Abstract
In Mexico, HPV vaccines available immunize against genotypes 16/18 and 16/18/6/11; however, there is limited surveillance about carcinogenic subtypes in different states of the country that allow evaluating the effectiveness of vaccination and cervical cancer screening programs. Here, we report the regional and age-specific prevalence of 14 hr-HPV genotypes as well as their prevalence in abnormal cytology (from ASCUS to cervical cancer) among Mexican women which were undergoing from cervical cancer screening in the Salud Digna clinics in 20 states of the country. This study includes women with social security from the majority of public health institutions (IMSS, ISSSTE, SEMAR, and PEMEX), and women without social security. For cervical cancer screening, we used the SurePath liquid-based cytology and the BD Onclarity HPV Assay. From December 1, 2016, to August 2, 2018, the hr-HPV prevalence among 60,135 women was 24.78%, the most prevalent types were HPV 16 (4.13%), HPV 31 (4.12%) and HPV 51 (3.39%), while HPV 18 (1.70%) was less prevalent among infected women. Interestingly, the genotypes not covered by current vaccines in Mexico were commonly found in precancerous lesions, evidencing their carcinogenic potential, so it is necessary to increase their surveillance and inclusion in cervical cancer screening triage.
Similar content being viewed by others
Introduction
The human papillomavirus (HPV) is the leading cause of cervical cancer and recently, has increased the evidence that relates HPV infections with anogenital, head, and neck cancers1. Carcinogenic types include predominantly, alpha 9 (HPV 16/31/33/35/52/58), alpha 7 (HPV 18/39/45/59/68), alpha 6 (HPV 56/66), and alpha 5 (HPV 51) genus2,3,4. These families differ in their mucotropisms, for example, members of alpha 7 genus have been associated with glandular lesions and adenocarcinoma of the cervix; meanwhile, alpha 9 genus has been found in squamous intraepithelial lesions and squamous cell carcinoma5,6.
Variability and prevalence of alpha members among populations would have an epidemiological impact in the incidence of some types of cancer. So that, screening programs, as well as preventive vaccination, have to be implemented in each population to reduce the burden of HPV-related cancers. Vaccination has reduced the abnormal cytology rate7, as well as screening programs had contributed to the reduction of cervical cancer mortality8,9; it might expect that together vaccination and screening contributes to reducing most cervical cancers.
The study of HPV genotypes allows identifying oncogenic strains present in human populations; since it has been reported that its prevalence and distribution vary according to countries and continents10. This differences in genotypes distribution may affect the effectiveness of the HPV vaccines in different populations. Regional data on the prevalence and distribution of HPV are essential for estimating the impact of vaccines and cervical cancer screening program (CCSP) in each country.
In Mexico, two previous population-based studies showed the usefulness of the HPV DNA test in CCSP11,12; this information has contributed to changing cancer screening from cytology to HPV triage. Regarding HPV prevalence, different works had been carried out in some states of the country (Supplementary Table S1); the majority analyzed women with social security. However, HPV infections in women without social security in Mexico has not been addressed.
Additionally, a detailed study of the distribution of HPV genotypes in the country is not available, which makes it difficult to select suitable vaccines according to the subtypes profile in the country. Despite that, in 2011, the Ministry of Health in Mexico approved the introduction of two prophylactic vaccines against HPV16/18 and HPV6/11/16/18 into the national vaccination program to prevent cervical cancer13. These vaccines were selected by the growing evidence of the carcinogenic potential of HPV16/18, which has been found in more than 70% of cervical cancer cases globally14.
However, it might be that the diversity of alpha members would impact vaccination effectiveness since the available vaccines in the market, not cover all potentially carcinogenic subtypes. For that reason, it is essential the surveillance of HPV genotypes, to collect clinical and epidemiology information that allows to evaluate the effectiveness of vaccination programs and help in the selection of suitable vaccines in each country.
In this study, we investigate the prevalence and distribution of circulating high risk-HPV genotypes (hr-HPV) in 60,135 women (including women with or without social security) from 20 states of Mexico. Also, we identified the most common genotypes present in precancerous lesions. This study, together with previous works can serve as a reference to guide cervical cancer screening and HPV vaccination programs in Mexico.
Material and Methods
Salud Digna is a private not for profit and non-government institution which provides diagnostic services in Mexico through outpatient diagnostic clinics located in 20 of 32 states of the country, in which could are attendant all people, no matter their socio-economic status, or if they have social security or not.
