme-linked immunosorbent assay. Patients with AE-IPF showed higher levels of serum adiponectin and leptin than those at initial diagnosis of IPF (p = 0.007 and p = 0.027, respectively). Serum adiponectin/leptin (A/L) ratio was negatively correlated with body mass index at AE-IPF (r = −0.456, p = 0.003) and PaO2 before AE-IPF (r = −0.498, p = 0.034), and positively correlated with C-reactive protein at AE-IPF (r = 0.316, p = 0.049). Patients with higher A/L ratios had worse survival than those with lower A/L ratios (log-rank, p = 0.026). Further, in multivariate analysis, serum A/L ratio was a significant prognostic factor in patients with AE-IPF (HR 2.60, p = 0.042). In conclusion, the higher adiponectin/leptin ratio may be associated with a poor prognosis in patients with AE-IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) shows a catastrophic prognosis1, and one of the leading causes of death in patients with IPF is acute exacerbation of IPF (AE-IPF)2. AE occurs more frequently in patients with IPF than in those with other interstitial pneumonias such as connective tissue disease-associated interstitial pneumonia3,4, and causes 30–40% of all deaths5,6. In patients with IPF, AE more often occurs in the advanced stage of IPF than in its early stages7. Patients with IPF lose further weight as the disease progresses. Therefore, the amount of adipose tissue should decrease in the advanced stage of IPF.
Adiponectin and leptin are adipokines produced from adipose tissue and are related to obesity and emaciation. These adipokines have pleiotropic functions, and affect lymphocytes, macrophages, epithelial cells, endothelial cells, and smooth muscle cells8,9. Additionally, the regulation of inflammation centering on the transcription factor peroxisome proliferator-activated receptor γ (PPARγ) has been reported in the biology of these adipokines8,9. Adiponectin and leptin have the opposite effect on PPARγ expression: adiponectin increases the level of PPARγ and leptin conversely decreases it8. Regarding fibrosis, decreased adiponectin levels were recently reported in patients with systemic sclerosis and interstitial pneumonia10,11. Furthermore, leptin levels in bronchoalveolar lavage fluid reportedly increased in patients with acute respiratory distress syndrome (ARDS), and higher leptin levels were associated with higher mortality in nonobese patients12. In addition, Cao et al. reported that plasma leptin levels increased at AE-IPF, and AE-IPF patients with higher leptin levels at AE showed worse prognosis than those with lower levels13. These results lead us to hypothesize that not only leptin but also adiponectin, which have opposite effects on PPARγ expression, may be related to pathogenesis of AE-IPF in the advanced stage of IPF. Further, adiponectin levels in patients with AE-IPF and their mechanism of action have been unknown.
In the current study, we retrospectively studied patients with AE-IPF and evaluated serum concentrations of these adipokines. To the best of our knowledge, this is the first study to show the relationship between these two adipokines, and the relationship between adipokines and clinical parameters/prognosis in patients with AE-IPF.
Results
Clinical characteristics; laboratory, physiological, radiological findings; and treatments of patients with AE-IPF
Clinical characteristics of all patients (39 episodes) are shown in Table 1 and Supplementary Table S1. Thirty-two patients presented their first AE episode and 7 with their second episode. Median body mass index (BMI) and body surface area (Du Bois formula) were 21.9 and 1.59, respectively. The percent predicted forced vital capacity (FVC) and diffusion lung capacity for carbon monoxide (DLCO) within 12 months before AE-IPF onset was moderately impaired (median, 57.6% and 57.1%, respectively). The most frequent IPF severity grades were GAP III (36%), and Japanese Respiratory Society (JRS) grade IV (36%), which is the most severe grade in each grading system. Twenty-four of 39 patients (62%) underwent treatment for IPF before AE-IPF, including corticosteroids, immunosuppressants, and pirfenidone. Thirteen patients (33%) underwent long-term oxygen therapy before AE-IPF. At the diagnosis of AE-IPF, the median number of peripheral blood white blood cells was 9755/μL and the median PaO2/FiO2 (P/F) ratio was 176. Steroid pulse therapy was administered to all patients. Treatments for AE-IPF began as soon as possible after admission concomitantly with antibiotics administration. The period from admission to beginning treatments for AE showed a median of one day.
