Abstract
Single nucleotide polymorphisms (SNPs) in TLR genes may serve as a crucial marker for early susceptibility of various cancers including cervical cancer. The present study was therefore designed to ascertain the role of TLR4 and TLR9 SNPs and haplotypes to hrHPV infection and cervical cancer susceptibility. The study included 110 cervical cancer biopsies and 141 cervical smears from age-matched healthy controls of Gujarati ethnicity of Western India. hrHPV 16 and 18 were detected using Real-time PCR. Eight SNPs, four each in TLR4 and TLR9 were analyzed using Polymerase Chain Reaction-Restriction Fragment Length Polymorphism and Allele-Specific PCR. HPV 16 and 18 were detected in 68% cervical cancer cases. TLR4 rs4986790, rs1927911 and TLR9 rs187084 showed association with HPV 16/18 infection. CC and CT genotypes of TLR4 rs11536889 and rs1927911 respectively, and TC, CC genotypes of TLR9 rs187084, as well as minor alleles of TLR4 rs4986790 and TLR9 rs187084, were associated with the increased risk of cervical cancer. Stage-wise analysis revealed TLR9 rs187084 and rs352140 to be associated with early-stage cancer. TLR4 haplotype GTAC and TLR9 haplotype GATC were associated with the increased risk of cervical cancer while TLR4 haplotype GCAG was associated with the decreased risk. TLR4 haplotype GCAG and TLR9 haplotype GATC showed association with increased susceptibility to hrHPV infection. In conclusion, the present study revealed association of TLR4 and TLR9 polymorphisms and haplotypes with hrHPV infection and cervical cancer risk. Further evaluation of a larger sample size covering diverse ethnic populations globally is warranted.
Similar content being viewed by others
Introduction
With respect to gender-specific cancers, cervical cancer is the next major cause of global cancer deaths among women, after the cancer of the breast, wherein India accounts for almost one-fourth of total cervical cancer-related mortalities1. Human papillomavirus (HPV) infection is considered as the most vital event in the development and progression of cervical cancer, as the HPV DNA has been detected in almost all of the cervical tumors globally2. With more than 200 HPV types known till date, fifteen (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) designated as high-risk types have been found to be associated with cervical cancer and precancerous lesions3. Of the various high-risk types, the combined prevalence of HPV 16 and 18 is estimated to be approximately 70% worldwide4. The key targets of HPV are epithelial cells of the skin and mucosae undergoing differentiation5. The integration of the high-risk HPV (hrHPV) DNA results in the constitutive expression of its oncogenes E6 and E7. Briefly, E6 oncoprotein binds to the cellular tumor suppressor protein p53 and directs its ubiquitin-mediated proteolytic degradation whereas E7 binds to and inactivates another cellular tumor suppressor protein Rb, thereby interfering the cell cycle control which leads to oncogenic growth6,7,8.
Although persistent hrHPV infection has become a well-established cause of cervical carcinogenesis, not all women infected with HPV develop cervical cancer, whereas women without HPV infection also develop cervical cancer9. This indicates the crucial role being played by variability in the host genetic factors, affecting women’s susceptibility to HPV infection and cervical cancer. One such factor is pathogen recognition receptors of the innate immune system, where Toll-like receptors (TLRs) have been identified as a key component playing a crucial role in the pathophysiology of varied human diseases, including cancer10.
TLRs are a part of innate immune system and significantly contribute in battling bacteria, viruses and other pathogens, and provide anti-tumor immunity11. TLRs serve as the initiator of inflammatory response generated by various factors including infection and tissue injury. Briefly, TLRs after binding to exogenous microbial or endogenous-tissue injury generated ligands activate transcription factors via adaptor protein myeloid differentiation factor 88 (MyD88) or MyD88 adaptor-like/Toll-interleukin 1 receptor domain–containing adaptor protein (Mal/TIRAP) leading to cytokines production and activation of adaptive immune response12.
To date, ten functional TLRs designated as TLR1 to TLR10 are expressed in humans by immune and certain non-immune cells. Of these TLRs, TLR1, 2, 4, 5, 6 and 10 are found on the cell surfaces whereas TLR3, 7, 8 and 9 are located in the endosomes or endoplasmic reticulum13. TLRs have also been implicated in the initiation, progression and metastasis of tumors14,15. Aberrant expression of different TLRs including TLR4 and TLR9 have been detected in gastric16,17, ovarian18, colorectal19, lung20, breast21, prostate22 as well as cervical cancers23. Furthermore, Hasan et al.24, reported the involvement of HPV16 E6 and E7 oncoproteins in the inhibition of TLR9 transcription, leading to decreased immune response and escape for HPV16.
Moreover, as inflammation is now considered as one of the crucial carcinogenic factors12,25, genetic variability in inflammation-associated TLR genes has revealed their potential role in influencing the susceptibility to pathogenic infections and development of cancer10. Of the various TLRs, TLR4 is known to recognise exogenous ligands such as lipopolysaccharide (LPS), fusion (F) protein of respiratory syncytial virus as well as endogenous ligand like heat shock proteins (HSP60, HSP70) and high mobility group box 1 (HMGB1)26,27,28, whereas TLR9 recognizes unmethylated CpG-rich bacterial and viral DNA29.