Study design and population
For this retrospective cross-sectional study, we analyzed anonymized electronic health records of cervical cancer screening from patients whose where attendant in the outpatient care clinics Salud Digna in 20 states of Mexico from December 01, 2016 and 02 August 2018. We include all available data collected during gynecological examinations, including data on demographics, gynecologic, and obstetric information. A unique standardized and validated survey was applied for data collection in all clinics. The cases included were from adult women (18 to 90 years), which were screened for cervical cancer. The exclusion criteria were the following: women younger than 18 years, pregnant women, and women with hysterectomy. For geographical analysis, we included states with 140 or more subjects. This threshold was considered from the sample size calculations for cross-sectional studies15; in this case, for an expected HPV-infections prevalence of 10% based on the mean of prevalence reported by population-based studies in Mexico12,16,17.
Consent for using information, handling data and protecting information privacy
The consent for the use of information from health records was obtained according to the Mexican Federal Law on Personal Data Protection (LFPDPPP, by its acronyms in Spanish). People who are attendant in the Salud Digna clinics accept our privacy policy which includes the use of their information for scientific research purposes; by the above, we do not need specific informed consent from each people included in this work, because this study is a cross-sectional analysis of an electronic health registry. We handled data protection and privacy of its, according to national laws and guidelines in Mexico. Data obtained was anonymized by the assignation of a unique ID code to protect the identity of people and to prevent data duplication in subsequent analysis. Aggregation of information was used to enhance data protection.
Ethical statement
This study was approved by the Ethical Review and Research Board of Salud Digna. All methods were carried out by the approved guidelines and the Declaration of Helsinki.
Procedures
The procedures for the Pap test and HPV genotyping used to generate the information collected in the electronic health records used in this study are described below. Cervical cancer screening results from the registry were obtained from the samples collected in the BD SurePath liquid-based cytology collectors during gynecological examinations in each clinic, then, were processed in the Cytology unit, and Molecular biology laboratory at the National Reference Center of Salud Digna in Culiacan, Sinaloa. Cytology samples were processed into the BD Totalys MultiProcessor (BD Diagnostics, Sparks, USA) for the cell enrichment of cervical samples (automated sample transfer, cells centrifugation, liquid decanting, and cells aspiration) as well as to make aliquots for an HPV test.
The slides preparation and staining for Pap smears were performed into the BD Totalys SlidePrep (BD Diagnostics, Sparks, USA) according to the manufacturer´s recommendations. Slides were visually inspected in Carl Zeiss Primo Star HD microscopes (Carl Zeiss Microscopy, LLC, USA) by 13 certified cytotechnologists; all abnormalities observed were confirmed for certified pathologists and 14% of total slides inspected by cytotechnologists were re-analyzed by independent pathologists (Pathologists were blinded for the commentaries of cytotechnologist). Pap test was performed according to the 3rd ed. of The Bethesda System for Reporting Cervical Cytology18.
The HPV DNA testing was performed using the automated workflow on the Viper LT system. HPV type was determined using the real-time PCR BD Onclarity HPV Assay (BD Diagnostics, Sparks, USA) which detects and amplify HPV type-specific E6 and E7 genes19. This assay simultaneously detects 14 high-risk HPV types, in three different reactions. R1 detects individually HPV 16/18/45, R2 detects individually HPV 31 and two groups: G1 (HPV 33/58), and G2 (HPV 56/59/66); R3 detects individually HPV 51/52 and G3 (HPV 35/39/68); all reactions use the human β-globin gene as an internal control.
Statistical analysis
Descriptive statistics were performed on all data sets. For categorical data, the Wilson score method without continuity correction was performed to calculate 95% CI. Genotypes detected in the G1, G2, and G3 had regarded as a single unit for results descriptions. We evaluated the associations between demographic, gynecobstetrics, and other factors to HPV infections using a stepwise multivariable logistic regression model. All variables significantly associated in the univariable analysis (p < 0.05) were selected for inclusion in the multivariate logistic regression model, which was used to control for potentially confounding factors. The adjusted odds ratio (OR) were calculated from age-adjusted multivariate logistic regression. To analyze the oncogenicity of HPV genotypes, we used registries from women undergoing both tests (HPV and Pap test); odds ratio (OR) of each genotype were calculated from the univariate logistic regression model. The analyses were done in SPSS V. 24 (IBM, USA).