Serum adiponectin and leptin levels at the diagnosis of AE-IPF
Serum adiponectin and leptin levels were evaluated in 39 episodes of AE-IPF, for which serum was available at the diagnosis of AE-IPF (Fig. 1). Median adiponectin and leptin concentrations at AE-IPF were 7892 ng/mL and 8.72 ng/mL, respectively. Serum adiponectin level tended to be higher than that at the initial diagnosis of IPF, although the difference was not significant (Fig. 1a, p = 0.148). The serum leptin level at AE-IPF was significantly higher than that at the initial diagnosis of IPF (n = 16) and that in age-matched healthy control (n = 48) (Fig. 1b; p = 0.021 and p < 0.0001, respectively). Regarding serial change in identical patients (n = 16), both adiponectin and leptin levels significantly increased at AE-IPF compared with those at initial IPF diagnosis (Fig. 1c, p = 0.007; Fig. 1d, p = 0.027, respectively).
Serum adiponectin and leptin concentrations at the diagnosis of AE-IPF. Serum adiponectin and leptin levels were evaluated in 39 episodes of AE-IPF. Median adiponectin and leptin concentrations at AE-IPF were 7892 ng/mL and 8.72 ng/mL, respectively. Serum adiponectin levels tended to be higher than those at the initial diagnosis of IPF (a; p = 0.148). Serum leptin levels at AE-IPF were significantly higher than those at the initial diagnosis of IPF (n = 16) and those of healthy controls (n = 48) (b; p = 0.021 and p < 0.0001, respectively). Regarding serial change in identical patients (n = 16), both adiponectin and leptin levels significantly increased at AE-IPF compared with those at initial IPF diagnosis (c, p = 0.007; d, p = 0.027, respectively).
Relationships between serum adiponectin/leptin levels and several clinical parameters
The correlations between serum adiponectin/leptin levels at AE-IPF and several clinical parameters are shown in Fig. 2. There was no correlation between adiponectin and leptin levels (Fig. 2a). Next, relationships between serum adiponectin/leptin (A/L) ratio and clinical parameters were evaluated because adiponectin and leptin show vastly different serum concentrations in obesity and opposite effects on PPARγ expression8. A negative correlation was found between A/L ratio and BMI (Fig. 2b; p = 0.003, r = −0.456). The A/L ratio was negatively correlated with the period from IPF-diagnosis to AE (Fig. 2c; p = 0.042, r = −0.322) and PaO2 before AE (Fig. 2d; p = 0.034, r = −0.498). At AE-IPF, the A/L ratio showed no relationship with PaO2/FiO2 (P/F) ratio (Fig. 2e; p = 0.979, r = −0.004) or HRCT extent score at AE (Fig. 2f; p = 0.125, ρ = −0.260). Conversely, the A/L ratio was positively correlated with serum C-reactive protein (CRP) (Fig. 2g; p = 0.049, r = 0.316) and negatively correlated with serum KL-6 at AE (Fig. 2h; p = 0.035, r = −0.357). The A/L ratio showed no relationships with age (p = 0.545, r = −0.099), smoking pack years (p = 0.080, r = −0.280), %FVC (p = 0.711, r = −0.077), GAP stage (p = 0.301, ρ = −0.181), JRS severity stage (p = 0.937, ρ = −0.014), or serum surfactant protein-D (SP-D) level (p = 0.162, r = −0.242) (Supplementary Fig. S1).
Relationships between serum adiponectin/leptin levels and several clinical parameters. The relationships between serum adiponectin/leptin (A/L) ratio at AE-IPF and clinical parameters are shown. There was no correlation between adiponectin and leptin levels (a). A negative correlation was found between A/L ratio and body mass index (BMI) (b; p = 0.003, r = −0.456). The A/L ratio was negatively correlated with the period from IPF-diagnosis to AE (c; p = 0.042, r = −0.322) and PaO2 before AE (d; p = 0.034, r = −0.498). At AE-IPF, the A/L ratio was not related to PaO2/FiO2 (P/F) ratio (e; p = 0.979, r = −0.004) or HRCT extent score at AE (f; p = 0.269, r = −0.187). The A/L ratio was positively correlated with serum C-reactive protein (CRP) (g; p = 0.049, r = 0.316) and negatively correlated with serum KL-6 at AE (h; p = 0.035, r = −0.357). “PaO2 before AE” was evaluated within 12 months before AE-IPF.