Reports on the influence of TLR4 and TLR9 single nucleotide polymorphisms (SNPs) in cervical cancer susceptibility are limited as well as conflicting30,31,32. In the case of TLR4 polymorphisms, Asp299Gly (rs4986790) and Thr399Ile (rs4986791) were shown to be associated with tumor progression, however, no direct association of these SNPs was found in case-control set up33,34. Among the common TLR9 polymorphisms -1486 T/C (rs187084) and C2848T (rs352140) polymorphisms were found to be the risk factors for cervical cancer35,36,37. Conversely, a study by Pandey et al.38 reported no association of TLR9 C2848T polymorphism with cervical cancer, however, the same SNP was marginally associated with advanced cancer stages. Jin et al.39 reported a significant difference in the distribution of minor alleles of TLR4 3′ UTR SNP rs7873784 C/G and TLR9 SNP G2848A in cervical cancer and HPV positive cases. However, in the same study group, the other TLR4 SNPs (rs4986791, rs11536889) were not associated with cervical cancer.
Considering the importance of chronic inflammation in carcinogenesis as well as the influence of TLR genes’ polymorphisms in inflammation and cancer susceptibility, the present study was designed to investigate the role of four TLR4 (rs4986790, rs10759931, rs11536889 and rs1927911) and equal number of TLR9 (rs187084, rs5743836, rs352140 and rs352139) SNPs in HPV infection and cervical cancer susceptibility.
Results
Clinico-demographic characteristics
Mean age of cervical cancer patients (52.4 ± 11.6 years) and controls (51.8 ± 11.8 years) was comparable without any statistically significant difference (p = 0.625). However, features such as age at marriage (p < 0.001), age at first childbirth (p < 0.001) and parity (p < 0.0001) showed statistically significant difference between the cases and controls. All the cervical cancer cases were histopathologically diagnosed as squamous cell carcinoma type. Clinical staging of cervical cancer biopsies was performed as per the FIGO guidelines that revealed 9 (8.2%), 39 (35.5%), 55 (50%) and 7 (6.3%) patients in Stage I, II, III and IV respectively. The detailed demographic and clinicopathologic features of patients are presented in Table 1.
HPV 16 and 18 prevalence
Prevalence of HPV as revealed by consensus primers in the cervical cancer cases was 81.6% (90/110), of which high-risk types 16 and 18 were detected in 64% (71/110) and 3.6% (4/110) cases. The combined frequency of HPV 16 and 18 was found to be 68% (75/110). Moreover, two out of 141 control subjects (1.4%) were also detected positive for HPV consensus sequences, of which one (0.7%) carried HPV16 DNA.
Genotype distributions
All the TLR4 and TLR9 SNPs within the control population were in agreement with the Hardy-Weinberg equilibrium except for TLR4 SNP rs11536889. However, the polymorphism was retained as its homozygous genotype GG was not detected in any of the study subjects which could be a probable reason for its deviation from the Hardy-Weinberg equilibrium.
A significant difference in the distribution of genotype frequencies between the cases and the controls were observed for TLR4 SNPs rs11536889 (p = 0.013) and rs1927911 (p = 0.04) as well as TLR9 SNPs rs187084 (p = 0.01) and rs352139 (p = 0.04). The distribution of genotypes for TLR4 and TLR9 are shown in Supplementary Table S1. Association of TLR4 and TLR9 polymorphisms with HPV 16 and 18 infections is shown in Table 2. Individuals carrying heterozygous genotype of rs4986790 [p = 0.033, age-adjusted OR = 1.693 (1.043–2.747)], rs1927911 [p = 0.032, age-adjusted OR = 1.896 (1.055–3.406)] and rs187084 [p = 0.001, age-adjusted OR = 2.915 (1.508–5.635)] showed significant association with the presence of HPV 16 and 18 infections. Analysis of alleles among HPV 16/18 infected cases compared to controls revealed association of minor allele of rs4986790 [p = 0.031, age-adjusted OR = 1.789 (1.055–3.034)], rs1927911 [p = 0.023, age-adjusted OR = 1.653 (1.072–2.549)] and rs187084 [p = 0.036, age-adjusted OR = 1.538 (1.028–2.302)] SNPs with HPV 16/18 infection. Homozygous variant genotypes of rs1156889 [p = 0.013, age-adjusted OR = 1.948 (1.149–3.305)], rs187084 [p = 0.049, age-adjusted OR = 2.040 (1.009–4.126)] and heterozygous genotypes of rs1927911 [p = 0.003, age-adjusted OR = 2.248 (1.328–3.806)], rs187084 [p = 0.0002, age-adjusted OR = 3.004 (1.668–5.413)] were found to be associated with the increased risk of developing cervical cancer. Frequencies of the minor allele of TLR4 SNPs rs4986790 [p = 0.033, age-adjusted OR = 1.693 (1.043–2.747)], rs1927911 [p = 0.013, age-adjusted OR = 1.635 (1.109–2.410)] and the major allele of TLR9 SNP rs187084 were also varied significantly between patients and controls, conferring their association with the cervical cancer risk. Genotypic and allelic association between TLR4 and TLR9 variants and cervical cancer risk is presented in Table 3. A comparative analysis between early (stage I + II) and late (stage III + IV) stages revealed heterozygous genotypes of TLR9 rs187084 [p = 0.011, age-adjusted OR = 0.283 (0.107–0.749)] and rs352140 [p = 0.015, age-adjusted OR = 0.304 (0.117–0.790)] to be associated with early stage cervical cancer. However, none of the TLR4 SNPs shown significant association with early or late stages of cancer (Table 4).