Results
Characteristics of the population
Between December 01, 2016 and 02 August 2018, 60,328 women were attendant for cervical cancer screening in outpatient diagnostic clinics Salud Digna located in 20 of 32 states of the country. 5 registries (belonging women younger than 18 years) were excluded from the dataset; also 188 registries were excluded because they belong from states with less than 140 women screened. For the HPV prevalence as well as geographical and age-specific analysis, 60,135 registries were included. For risk factors analysis and the relationship of HPV genotypes according to cytology results, 52,415 registries were included, and 7,721 registries with incomplete gynecological and clinical information were excluded (in each analysis missed values and exclusions are declared).
In this study, women from 18 to 88 years were screened for cervical cancer; the mean age was 38.76 years; 53.69% of women had their first sexual intercourse at 18 years or older. Also, the majority of women declared not to use tobacco (75.59%). Most of the women studied have been pregnant (71.64%), 51.34% declared to used contraceptive methods, from these, 21.74% corresponding to condom use; 70.72% of women previously have been undergoing to Pap test. It is important to note that most of the women declared not having social security (70.81%). A summary of the demographic characteristics of women included in this study is shown in Table 1.
Prevalence of circulating hr-HPV genotypes
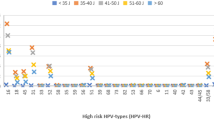
The prevalence of hr-HPV in 60,135 women was 24.78% (Fig. 1); the most prevalent genotypes were HPV 16 (4.13%), HPV 31 (4.12%), and HPV 51 (3.39%), while HPV 18 was less prevalent (1.70%) (Table 2). From the genotypes detected in groups, G2 (HPV 56/59/66) was the most prevalent (9.05%), followed by G3 (HPV 35/39/68) with 5.59% (Table 2). Moreover, women without social security had a higher prevalence of infections (25.72%) than women with social security (22.48%) (Supplementary Table S2). In contrast, no significant differences were observed in the prevalence of hr-HPV genotypes between women with or without social security and between women belonging to different public health institutions (Supplementary Table S3).
Prevalence of hr-HPV infections in 60,135 women from 20 states of Mexico. Coropletic histogram (A) and map (B) shows the prevalence of hr-HPV infections in 20 states of Mexico. The color red indicates the states with the highest prevalence of infections, while yellow shows the states with the lowest prevalence. Gray color (in the map) shows the states that are not included in this work. (C) The 5-year prevalence of hr-HPV infections in 60,135 women screened in 20 states of Mexico, the trend of age-specific prevalence was evaluated with the chi-squared (χ2) test for trend. The confidence intervals of the prevalence in each state are shown in Table 2.
Prevalence of HPV genotypes in 20 states of Mexico
In this work, we include those states in which the number of records (sample size for these states) was at least 140. This threshold was considered from the sample size calculations for cross-sectional studies15. The expected HPV prevalence was 10% based on the mean of prevalence previously reported by population-based studies in Mexico12,16,17. Among states analyzed, Chihuahua (28.74%), and Nayarit (28.33%) had the highest prevalence of infections, while in Quintana Roo (21.17%) and Sinaloa (21.54%) we found the lowest prevalence (Fig. 1). An age-standardized rate is shown in Supplementary Table S4. According to genotype, the HPV 16 was more prevalent in Sonora (5.70%), and Chihuahua (5.59%); meanwhile the lowest prevalence was found in Puebla (3.17%), and Sinaloa (3.42%).
We found that the HPV 31 was more prevalent in San Luis Potosi (6.07%) and Aguascalientes (5.20%); while the lowest prevalence was observed in Quintana Roo (1.95%), and Sinaloa (3.01%). The HPV 51 was more frequent in Nayarit (4.78%), and Nuevo Leon (4.39%), in contrast, we observed the lower prevalence in Quintana Roo (2.43%), and Durango (2.45%). The HPV 52 was most prevalent in Chihuahua (4.68%) and Veracruz (4.63%); in contrast in Sinaloa (2.84%), and Nuevo León (2.93%) it was observed the lowest frequencies (Table 2).