Comparisons of data between patients with higher and lower A/L ratio
Based on the median A/L ratio (median 905) at AE-IPF in 39 patients, the patients were divided into two groups. For the prognostic analysis, comparisons of clinical data between patients with higher A/L ratio (≥905) and lower A/L ratio (<905) in 32 patients without duplicate are shown in Supplementary Table S2. BMI was significantly lower in the higher A/L ratio group than in the lower group (p = 0.023). Regarding the severity of IPF before AE, FVC, JRS severity grades, or GAP stages were not significantly different between groups (p = 0.559, 0.205, and 0.626, respectively). The serum KL-6 level was significantly lower in the higher A/L ratio group than in the lower group (p = 0.022). The serum LDH and SP-D level also tended to be lower in the higher A/L ratio group than in the lower group. The proportion of patients with hyperlipidemia, diabetes mellitus, and administration of exogenous PPARγ agonists - which affect the biology of adiponectin and leptin - did not differ between groups. All patients received steroid pulse therapy, although fewer patients were administered immunosuppressants in the higher A/L ratio group than in the lower A/L ratio (p = 0.028). Other treatments and HRCT extent score did not differ between groups.
Impact of serum A/L ratio on survival
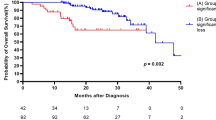
Among 32 patients with AE-IPF, seven died within 1 month of onset (mortality rate, 21.8%), 9 died within 3 months (mortality rate, 28.1%), and 23 died within 12 months (mortality rate, 71.8%) from the first episode of AE-IPF. Nineteen patients died of respiratory failure and four died of infectious pneumonia after beginning of steroid-treatment. Kaplan-Meier survival curves from the first AE-IPF onset are shown in Fig. 3. When patients were divided into two groups based on the median value of each adipokine, the 12-month survival rates were not significantly different among patients with higher and lower adiponectin or leptin (Fig. 3a; adiponectin, log-rank test, p = 0.120 and Fig. 3b; leptin, log-rank test, p = 0.850, respectively). However, the 12-month survival rate was significantly lower in patients with higher A/L ratios (≥905) than in those with lower A/L ratios (Fig. 3c, log-rank test, p = 0.026). The results of univariate Cox proportional hazards analyses for prognostic factors within 12 months of AE-IPF onset are shown in Supplementary Table S3. Serum adiponectin or leptin alone was not a significant prognostic factor (hazard ratio [HR] 1.000, p = 0.076 and HR 0.930, p = 0.075, respectively). However, A/L ratio itself and higher A/L ratio (≥905) were both significant factors of worse prognosis (HR 1.000, p = 0.006 and HR 2.729, p = 0.032, respectively). Additionally, peripheral blood neutrophils at AE (HR 1.000, p = 0.027) and period from admission to the beginning of AE treatment (HR 1.126, p = 0.032) were also significant prognostic factors. The results of multivariate Cox proportional hazards analyses, which show factors that predict survival adjusted for age, are shown in Table 2. A/L ratio itself and higher A/L ratio (≥905) were still significant factors of worse prognosis (HR 1.000, p = 0.012 and HR 2.600, p = 0.042, respectively), although adiponectin or leptin alone was not significant. Furthermore, JRS severity grade (HR 1.610, p = 0.040), preceding oxygen therapy (HR 2.628, p = 0.035), peripheral blood neutrophils at AE (HR 1.000, p = 0.020), and period from admission to the beginning of AE treatment (HR 1.125, p = 0.035) were also significant factors. Although A/L ratio was moderately and negatively associated with BMI (Fig. 2b), even in multivariate Cox proportional hazards analyses adjusted for BMI, A/L ratio itself and higher A/L ratio (≥905) were significant factors of worse prognosis (Supplementary Table S4; HR 1.000, p = 0.011 and HR 2.680, p = 0.046, respectively).