Haplotype analysis
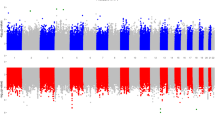
Linkage disequilibrium (LD) analysis revealed two SNPs of each TLR4 (rs10759931 aka rs11536858, rs1927911) and TLR9 (rs352139, rs187084) genes in strong LD (Fig. 1). The haplotypes were generated using the four SNPs of each TLR4 and TLR9 genes among the cases and controls (Table 5). Six common haplotype of TLR4 (frequency > 5%) and TLR9 (frequency > 2.5%) showed an accumulated frequency of 86.1% and 79.6% respectively in controls. Among HPV 16 and 18 positive patients TLR4 and TLR9 haplotypes revealed an accumulated frequency of 85.5% and 84.7% respectively. Distribution of TLR4 haplotypes differed significantly in HPV 16 and 18 infected cases (Pglobal = 0.045) as compared to control, whereas no such difference was detected while evaluating the TLR9 haplotypes (Pglobal = 0.493) (Table 6). TLR4 haplotype GCAG [p = 0.0035, OR = 0.44 (0.20–0.96)] was associated with the decreased risk whereas TLR9 haplotype GATC [p = 0.018, OR = 4.15 (1.16–14.80)] was associated with the increased risk of acquiring HPV 16 and 18 infection compared with controls.
TLR4 and TLR9 haplotype block structures and linkage disequilibrium plots generated by Haploview and Locusview. (a) TLR4 and (b) TLR9 haplotype block structures, linkage disequilibrium plot and pairwise D′ value. The level of pair-wise D′ indicates the degree of linkage disequilibrium between two SNPs.
Among cervical cancer cases, TLR4 and TLR9 haplotypes revealed an accumulated frequency of 85% and 83.1% respectively. Results of the global test score showed a significant difference in haplotype distribution between patients and controls in the case of TLR4 variants (Pglobal = 0.0033), while no significant difference was obtained for TLR9 variants (Pglobal = 0.227) (Table 5). Furthermore, the TLR4 haplotype GTAC [p = 0.047, OR = 1.77 (1.00–3.13)] and TLR9 haplotype GATC [p = 0.019, OR = 3.95 (1.15–13.50)] were found to be associated with the increased risk of cervical cancer whereas the TLR4 haplotype GCAG [p = 0.0076, OR = 0.39 (0.19–0.79)] was significantly associated with decreased risk of cervical cancer. Furthermore, within cases, haplotypes analysis did not reveal an association of either TLR4 (Pglobal = 0.733) or TLR9 (Pglobal = 0.546) haplotypes with the early or late stages of cervical cancer (Supplementary Table S2).
Discussion
The influence of TLR polymorphisms is gradually increasing in the field of biomarkers study in various diseases including cancer10. In the present study, we investigated the role of the common TLR4 and TLR9 SNPs in susceptibility to HPV infection and cervical cancer among the study subjects from Gujarat, India. Considering the influence of hrHPVs in cervical carcinogenesis, we first analyzed the prevalence of two major hrHPVs HPV 16 and 18 that revealed a frequency of 68% as compared to nearly 71% and 78% prevalence globally as well as in India respectively40. However, a previous report41, from the same geographic region as of ours found 60% of the patients to be infected with HPV 16 and 18. The difference in the percentage of hrHPV detection, though not very high, can be attributed to the variation in the sample size as the number of patients in the present study were more than double as reported by Patel et al.41. A higher prevalence of approximately 21% HPV infection other than HPV 16 and 18 in our study subjects highlights the necessity of genotyping other hrHPVs to identify additional prevailing HPVs.
We further analyzed polymorphisms present in UTRs, exons, and introns of TLR4 and TLR9 genes. The variations in UTRs are known to influence ribosome recognition, termination and post-transcriptional modification which may alter the expression and functionality of a particular protein42. We found a mixed association of different 3′ UTR and 5′ UTR SNPs of TLR4 and TLR9 genes in our study subjects, suggesting a probable role of these SNPs in disease susceptibility.