The HPV 18 was more prevalent in Nayarit (2.85%) and Nuevo Leon (2.66%) than in Quintana Roo (0.97%), and Durango (1.15%) in which was less prevalent. In Chihuahua (3.60%) and Nayarit (2.26%), HPV 45 was the most prevalent; meanwhile in Quintana Roo (0.73%), and Durango (1.15%) was less prevalent. The G2 (HPV 56/59/66) was more prevalent in Nuevo Leon (11.32%), and San Luis Potosi (10.90%), and less frequent in Quintana Roo (6.81%) and Sinaloa (7.44%). The G3 (HPV 35/39/68) was more prevalent in Veracruz (7.48%), and Chihuahua (6.85%), while in Guanajuato (4.83%), and Coahuila (4.86%) was less prevalent. Finally, the G1 (HPV 33/58) was more prevalent in Chiapas (4.86%), and Michoacan (4.37%), and less prevalent in Quintana Roo (2.19%), and Nuevo Leon (2.40%) (Table 2).
Age-distribution of hr-HPV genotypes
The age-prevalence of HPV infections is shown in Fig. 1C; the higher prevalence was observed in women younger than 25 years (47.79%) and subsequently, infections decreases; the second peak of infections was observed in women older than 70 years (12.79%). A similar trend was observed in all HPV types. For example, HPV 45 reaches their higher prevalence in women with 20 to 30 years; only HPV 16/52/18, G2 (HPV 56/59/66) and G3 (HPV 35/39/68) were present in women older than 70 years (Supplementary Fig. S1).
Association between selected risk factors to hr-HPV infections
Moreover, we assessed the relationships between gynecologic, obstetrics, and lifestyle characteristics for HPV infections. We found that women without social security showed an increased the odds to get infected (OR = 1.10; 95%CI = 1.05–1.16). Similarly, sexual intercourse before 18 years was significantly associated (OR = 1.15; 95%CI = 1.10–1.20). However, menarche age lower than 12 years did not show a significant association with HPV infections (OR = 0.97; 95%CI = 0.92–1.01) (Table 3).
Furthermore, we found that women that never had been pregnant showed increasing odds to be infected (OR = 1.84; 95%CI = 1.66–2.02) than women with a history of pregnancies. In this way, women who have had more than one pregnancy showed an odds reduction to get infected. Regarding birth types, the cesarean surgeries reduced the odds of infections (OR = 0.86; 95%CI = 0.82–0.90); however, births were not related (OR = 1.03; 95%CI = 0.98–1.08). Women with a history of abortions slightly increased their odds (OR = 1.09; 95%CI = 1.04–1.15) (Table 3).
The tobacco consumption (OR = 1.24; 95%CI = 1.17–1.31) as well as sexually transmitted diseases (STD) were significantly associated to HPV infections (OR = 1.21; 95%CI = 1.09–1.34). Of the STD’s, infections with Trichomonas vaginalis (OR = 1.52; 95%CI = 1.29–1.78), Candida (OR = 1.12; 95%CI = 1.04–1.21) or Gardnerella vaginalis (OR = 1.51; 95%CI = 1.41–1.61) showed significant odds (Table 3).
Association between cervical cytology and HPV genotypes
We analyzed the prevalence of hr-HPV according to cytological results in women undergoing co-testing procedures (Pap test and HPV typing at the same time). We found that the prevalence of hr-HPV was 21.21% in women negative for intraepithelial lesions or malignancy (NILM), 73.04% in atypical squamous cells with undetermined significance (ASCUS), 82.14% in atypical squamous cells cannot exclude high-grade squamous intraepithelial lesions (ASC-H), 73.68% in atypical glandular cells (AGC), 84.09% in low-grade squamous intraepithelial lesions (LSIL), 92.22% in high-grade squamous intraepithelial lesions (HSIL), and 78.57% in cervical cancer cases (Table 4).
The most prevalent genotypes from NILM to HSIL were HPV 16 (NILM: 3.21%, HSIL: 48.88%), HPV 31 (NILM: 3.48%, HSIL: 13.33%), HPV 51 (NILM: 2.69%, HSIL: 5.55%) and HPV 52 (NILM: 2.75%, HSIL: 7.77%). In the cervical cancer cases, HPV 16 (42.86%) and HPV 31 (21.43%) were the most prevalent genotypes (Table 4). Regarding genotypes detected in groups, they were more prevalent in abnormal cytology and HSIL, but low prevalent among cervical cancer (Table 4).
The analysis by HPV genus showed that members of the alpha 9 genus, (e.g., HPV 16/31 and HPV 33/58) were more prevalent in all cytological abnormalities including cervical cancer; while the alpha 7 genus (e.g., HPV 18/45, and HPV 39/59/68) were less frequent among cytological abnormalities and cervical cancer. Despite that genotypes of the alpha 6 genus (e.g., HPV 56/66) were the most prevalent in all categories of the cytology test; these genotypes were not associated with precancerous lesions and cervical cancer. Similarly, HPV 51 (from alpha 5 genus) was not significantly associated with precancerous lesions and was absent in cervical cancer cases (Table 4).