Impact of serum A/L ratio on survival. Among 32 patients with AE-IPF, Kaplan-Meier survival curves from the first AE-IPF onset are shown. When patients were divided into two groups based on median value of each adipokine, the 12-month survival rates were not significantly different between patients with higher and lower adiponectin or leptin (a; adiponectin, log-rank test, p = 0.120 and b; leptin, log-rank test, p = 0.850, respectively). The 12-month survival rate was significantly lower in patients with higher A/L ratio (≥905) than in those with lower A/L ratio (c, log-rank test, p = 0.026).
Discussion
In this study, we evaluated serum adipokine concentrations and determined whether adipokines were important to predict prognosis in patients with AE-IPF. Patients with AE-IPF had increased levels of serum adiponectin and leptin compared with those at first diagnosis of IPF. A/L ratio negatively correlated with BMI and PaO2 before AE-IPF, and positively correlated with CRP. Finally, A/L ratio was a significant prognostic factor in patients with AE-IPF. To the best of our knowledge, this is the first study to show the relationship between adiponectin and leptin, and the significance of these two adipokines in patients with AE-IPF.
Adiponectin and leptin are produced from adipose tissue, and these adipokines are related to obesity and type II diabetes mellitus9. In patients with obesity and type II diabetes mellitus, the serum adiponectin level decreases and that of leptin increases9. Conversely, in emaciated subjects, adiponectin increases and leptin decreases. Furthermore, the biological functions of both adipokines are closely linked to PPARγ activity and its anti-inflammation capacity8,9. In the current study, however, both adiponectin and leptin levels increased at AE-IPF, and the mechanism of action of these adipokines does not seem to be simple like in obesity or diabetes mellitus.
Leptin has proinflammatory effects via PPARγ suppression8,9. Leptin reportedly promotes profibrotic gene transcription related to transforming growth factor (TGF) β in patients with ARDS without obesity12. Further, Cao et al. reported that leptin levels increased at AE-IPF, and AE-IPF patients with higher leptin levels at AE had poorer prognosis than those with lower levels13. Even in the present study, the leptin level significantly increased at AE-IPF. However, higher leptin alone was not related to worse prognosis, and higher adiponectin rather seemed to be related to poor prognosis. Adiponectin has anti-inflammatory effects via activation of PPARγ8,9. Furthermore, adiponectin suppresses TGF β signaling centering on PPARγ8, and suppresses acute lung injury14. Reportedly, in patients with IPF15 or systemic sclerosis10,11, adiponectin levels significantly decreased. Conversely, several studies have shown that adiponectin has profibrotic effects. Higher adiponectin levels were positively related to advanced liver fibrosis in hepatitis B viral infection16,17, and lack of adiponectin was associated with dampened wound healing in diabetes mellitus18. Further, adiponectin promoted collagen production from fibroblast19,20, induced intercellular adhesion molecule (ICAM)-1 on fibroblast21, and then facilitated its adhesion capacity20. In the current study, both adipokines increased at AE-IPF, and higher A/L ratio was rather related to poorer prognosis. Although the exact mechanism related to these adipokines remains unknown in AE-IPF, these results imply that adiponectin’s anti-inflammatory (profibrotic) effects following leptin’s proinflammatory effects may play a role in the pathogenesis of AE-IPF. This pathogenesis seems to be similar to the activation of M2 (anti-inflammatory and profibrotic) alveolar macrophages following that of M1 (proinflammatory) macrophages in acute lung injury or ARDS22.
This study has several limitations. First, only a small number of patients with AE-IPF were enrolled. Second, the clinical and prognostic data were retrospectively analyzed. Finally, the treatment of AE-IPF was not uniform. A prospective and larger study should be conducted to determine the role of adipokines in patients with AE-IPF.
In conclusion, this study showed that serum adiponectin and leptin levels significantly increased in patients with AE-IPF. A/L ratio positively correlated with CRP, and A/L ratio was a significant prognostic factor in patients with AE-IPF. These adipokines are closely associated with the balance between inflammation and fibrosis, and may play a role in the pathogenesis of AE-IPF. A larger and prospective study is needed to precisely evaluate the role of these adipokines in the pathogenesis of AE-IPF.