TLR9 promoter SNP rs187084 (-1486T/C) conferred a increased risk to HPV 16 and 18 infection and cervical cancer. A similar association of TLR9 rs187084 polymorphism with an increased risk of cervical cancer was reported among Polish and Chinese women35,36. Our results on TLR9 rs187084 polymorphism are in good agreement with the recent meta-analyses30,31 that supported a significant role of rs187084 in cervical cancer risk. Within cases, TLR9 rs187084 showed over presentation in early-stage cancer compared with late stages. Interestingly, we did not find an association of another TLR9 promoter SNP rs5743836 (−1237T/C) with HPV infection and/ or cervical cancer risk. Our result supports the observation of Oliveira et al.43 who reported no association of TLR9 promoter SNP rs5743836 with HPV infection or clearance in healthy Brazilian women. Even though no direct role of TLR9 promoter SNPs has been reported in cervical cancer, the T allele of TLR9 promoter SNP rs187084 (−1486T/C) together with G allele of intronic rs352139 A/G SNP have been suggested to down regulate TLR9 expression in systemic lupus erythematosus44. The T allele of rs5743836 (−1237T/C) has been suggested to be associated with high basal promoter activity45 and C allele with higher affinity to NF-κB binding, causing increased production of proinflammatory cytokines46.
With regard to TLR4 promoter SNP rs10759931, no association was observed either with HPV infection or cervical cancer risk. However, the same SNP has been reported to be associated with prostate and gastric cancers risk47,48. The homozygous AA genotype of TLR4 rs10759931 has been reported to be associated with high TLR4 expression in symptomatic atherosclerotic patients compared to non-symptomatic and healthy individuals carrying GG or GA genotypes49. They found that the two alleles of rs10759931 differ in their binding affinity to GATA-2 transcriptional factor. Furthermore, we observed the 3′ UTR heterozygous genotype GC of TLR4 rs11536889 to be associated with increased risk of cervical cancer in our study subjects. A similar observation was found in bladder cancer50, however, the association status of this SNP with other cancers was inconsistent32. Moreover, the G allele of TLR4 rs11536889 3′ UTR SNP has been suggested to play a key role in inhibiting TLR4 translation in monocytes51. However, expression analysis of TLR4 and TLR9 genes may provide more insights into the functional role of these UTR SNPs in cervical cancer risk.
Additionally, we analyzed a synonymous and a non-synonymous SNP of TLR9 and TLR4 genes respectively. Even though a synonymous change does not alter incorporation of amino acid, it has been observed that such SNPs can alter mRNA splicing, stability, and structure as well as protein folding thereby affecting the function of the subsequent protein52. We did not find a significant effect of TLR9 synonymous SNP rs352140 (G2848A; Pro545Pro) with cervical cancer risk which is in good agreement with a recent meta-analysis by Tian et al.30. An association of G2848A SNP with early stages of cervical cancer was detected in our study subjects which is in contrast to the report of Pandey et al.38 who observed an association of the same SNP with the late stage cervical cancer in North Indian women. However, Roszak et al.35 reported an association of C2848T SNP along with –1486T/C SNP with cervical cancer risk in the Polish population. Similarly, the Han Chinese women carrying TLR9 rs352140 (G2848A) GA/AA genotype along with HPV16 infection showed an increased risk of cervical cancer compared to women with GG genotype35,53.
With regard to non-synonymous SNP rs4986790 (A896G; Asp299Gly) of TLR4, intriguingly, we found the heterozygous AG genotype (Asp/Gly) to be strongly linked to HPV 16/18 infection suggesting a queering effect of the amino acid change as no interaction of HPV capsid proteins with TLR4 is known yet. The amino acid change is reported to affect van der Waals interaction and hydrogen bonding in the leucine-rich repeats of TLR4, thereby modulating its surface properties that may affect the binding of TLR4 ligand such as LPS54. Although HPV is not a known TLR4 ligand, our paradoxical observation warrants a meticulous investigation. Furthermore, we observed a significant association of minor allele G (Gly) of Asp299Gly polymorphism with cervical cancer risk, however, no genotypic association was found. Similarly, in North Indian women, no association of TLR4 Asp299Gly polymorphism, in addition to another common TLR4 Thr399Ile polymorphism with cervical cancer risk was observed by Pandey et al.33. Moreover, Asp299Gly polymorphism has been found to be contradictorily associated with different cancer types including cervical cancer32.
A growing body of evidence suggests a potential role of intronic SNPs located either in exon/ intron boundaries, intron splice enhancer, branchpoint site or outside the exon-intron splice junctions in regulating gene expression55. It has also been observed that intronic SNPs in one gene can affect the expression of a far located gene55. Congruously, we observed a significant difference in the distribution of genotypes of TLR9 intronic rs352139 A/G SNP between cases and controls, however, none of its genotypes or allele was associated with cervical cancer risk. On the other hand, the heterozygous genotype of TLR4 intronic rs1927911 SNP was significantly associated with cervical cancer risk which is in agreement with the observation of Song et al.47 in prostate cancer. However, in hepatocellular carcinoma, the same SNP showed a protective effect56.