Discussion
This study is the first in Mexico that comprises a wide geographical dispersion, including 20 of 32 states of the country. In this work, we show the prevalence of carcinogenic HPV types among Mexican women undergoing cervical cancer screening in private outpatient care Salud Digna clinics. The study population consisted of gynecological outpatients and asymptomatic women; some of them are affiliated to public health institutions (such as IMSS, ISSSTE, PEMEX, SEDENA, and Seguro Popular), and others do not have social security (Table 1).
The prevalence of 14 hr-HPV in 60,135 women was 24.78%. Previous studies in Mexico in different health care institutions reported prevalences of 10–49.7%, which includes low and high-risk HPV types. There are some differences between previous works and this study that limits the comparison of the results, including the type and design of the study, and the intrinsic limitations of HPV test used in each work which has been further discussed20,21; the above can be reviewed in detail in the Supplementary Table S1.
One of the main differences is that we used the real-time PCR Onclarity HPV assay test for HPV typing which has been recently approved by the Food and Drug Administration (FDA) agency in the US for HPV test22. This assay detects 13 carcinogenic HPV types and 1 possible carcinogenic (HPV 66)23. Six genotypes are detected individually (16, 18, 31, 45, 51, and 52) and the others are detected in groups: G1 (HPV 33/58), G2 (HPV 56/59/66), and G3 (HPV 35/39/68), reducing the possibility of knowing the real prevalence of each genotype, representing a limitation for HPV genotypes surveillance, but not for clinical purposes22. The G2 and G3 groups have a lower risk of cervical lesions (among carcinogenic types) compared to HPV 16 according to globally reports10,14,23,24.
Moreover, this test targeting HPV DNA E6 and E7 genes, this implies that those infections in which the L1 gene has been lost by viral integration can be detected because the E6 and E7 genes are not affected by this event21; in contrast, L1-based typing tests could miss HPV infections in cervical samples. Also, a comparison between consensus PCR targeting L1 primers (MY09/11) and type-specific E6/E7 HPV PCR showed that L1 PCR failed to detect 10.9% of HPV infections25, also MY09/11 showed lower sensitivity (87.9% vs 98.3%) and specificity (38.7% vs 76.14%) than type-specific E6/E7 primers25. Another primer set is GP5+/GP6+, which also targeting L1 and has similar sensitivity than MY09/1126. However, this primer set to fail in the amplification of DNA from multiple HPV present in a single sample26. The above might explain why we observed a high prevalence of HPV infections using E6/E7-based typing test than previous works in the country that used L1-based typing tests (Supplementary Table S1) which might do not detect HPV infections correctly.
Furthermore, studies of HPV in women without social security in Mexico are limited. In this work, 70.81% of women studied had no social security, in which the prevalence of hr-HPV infections was higher (25.72%) than women with social security (22.48%) affiliated to the majority of public health institutions of the country (IMSS, ISSSTE, Seguro Popular, SEDENA, and PEMEX). Also, the age-distribution of HPV infections observed in this study was similar to previous studies in Mexico11,16,17,27 and other Latin-American countries10.
In this work, we found that women without social security increase their odds of getting HPV infections. The above shows the need to increase the accessibility to cervical cancer prevention programs to all women in Mexico. Additionally, sexually transmitted diseases were highly related to HPV infections which had been reported in other populations28,29,30. Interestingly, multiple pregnancies were associated with a reduction in the odds of infections. Also, cesarean surgeries and abortions were weakly associated with HPV infections. The information about lifetime-sexual partners is an important risk factor for HPV infection; however, we could not get that information during interviews for cervical cancer screening, which could confuse some of the factors studied here.
Regarding the prevalence of HPV genotypes, we found that HPV 16, 31 and 51 were the most prevalent, also G2 (HPV 56/59/66), G3 (HPV 35/39/68) were the commonest, while HPV 18 was less prevalent, which differs with the majority of previous reports in the country (Supplementary Table S1). In Mexico, the National Immunization Council in 2011 approved the nationwide expansion of school-based HPV vaccination program which includes all girls aged 9 years and unschooled girls aged 11 years13; this program used vaccines against HPV 16/18 and HPV 6/11/16/18.