Methods
Study design and patients
Thirty-two patients who were diagnosed with AE-IPF at our hospital between 1997 and 2016 were studied. Seven patients had two episodes of AE-IPF (Table 1). In sum, 39 episodes of AE, for which serum samples were available, were retrospectively reviewed. Twenty patients were thoroughly examined by surgical lung biopsy before developing AE-IPF and met the 2011 international consensus criteria for IPF1. The remaining 12 patients showed clinical and high-resolution computed tomography (HRCT) features identical to IPF and were diagnosed with IPF without surgical lung biopsy1. Patients diagnosed with any connective tissue disorders were excluded from the study.
AE-IPF was diagnosed according to the modified diagnostic criteria as previously described23,24,25,26. Patients enrolled in this study also met the 2016 AE-IPF criteria27. The study protocol was approved by the Ethical Committee of Hamamatsu University School of Medicine (approval number 18–085 and 15–165), and this study was carried out in accordance with the approved protocol. The need for patient approval and informed consent was waived due to the retrospective nature of the study.
Data collection
Clinical data were obtained from the medical records. Disease severity of IPF within 12 months before the AE event was assessed using the GAP-staging system28 and the Japanese Respiratory Society (JRS)-severity scale of interstitial pneumonia7. The former considers gender, age, and two lung physiology variables: FVC and DLCO28. The latter is based on PaO2 at rest and minimum SpO2 during the 6-minute walking test (6MWT)7.
The extent of lung opacities was measured on three HRCT slices as previously described29,30. The sum of the scores from five lobes (0–25) was used to express the extent of lung opacities in each patient. The HRCT patterns of AE-IPF were evaluated as reported by Akira31. The HRCT findings were reviewed by two observers.
Measurement of serum adiponectin and leptin concentrations
All blood samples were collected on the first or second day of admission before starting treatments for AE-IPF. Serum adiponectin and leptin concentrations were measured with enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
Statistical analyses were performed using StatView J-4.5 and JMP-13.1.0 (SAS Institute Inc., Cary, NC, USA). Categorical data were compared using the Chi-square test or Fisher’s exact probability test for independence, and continuous data were compared using the unpaired t-test. Continuous data at different time points in the same patient were compared using the paired t-test. The relationship between adiponectin/leptin ratio and serial data was analyzed using Pearson’s correlation coefficient, and that between adiponectin/leptin ratio and discrete variable data was analyzed using Spearman’s rank correlation coefficient. Survival of patient groups was evaluated with Kaplan-Meier curves, and was compared between groups using the log-rank test. The relationships between variables and mortality were assessed by the Cox proportional hazards regression analysis. All tests were two-sided and statistical significance was set at p < 0.05.
References
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine 183, 788–824, https://doi.org/10.1164/rccm.2009-040GL (2011).
Kondoh, Y. et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 103, 1808–1812 (1993).
Suda, T. et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 103, 846–853, https://doi.org/10.1016/j.rmed.2008.12.019 (2009).
Song, J. W., Hong, S. B., Lim, C. M., Koh, Y. & Kim, D. S. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 37, 356–363, https://doi.org/10.1183/09031936.00159709 (2011).
Ley, B., Collard, H. R. & King, T. E. Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183, 431–440, https://doi.org/10.1164/rccm.201006-0894CI (2011).
Natsuizaka, M. et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 190, 773–779, https://doi.org/10.1164/rccm.201403-0566OC (2014).
Homma, S., Sugino, K. & Sakamoto, S. Usefulness of a disease severity staging classification system for IPF in Japan: 20 years of experience from empirical evidence to randomized control trial enrollment. Respir Investig 53, 7–12, https://doi.org/10.1016/j.resinv.2014.08.003 (2015).
Dantas, A. T. et al. The Role of PPAR Gamma in Systemic Sclerosis. PPAR Res 2015, 124624, https://doi.org/10.1155/2015/124624 (2015).
Haley, S., Shah, D., Romero, F. & Summer, R. Scleroderma-related lung disease: are adipokines involved pathogenically? Curr Rheumatol Rep 15, 381, https://doi.org/10.1007/s11926-013-0381-8 (2013).
Arakawa, H. et al. Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients. Exp Dermatol 20, 764–766, https://doi.org/10.1111/j.1600-0625.2011.01310.x (2011).
Masui, Y. et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Mod Rheumatol 23, 323–329, https://doi.org/10.1007/s10165-012-0660-7 (2013).
Jain, M. et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-gamma. Am J Respir Crit Care Med 183, 1490–1498, https://doi.org/10.1164/rccm.201009-1409OC (2011).