As haplotypes are considered more informative than SNPs57, we generated haplotypes from different combinations of TLR4 and TLR9 SNPs. The TLR4 haplotype GTAC was linked with a significant increase in cervical cancer risk in addition to the TLR9 haplotype GATC that also showed association with increased HPV 16 and 18 infections. Intriguingly, another TLR4 haplotype GCAG showed a significant association with decreased cervical cancer risk as well as acquiring the hrHPV infection, suggesting its protective role. Moreover, to understand the influence of TLR4 and TLR9 haplotypes on tumor progression, we correlated the haplotypes with early (I and II) and late (III and IV) tumor stages. However, none of the haplotypes showed association with clinical aggressiveness. Since these haplotypes included both risk as well as protective alleles, a crucial role of TLR4 and TLR9 polymorphisms may be envisaged towards HPV infection and cervical cancer susceptibility.
To identify the strong coinheritance of the SNPs we calculated linkage disequilibrium of TLR4 and TLR9 SNPs, wherein TLR4 rs10759931 and rs1927911, and TLR9 rs187084 and rs352139 were in strong LD, evincing strong influence of these inherited variations in cervical cancer. Intriguingly, we observed that in both the genes strong LD was detected between SNPs of 5′ UTR and the first intron only. Conceptually there should be a decrease in linkage disequilibrium with a decrease in distance between two loci. However, our study revealed SNP pairs in both TLR4 and TLR9 genes that did not follow the standard notion. For example, in TLR4, SNP pair rs10759931:rs4986790 with a distance of 11.1 Kb showed strong LD (D′ = 0.54) as compared to another SNP pair rs4986790:rs11536889 that had a shorter distance of 2.8Kb (D′ = 0.12). Similarly, TLR9 SNP pair rs352140:rs187084 (distance = 4.3 kb) was in strong LD (D′ = 0.5) compared to SNP pair rs5743836:rs187084 (D′ = 0.04) with shorter distance of 0.24 kb among them. Our LD analysis is in agreement with the observations of Stephens et al.57 who suggested that distance between the SNPs does not have a significant impact on the level of LD. Various SNP pairs of TLR4 and TLR9 genes, their genetic distance and D′ values are shown in Supplementary Table S3.
Although our results suggest a significant role of TLR4 and TLR9 polymorphisms in cervical cancer, the study has some vital limitations too. Firstly, the selection bias cannot be excluded as it was a hospital-based case-control study, Moreover, the size of the study population needed augmentation to increase the statistical power, which is one of the major limiting factors among the numerous cancer case-controls studies worldwide. Additionally, in vivo expression analysis would have reflected the effect of SNPs on the expression pattern of TLR4 and TLR9.
To our knowledge, this is the first comprehensive analysis of TLR4 and TLR9 SNPs and haplotypes to understand their role in cervical cancer. Our results suggest moderate to strong impact of TLR4 and TLR9 polymorphisms in susceptibility to hrHPV infection and cervical cancer. Additional research on large and varied ethnic populations is warranted to precisely understand the impact of both the genes in HPV infection and cervical cancer risk.
Methods
Study subjects
The study comprised of 110 untreated cervical cancer patients and 141 healthy controls recruited from 2012 to 2017; from Shree Krishna Hospital, Karamsad, Anand; and Sir Sayajirao General Hospital and Medical College, Vadodara, India. The sample types included primary histopathologically diagnosed cervical cancer biopsies and cytologically confirmed normal cervical smears from healthy controls. The clinical staging of cervical cancer samples was done as per The International Federation of Gynecology and Obstetrics (FIGO) recommendations. The study subjects belonging to Gujarati ethnicity were comparable in age and non-relatives of each other. The patients manifesting multiple cancers and those who underwent radiation or chemotherapy were excluded from the study. The inclusion criteria of healthy controls included the absence of cancer history in family and cervix related disorders such as cervicitis, warts, pre-cancerous and cancerous lesions. Additionally, sample collection was avoided from the women undergoing menstruation. All experiments were performed in accordance with the relevant guidelines and regulations. The study was approved by the Institutional Review Board, Ashok and Rita Patel Institute of Physiotherapy, CHARUSAT, Changa, Anand; Institutional Ethics Committee, HP Patel Centre for Medical Care and Education, Karamsad and Institutional Ethics Committee for Human Research (IECHR) Medical College and SSG Hospital, Vadodara, India. Written informed consent was obtained from all the study subjects.
DNA extraction
The samples were collected in chilled phosphate buffered saline and were either processed immediately or stored at −20 °C till further processing. DNA was isolated using standard Proteinase-K phenol-chloroform extraction method. In the case of a low number of cervical cells, spin-column based DNA isolation kit (NucleoSpin Tissue, Macherey-Nagel, Germany) was utilized. The quality and quantity of extracted DNA were determined using ethidium bromide-stained 1% agarose gel on a GelDoc system (BioRad, USA) and NanoDrop 2000 (Thermofisher, USA).
HPV detection
HPV detection was first carried out using consensus Gp5+/Gp6+ primers followed by type-specific primers for the detection of hrHPV 16 and 18, on a Real-time PCR platform (7500 Real-Time PCR System, Applied Biosystems, USA) using SYBR Premix Ex Taq II (Tli RNaseH Plus) kit (Takara, Japan). Typically, a 20 μl real-time PCR mix comprised of 1X SYBR Premix Ex Taq (Tli RNAse H Plus), 0.2 µM of each forward primer and reverse primer, 1X ROX reference Dye II and 25 ng of template DNA. The positive controls for HPV 16 and 18 were obtained as a part of participation in the Global HPV Proficiency Study, Equalis, Uppsala, Sweden. β-globin gene served as an internal control while in the negative control DNA was replaced with PCR grade nuclease-free water. All the reactions were performed in duplicates. Touchdown thermal profile for HPV detection by consensus primers and thermal cycling conditions for HPV 16 and 18 detections along with the details of primer sequence and amplicon size is mentioned in Supplementary Table S4.