The first generation of women vaccinated in 2011 has reached the age of 18–20 years; this age group represents 12.08% of the women studied in this work so that we expect that in our work the majority of women are not vaccinated (87.92%). In consequence, we consider that the low prevalence of HPV 18 observed would be explained by different factors such as mucotropism of HPV genotypes5,6, and ethnicity31,32,33 rather than vaccination.
Additionally, we found a difference in the prevalence of hr-HPV genotypes between young and old women (see Supplementary Fig. S1); despite the low prevalence observed in old women, the genotypes observed in this age group had been associated with cervical cancer34,35,36, this might explain the raising of cancer cases in old women in the country37. Furthermore, we found that the most prevalent genotypes from normal to abnormal cytology were HPV 16/31/51, which was similar to previous reports in women with normal cytology10,35,36,38 and cervical cancer cases14,24,36,39. However, HPV 18 and 45 were less frequent in this population. In contrast, genotypes detected in groups (56/59/66 and 35/39/68) were commonly found in normal cytology and LSIL, but lower in HSIL and CC cases, showing a lower risk for high-grade cervical lesion promoted by this genotypes; which is consistent with worldwide reports14,24.
One of the limitations of this work is the low number of cancer cases reported and the lack of information about colposcopy and biopsy results since this work studied an ambulatory population, women that need a colposcopy were referred to other health institutions and specialized hospitals. For the above is not possible to assess the contribution of HPV types in cervical cancer as well as to evaluate the impact of vaccination in the reduction of cervical cancer.
It is important to know that we found a high prevalence of HPV 31 and HPV 33/58 in normal and abnormal cytology; a previous work found that these genotypes had a comparable risk for HSIL development that of HPV 1840. However, these genotypes are not included in the current vaccines available in the country (Cevarix and Gardasil4). Recently, a nine-valent vaccine (Gardasil9) which immunizes against HPV 6/11/16/18/31/33/45/52/58 has been tested to assess their safety and efficacy to reduce precancerous lesions41,42,43 and has been approved for use in Europe, Canada, Australia, and the US44. This vaccine immunizes against the most prevalent carcinogenic genotypes found in this work and other previous studies in the country, so that could be suitable for vaccination in Mexico.
Moreover, with the development and validation of HPV tests, different screening algorithms for cervical cancer had been proposed45, including HPV 16/18 typing to refers women to colposcopy45,46,47,48,49. In Mexico, the HPV triage coupled to hr-HPV 16/18 genotyping shown a good cost-effectiveness relationship than hr-HPV plus pap test50. A recent analysis showed that HPV 31/33 have higher positive predictive values for CIN3 and CIN2 than HPV 1851. Taking into account the results shows here, and the previous works in the country, we consider that the inclusion of HPV 31 in the HPV typing triage in Mexico would improve cervical cancer screening due to the high prevalence of this genotype among cervical neoplasias.
The information showed in this work does not represent the Mexican population entirely; however, this is one of the first approaches to known the circulating hr-HPV genotypes in Mexico. Our results show a growing prevalence of HPV genotypes not included in the current vaccines applied in the country. Together all work on HPV in Mexico in women from different regions of the country, with or without social security and with different population profiles provides valuable information that will help to guide the future cervical cancer screening programs and HPV vaccination in Mexico. Also, it is necessary to extend and continue the surveillance of circulating HPV genotypes to evaluate the effectiveness of vaccination and screening programs to reduce of cervical cancer and other HPV related cancers in Mexico in the coming years and long-term.
Data Availability
All relevant data concerning this work are published in this article and in the supplementary material.
References
Bruni L. et al. Human Papillomavirus and Related Diseases in the World. (ICO Information Centre on HPV and Cancer (HPV Information Centre), 2017).
Chen, Z. et al. Evolution and Taxonomic Classification of Human Papillomavirus 16 (HPV16)-Related Variant Genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS One 6, e20183 (2011).
Burk, R. D., Harari, A. & Chen, Z. Human papillomavirus genome variants. Virology 445, 232–243 (2013).
Bzhalava, D. et al. Deep sequencing extends the diversity of human papillomaviruses in human skin. Sci. Rep. 4, 1–7 (2014).
Bulk, S. et al. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br. J. Cancer 94, 171–175 (2006).
Clifford, G. & Franceschi, S. Members of the human papillomavirus type 18 family (alpha-7 species) share a common association with adenocarcinoma of the cervix. Int. J. Cancer 122, 1684–1685 (2008).