Cao, M. et al. Plasma Leptin Is Elevated in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Mediators Inflamm 2016, 6940480, https://doi.org/10.1155/2016/6940480 (2016).
Konter, J. M. et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol 188, 854–863, https://doi.org/10.4049/jimmunol.1100426 (2012).
Neumann, E. et al. Adipokine expression in systemic sclerosis lung and gastrointestinal organ involvement. Cytokine 117, 41–49, https://doi.org/10.1016/j.cyto.2018.11.013 (2019).
Hui, C. K. et al. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol 47, 191–202, https://doi.org/10.1016/j.jhep.2007.02.023 (2007).
Hsu, C. S. et al. Adipocytokines and liver fibrosis stages in patients with chronic hepatitis B virus infection. Hepatol Int 9, 231–242, https://doi.org/10.1007/s12072-015-9616-2 (2015).
Boulenouar, S. et al. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity 46, 273–286, https://doi.org/10.1016/j.immuni.2017.01.008 (2017).
Nakasone, H. et al. Impact of high-/middle-molecular-weight adiponectin on the synthesis and regulation of extracellular matrix in dermal fibroblasts. Exp Hematol 42, 261–273, https://doi.org/10.1016/j.exphem.2013.12.009 (2014).
Fang, C. L., Huang, L. H., Tsai, H. Y. & Chang, H. I. Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin. Int J Mol Sci 17, https://doi.org/10.3390/ijms17071129 (2016).
Chen, H. T. et al. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS One 9, e92741, https://doi.org/10.1371/journal.pone.0092741 (2014).
Aggarwal, N. R., King, L. S. & D’Alessio, F. R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306, L709–725, https://doi.org/10.1152/ajplung.00341.2013 (2014).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine 176, 636–643, https://doi.org/10.1164/rccm.200703-463PP (2007).
Enomoto, N. et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med 15, 15, https://doi.org/10.1186/s12890-015-0004-4 (2015).
Enomoto, N. et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J 12, 2378–2389, https://doi.org/10.1111/crj.12918 (2018).
Enomoto, N. et al. Differences in clinical features of acute exacerbation between connective tissue disease-associated interstitial pneumonia and idiopathic pulmonary fibrosis. Chron Respir Dis 16, 1479972318809476, https://doi.org/10.1177/1479972318809476 (2019).
Collard, H. R. et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 194, 265–275, https://doi.org/10.1164/rccm.201604-0801CI (2016).
Ley, B. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 156, 684–691, https://doi.org/10.7326/0003-4819-156-10-201205150-00004 (2012).
Enomoto, N. et al. Amount of elastic fibers predicts prognosis of idiopathic pulmonary fibrosis. Respir Med 107, 1608–1616, https://doi.org/10.1016/j.rmed.2013.08.008 (2013).
Enomoto, N. et al. Quantitative analysis of lung elastic fibers in idiopathic pleuroparenchymal fibroelastosis (IPPFE): comparison of clinical, radiological, and pathological findings with those of idiopathic pulmonary fibrosis (IPF). BMC Pulm Med 14, 91, https://doi.org/10.1186/1471-2466-14-91 (2014).
Akira, M., Kozuka, T., Yamamoto, S. & Sakatani, M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine 178, 372–378, https://doi.org/10.1164/rccm.200709-1365OC (2008).
Acknowledgements
We thank Dr Andrew Stow from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This study was not funded. This study was supported by the Study Group on Diffuse Lung Disease, and the Scientific Research/Research on Intractable Diseases in the Ministry of Health, Labour and Welfare of Japan. Results of this study were previously presented at the ATS International Conference 2017.
Author information
Authors and Affiliations
Contributions
Conception and design: N.E. Analysis and interpretation: N.E., Y.O., H.Y., M.K., H.H., Y.S., M.K., K.F., T.F., N.I., Y.N. and T.S. Drafting of the manuscript for important intellectual content: N.E., Y.N. and T.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enomoto, N., Oyama, Y., Yasui, H. et al. Analysis of serum adiponectin and leptin in patients with acute exacerbation of idiopathic pulmonary fibrosis. Sci Rep 9, 10484 (2019). https://doi.org/10.1038/s41598-019-46990-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46990-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.