Genotype analyses
A total of eight SNPs, four each of TLR4 (rs4986790, rs10759931, rs11536889, rs1927911) and TLR9 (rs187084, rs5743836, rs352140, rs352139) genes were analyzed either using Polymerase Chain Reaction and Restriction Fragment Length Polymorphism (PCR-RFLP) or Allele-Specific PCR (AS-PCR). The selection of SNPs was carried out using SNP database of NCBI (https://www.ncbi.nlm.nih.gov/snp/). The SNPs were selected on the basis of (1) Genetic region: In this criteria the SNPs were selected to cover different regions of gene, for example, exon, intron and UTRs, (2) Global minor allele frequency: The SNPs with minor allele frequency > 5% were evaluated for association analysis (3) Frequent association of SNPs with different inflammation associated cancers: To fulfil the above criteria literature survey was conducted using PubMed and random web search. The characteristics of TLR4 and TLR9 SNPs included in this study are shown in Supplementary Table S5. Sequences of primers specific for each SNP, amplicon size and thermal profile is mentioned in Supplementary Table S6. A typical PCR of 25 µl contained 50 to 100 ng genomic DNA, 0.1 mM dNTP mix, 0.1 µM of each oligonucleotide primer and 0.8U Taq DNA polymerase (Kapabiosystems, USA). All the reactions were performed on an MJ Mini Thermal Cycler (BioRad, USA). Except for TLR9 rs352139 polymorphism that was genotyped using AS-PCR, the rest of the SNPs were subjected to restriction digestion using 5U of respective restriction enzymes procured from New England Biolabs, USA. For the identification of SNPs by RFLP, the associated restriction enzymes, incubation temperature and time, digested products, genotypes and mode of visualization is detailed in Supplementary Table S7. The amplified, as well as restriction digested products, were visualized on a GelDoc system (BioRad, USA).
Statistical analysis
Alterations in demographic features among cases and controls were compared using student t-test and chi-square test for continuous and categorical variables respectively. Age of study subjects was expressed as mean ± standard deviation. Deviation from Hardy–Weinberg equilibrium was determined by the χ2 goodness-of-fit test. Pearson’s χ2 test was used to evaluate the difference of the SNP distribution among cases and controls. Genotypic and allelic association of SNPs with the disease were estimated using χ2 and Fisher’s exact test. Unconditional logistic regression analysis was performed to compute age-adjusted odds ratio (OR). All the statistical analysis was performed on the Statistical Package for Social Sciences version 24.0 (SPSS, USA). Tests of statistical significance were two-sided and taken as significant when the p-value was less than 0.05. Haplotype block structure and linkage disequilibrium (LD) structure were determined by Haploview (v4.2) and Locusview (v2.0). The D′ values were computed using the default algorithm created by Gabriel et al.58 at 95% confidence interval. Haplotypes were estimated using an accelerated EM algorithm similar to the partition/ ligation method as described by Qin et al.59. Sum of the fractional likelihoods of each individual for each haplotype was used to obtain a count for case-control association tests. Global score test was performed using FAMHAP software v19 to evaluate the differences in haplotype frequency distribution among cases and controls. Association of the individual haplotype with cervical cancer as well as HPV infection was measured by the χ2 test.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Change history
04 December 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Walboomers, J. M. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
Burd, E. M. Human Papillomavirus and Cervical Cancer. 16, 1–17 (2003).
Muñoz, N. et al. Incidence, Duration, and Determinants of Cervical Human Papillomavirus Infection in a Cohort of Colombian Women with Normal Cytological Results. J. Infect. Dis. 190, 2077–2087 (2004).
Gómez, D. T. & Santos, J. L. Human Papillomavirus Infection and Cervical Cancer: Pathogenesis and Epidemiology. Communicating current research and educational topics and trends in applied microbiology 1, 680–688 (2007).
Werness, B. A., Levine, A. J. & Howley, P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 248, 76–79 (1990).
Dyson, N. et al. The Human Papilloma Virus-16E7 Oncoprotein is Able to Bind to the Retinoblastoma Gene Product. Science. 243, 934–936 (1989).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Rodríguez-Carunchio, L. et al. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG An Int. J. Obstet. Gynaecol. 122, 119–127 (2015).
Misch, E. A. & Hawn, T. R. Toll-like receptor polymorphisms and susceptibility to human disease. Clin. Sci. 114, 347–360 (2008).
Yang, X., Cheng, Y. & Li, C. The role of TLRs in cervical cancer with HPV infection: a review. Nat. Publ. Gr. 2, 17055 (2017).