Ueda, Y. et al. Dynamic changes in Japan’s prevalence of abnormal findings in cervical cervical cytology depending on birth year. Sci. Rep. 8, 5612 (2018).
Lazcano-Ponce, E. et al. Decreasing cervical cancer mortality in Mexico: Effect of papanicolaou coverage, birthrate, and the importance of diagnostic validity of cytology. Cancer Epidemiol. Biomarkers Prev. 17, 2808–2817 (2008).
Landy, R., Pesola, F., Castañón, A. & Sasieni, P. Impact of cervical screening on cervical cancer mortality: Estimation using stage-specific results from a nested case-control study. Br. J. Cancer 115, 1140–1146 (2016).
Bruni, L. et al. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta-Analysis of 1 Million Women with Normal Cytological Findings. J. Infect. Dis. 202, 1789–1799 (2010).
Lazcano-Ponce, E. et al. Specimen self-collection and HPV DNA screening in a pilot study of 100,242 women. Int. J. Cancer 135, 109–116 (2014).
Torres-Ibarra, L. et al. Triage strategies in cervical cancer detection in Mexico: methods of the FRIDA Study. Salud Publica Mex. 58, 197–210 (2016).
Programa de vacunación universal - lineamientos 2014. Available at, http://www.censia.salud.gob.mx/contenidos/descargas/vacunas/LINEPVU2014SF.pdf (2014).
Guan, P. et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 131, 2349–2359 (2012).
Pourhoseingholi, M. A., Vahedi, M. & Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. from Bed to Bench 6, 14–17 (2013).
Flores, Y. et al. Improving cervical cancer screening in Mexico: Results from the Morelos HPV Study. Salud Publica Mex. 45, Suppl 3:S388–98 (2003).
Lazcano-Ponce, E. et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer 9, 412–420 (2001).
Ritu, N. & Wilbur, D. C. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. (Springer, 2015).
Calva-Espinosa, D. Y. et al. Comparison of automated platforms for the HPV genotypification. Ginecol. Obstet. Mex. 85, 569–577 (2017).
Abreu, A. L. P., Souza, R. P., Gimenes, F. & Consolaro, M. E. L. A review of methods for detect human Papillomavirus infection. Virol. J. 9, 262 (2012).
Tjalma, W. A. A. & Depuydt, C. E. Cervical cancer screening: Which HPV test should be used - L1 or E6/E7? Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 45–46 (2013).
Stoler, M. H. et al. The Onclarity Human Papillomavirus Trial: Design, methods, and baseline results. Gynecol. Oncol. 149, 498–505 (2018).
Bouvard, V. et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 10, 321–322 (2009).
Li, N., Franceschi, S., Howell-Jones, R., Snijders, P. J. F. & Clifford, G. M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 128, 927–935 (2011).
Depuydt, C. E. et al. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J. Cell. Mol. Med. 11, 881–891 (2007).
Qu, W. et al. PCR detection of human papillomavirus: Comparison between MY09/MY11 and GP5+/GP6+ primer systems. J. Clin. Microbiol. 35, 1304–1310 (1997).
Jácome-Galarza, I. et al. Prevalence of human papillomavirus in women from the state of Michoacan, Mexico, Showed high frequency of unusual virus genotypes. Rev. Investig. Clin. 69, 262–269 (2017).
Lazenby, G. B. et al. An association between trichomonas vaginalis and high-risk human papillomavirus in rural tanzanian women undergoing cervical cancer screening. Clin. Ther. 36, 38–45 (2014).
Ghosh, I., Mandal, R., Kundu, P. & Biswas, J. Association of genital infections other than human papillomavirus with pre-invasive and invasive cervical Neoplasia. J. Clin. Diagnostic Res. 10, XE01–XE06 (2016).
Verteramo, R. et al. Human Papillomaviruses and genital co-infections in gynaecological outpatients. BMC Infect. Dis. 9, 1–7 (2009).
Guardado-Estrada, M. et al. The amerindian mtDNA haplogroup B2 enhances the risk of HPV for cervical cancer: De-regulation of mitochondrial genes may be involved. J. Hum. Genet. 57, 269–276 (2012).
Badano, I. et al. Mitochondrial DNA ancestry, HPV infection and the risk of cervical cancer in a multiethnic population of northeastern Argentina. PLoS One 13, 1–16 (2018).