Ioannou, S. & Voulgarelis, M. Toll-Like Receptors, Tissue Injury, and Tumourigenesis. Mediat. Inflamm. 2010: 581837 (2010).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Sato, Y., Goto, Y., Narita, N. & Hoon, D. S. B. Cancer cells expressing toll-like receptors and the tumor microenvironment. Cancer Microenviron. 2, 205-214 (2009).
Oblak, A. & Jerala, R. Toll-like receptor 4 activation in cancer progression and therapy. Clin. Dev. Immunol. 2011:609579 (2011).
Fukata, M. & Abreu, M. T. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 27, 234 (2008).
Pimentel-Nunes, P. et al. Increased expression of toll-like receptors (TLR) 2, 4 and 5 in gastric dysplasia. Pathol. Oncol. Res. 17, 677–83 (2011).
Woods, D. C., White, Y. A., Dau, C. & Johnson, A. L. TLR4 activates NF-κB in human ovarian granulosa tumor cells. Biochem. Biophys. Res. Commun. 409, 675–80 (2011).
Eiró, N. et al. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J. Clin. Immunol. 32, 848–854 (2012).
Samara, K. D. et al. Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int. J. Oncol. 40, 1397–1404 (2012).
Cai, Z. et al. Activation of Toll-Like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 71, 2466–2475 (2011).
González-Reyes, S. et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol. Immunother. 60, 217–226 (2011).
De Matos, L. G. G. et al. Associaton between Toll-like receptor and tumor necrosis factor immunological pathways in uterine cervical neoplasms. Tumori 103, 81–86 (2017).
Hasan, U. A. et al. TLR9 Expression and Function Is Abolished by the Cervical Cancer-Associated Human Papillomavirus Type 16. J. Immunol. 178, 3186–3197 (2007).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436 (2008).
Yu, L., Wang, L. & Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 14, 2592–2603 (2010).
Su, B., Ceponis, P. J. M., Lebel, S., Huynh, H. & Sherman, P. M. Helicobacter pylori Activates Toll-Like Receptor 4 Expression in Gastrointestinal Epithelial Cells. Infect Immun 71, 3496–3502 (2003).
Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. Cutting Edge: Heat Shock Protein 60 Is a Putative Endogenous Ligand of the Toll-Like Receptor-4 Complex. J. Immunol. 164, 558–561 (2000).
Medzhitov, R. & Janeway, C. The Toll receptor family and microbial recognition. Trends Microbiol. 8, 452–456 (2000).
Tian, S. et al. The Associations between Toll-Like Receptor 9 Gene Polymorphisms and Cervical Cancer Susceptibility. 2018, 9127146 (2018).
Yang, S., Liu, L., Xu, D. & Li, X. The Relationship of the TLR9 and TLR2 Genetic Polymorphisms with Cervical Cancer Risk: a Meta-Analysis of Case-Control Studies. Pathol. Oncol. Res. https://doi.org/10.1007/s12253-018-0465-x (2018).
Pandey, N., Chauhan, A. & Jain, N. TLR4 Polymorphisms and Expression in Solid Cancers. Mol. Diagn. Ther. 22, 683–702 (2018).
Pandey, S. et al. Gynecologic Oncology Impact of Toll-like receptors [TLR] 2 (−196 to −174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecol. Oncol. 114, 501–505 (2009).
Zidi, S., Sghaier, I., Gazouani, E., Mezlini, A. & Yacoubi-Loueslati, B. Evaluation of Toll-Like Receptors 2/3/4/9 Gene Polymorphisms in Cervical Cancer Evolution. Pathol. Oncol. Res. 22, 323–330 (2016).
Roszak, A., Lianeri, M. & Sowin, A. Involvement of toll-like receptor 9 polymorphism in cervical cancer development. Mol Biol Rep 39, 8425–8430 (2012).
Chen, X. et al. A Genetic Variant in the Promoter Region of Toll-Like Receptor 9 and Cervical Cancer Susceptibility. DNA Cell Biol. 31, 766–771 (2012).
Martínez, C., Margarita, C., Román, B. & Torres, K. TLR9 gene polymorphism −1486T/C (rs187084) is associated with uterine cervical neoplasm in Mexican female population. J Cancer Res Clin Oncol 143, 2437–2445 (2017).
Pandey, S., Mittal, B. & Srivastava, M. Evaluation of Toll-like receptors 3 (c. 1377C/T) and 9 (G2848A) gene polymorphisms in cervical cancer susceptibility. Mol Biol Rep 38, 4715–4721 (2011).
Jin, Y., Qiu, S., Shao, N. & Zheng, J. Association of toll-like receptor gene polymorphisms and its interaction with HPV infection in determining the susceptibility of cervical cancer in Chinese Han population. Mamm. Genome 28, 213–219 (2017).
Martel, C. D., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 141, 664–670 (2017).
Patel, K. R. et al. Prevalence of high-risk human papillomavirus type 16 and 18 in oral and cervical cancers in population from Gujarat, West India. J Oral Pathol Med 43, 293–297 (2014).
Barrett, L. W., Fletcher, S. & Wilton, S. D. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell. Mol. Life Sci. 69, 3613–3634 (2012).
Oliveira, L. B., Louvanto, K., Ramanakumar, A. V., Franco, E. L. & Villa, L. L. Polymorphism in the promoter region of the Toll-like receptor 9 gene and cervical human papillomavirus infection. J Gen Virol 94, 1858–1864 (2013).
Kayoko, T. et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis 66, 905–909 (2007).
Novak, N. et al. Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy 62, 766–772 (2007).
Ng, M. T. et al. Increase in NF-kappaB binding affinity of the variant C allele of the toll-like receptor 9 -1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect Immun 78, 1345–1352 (2010).
Song, J. et al. The association between Toll-like receptor 4 (TLR4) polymorphisms and the risk of prostate cancer in Korean men. Cancer Genet. Cytogenet. 190, 88–92 (2009).
Castaño-Rodríguez, N. et al. Genetic polymorphisms in the Toll-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer. Hum. Immunol. 75, 808–815 (2014).
Ferronato, S. et al. Polymorphism À 2604G 4 A variants in TLR4 promoter are associated with different gene expression level in peripheral blood of atherosclerotic patients. J Hum Genet 58, 812–814 (2013).
Shen, Y., Liu, Y., Liu, S. & Zhang, A. Toll-like receptor 4 gene polymorphisms and susceptibility to bladder cancer. Pathol. Oncol. Res. 19, 275–80 (2013).
Sato, K. et al. A Single Nucleotide Polymorphism in 3 -Untranslated Region Contributes to the Regulation of Toll-like Receptor 4. J Biol Chem 287, 25163–25172 (2012).
Hunt, R., Sauna, Z. E., Ambudkar, S. V. & Gottesman, M. M. K.-S. C. Silent (synonymous) SNPs: should we care about them? Methods Mol Biol. 578, 23–39 (2009).
Lai, Z. Z., Ni-Zhang Pan, X. L. & Song, L. Toll-like receptor 9 (TLR9) gene polymorphisms associated with increased susceptibility of human papillomavirus-16 infection in patients with cervical cancer. J Int Med Res. 41, 1027–36 (2013).
Ohto, U., Yamakawa, N., Akashi-takamura, S., Miyake, K. & Shimizu, T. Structural analyses of human Toll-like receptor 4 polymorphisms D299G and T399I. J Biol Chem 287, 40611–40617 (2012).
Vaz, R., Noélia, D., Maria, C. & Fonseca, C. Deep intronic mutations and human disease. Hum. Genet. 136, 1093–1111 (2017).
Minmin, S. et al. Single nucleotide polymorphisms of toll-like receptor 4 decrease the risk of development of hepatocellular carcinoma. PLoS One 6, 2–8 (2011).
Stephens, J. C. et al. Haplotype Variation and Linkage Disequilibrium in 313 Human Genes. Science. 293, 489–493 (2001).
Gabriel, S. B. et al. The Structure of Haplotype Blocks in the Human Genome. Science. 296, 2225–2229 (2002).
Qin, Z. S., Niu, T. & Liu, J. S. Partition-Ligation–Expectation-Maximization Algorithm for Haplotype Inference with Single-Nucleotide Polymorphisms. Am. J. Hum. Genet 71, 1242–1247 (2002).
Acknowledgements
This work was financially supported by Charotar University of Science and Technology (CHARUSAT). Authors thank Dr. R.J. Verma, Dr. M.V. Rao and Dr. D.D. Jhala, Department of Zoology, Gujarat University, Ahmedabad for extending the Real-Time PCR facility, and Dr Anjana Chauhan, Gynec Cancer Surgeon, Ex-Associate Professor, Gujarat Cancer and Research Institute, Ahmedabad, India for discussions and suggestions. N.J. dedicates the article to his mentors Prof. Bhudev C. Das, Dean, Health and Allied Sciences, Amity University, Noida and Prof. Syed Akhtar Husain, Department of Biosciences, Jamia Millia Islamia, New Delhi, India.
Author information
Authors and Affiliations
Contributions
All the authors have contributed significantly, read the manuscript, and agrees to its submission to Gynecologic Oncology. In this study N.P. and A.C. have performed experimental work, analysis and interpretation of data, preparation and drafting of manuscript. N.R., P.P. and A.D. have supervised the study as clinical investigators and critically reviewed the study proposal. R.K., Y.C. and R.S.K. have contributed in sample/data collection and analysis, and reviewed the manuscript critically. The task of conceptualization, funding acquisition, project administration, supervision, validation, review and editing was performed by N.J.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandey, N.O., Chauhan, A.V., Raithatha, N.S. et al. Association of TLR4 and TLR9 polymorphisms and haplotypes with cervical cancer susceptibility. Sci Rep 9, 9729 (2019). https://doi.org/10.1038/s41598-019-46077-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46077-z
This article is cited by
-
Role of toll-like receptor in the pathogenesis of oral cancer
Cell Biochemistry and Biophysics (2024)
-
Polymorphisms in GSTT1 and GSTM1 genes as possible risk factors for susceptibility to breast cancer development and their influence in chemotherapy response: a systematic review
Molecular Biology Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.