Alaez-Verson, C. et al. HPV-16 and HLA-DRB1 Alleles Are Associated with Cervical Carcinoma in Mexican Mestizo Women. Arch. Med. Res. 42, 421–425 (2011).
Guardado-Estrada, M. et al. The distribution of high-risk human papillomaviruses is different in young and old patients with cervical cancer. PLoS One 9 (2014).
Aguilar-Lemarroy, A. et al. Human papillomavirus infections in Mexican Women with normal cytology, precancerous lesions, and cervical cancer: Type-specific prevalence and HPV coinfections. J. Med. Virol. 87, 871–884 (2015).
Illades-Aguiar, B. et al. Prevalence and distribution of human papillomavirus types in cervical cancer, squamous intraepithelial lesions, and with no intraepithelial lesions in women from Southern Mexico. Gynecol. Oncol. 117, 291–296 (2010).
Anaya-Ruiz, M., Vincent, A. K. & Perez-Santos, M. Cervical cancer trends in Mexico: Incidence, mortality and research output. Asian Pacific J. Cancer Prev. 15, 8689–8692 (2014).
Salcedo, M. et al. Human papillomavirus genotypes among females in Mexico: A study from the Mexican Institute for Social Security. Asian Pacific J. Cancer Prev. 15, 10061–10066 (2014).
Ciapponi, A., Bardach, A., Glujovsky, D., Gibbons, L. & Picconi, M. A. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: Systematic review and meta-analysis. PLoS One 6, 1–15 (2011).
Wright Thomas, C. et al. Clinical performance of the BD onclarity HPV assay using an adjudicated cohort of BD surepath liquid-based cytology specimens. Am. J. Clin. Pathol. 142, 43–50 (2014).
Huh, W. K. et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 390, 2143–2159 (2017).
Signorelli, C. et al. Human papillomavirus 9-valent vaccine for cancer prevention: A systematic review of the available evidence. Epidemiol. Infect. 145, 1962–1982 (2017).
Ruiz-Sternberg, Á. M. et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine in Latin American girls, boys, and young women. Papillomavirus Res. 5, 63–74 (2017).
Van Damme, P. et al. Use of the nonavalent HPV vaccine in individuals previously fully or partially vaccinated with bivalent or quadrivalent HPV vaccines. Vaccine 34, 4759–4760 (2016).
Cox, J. T. et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: Results from the ATHENA HPV study. Am. J. Obstet. Gynecol. 208, 184.e1–184.e11 (2013).
Wentzensen, N., Schiffman, M., Palmer, T. & Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 76, 49–55 (2016).
Lew, J. et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: eff ectiveness and economic assessment for the National Cervical Screening Program. 96–107, https://doi.org/10.1016/S2468-2667(17)30007-5 (2017).
Ogilvie, G. S. et al. Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months The HPV FOCAL Randomized Clinical Trial. JAMA 320, 43–52 (2018).
Ebisch, R. M. F. et al. The clinical value of HPV genotyping in triage of women with high-risk-HPV-positive self-samples. Int. J. Cancer 139, 691–699 (2016).
Beal, C. M. et al. Cost analysis of different cervical cancer screening strategies in Mexico. 56 (2014).
Jack, C. & Cosette, W. Need for expanded HPV genotyping for cervical screening. Papillomavirus Res. 2, 112–115 (2016).
Acknowledgements
We gratefully acknowledge to Iromy Meza, Jessica Avitia, and Oswaldo Carrillo for their technical support in obtaining databases during this project. Also, we want to thanks the staff of the Salud Digna clinics and the National Reference Center of Salud Digna for their support during this work. This work was funding by Salud Digna.
Author information
Authors and Affiliations
Contributions
A.C.R., M.A.L.R.E. and J.A.F. designed the study and contributed to data acquisition, evaluation, and analysis. D.Y.C.E., J.A.C.M., D.J.M.P. and J.L.M.C. managed data acquisition and analysis. K.S.A., A.L.F., M.A.A.A., F.A.M.M., and M.M.G. contributed to data analysis, discussion of the results and critically revised the manuscript. A.C.R. and J.A.F. did the literature search and drafted the manuscript, which was reviewed, modified, and approved by all coauthors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campos-Romero, A., Anderson, K.S., Longatto-Filho, A. et al. The burden of 14 hr-HPV genotypes in women attending routine cervical cancer screening in 20 states of Mexico: a cross-sectional study. Sci Rep 9, 10094 (2019). https://doi.org/10.1038/s41598-019-46543-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46543-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.