Abstract
Contemporary coexistence theory provides a framework for predicting invasiveness and impact of Invasive Non-Native Species (INNS) by incorporating differences in niche and fitness between INNS and co-occurring native species. The widespread invasive kelp Undaria pinnatifida is considered a high-risk INNS, although a robust evidence base regarding its invasiveness and impact is lacking in many regions. Invaded macroalgal canopies at nine coastal sites in the southwest UK were studied over three years to discern whether Undaria is coexisting or competing with native canopy-forming species across different habitat types. Spatial, temporal and depth-related trends in species distributions and abundance were recorded within intertidal and subtidal rocky reef as well as on marina pontoons. A primary succession experiment also examined competitive interactions between species. In rocky reef habitats, Undaria had lower fitness compared to long-lived native perennials, but was able to coexist due to niche dissimilarity between species. In contrast, Undaria was likely to be competing with short-lived native annuals on rocky reef due to large niche overlap and similar fitness. In marina habitats, Undaria dominated over all other canopy formers due to low niche diversification and higher fitness. Generalisations on INNS impact cannot be made across habitats or species, without considering many abiotic factors and biotic interactions.
Similar content being viewed by others
Introduction
The earliest works on invasion ecology predicted that the most successful invasive non-native species (INNS) would be taxonomically or functionally distinct from the recipient communities to which they are introduced1,2. This links to classic niche theory2,3,4, whereby a newly arriving species is predicted to be more successful if it occupies a vacant or under-used niche5. More recent theories in invasion ecology, based on factors such as resource utilisation and species diversity, are fundamentally linked to this ‘niche-space’ concept5,6,7,8. Niche theory does not, however, take into account that in order for an INNS to become successfully established within a recipient community, some overlap of potential niches will inevitably be present in terms of habitat, climate and other abiotic factors where native and non-native species co-occur9.
The invasiveness of a species (i.e. its potential to rapidly colonize large and/or multiple areas) is not necessarily linked to its ecological impact10. This is supported by niche theory, as competition with native species (and therefore potential for impact) is more likely to occur between functionally similar species or those occupying a similar niche, whereas niche dissimilarity would promote invasiveness but not necessarily competition11,12. Contemporary coexistence theory provides a framework for explaining both the invasiveness and impact of INNS at a given site by considering both niche and fitness differences between the invader and co-occurring native species13,14,15. Differences in potential niche promote coexistence, and can be quantified by the degree to which population growth is limited by intraspecific, rather than interspecific, competition. Fitness differences drive competition and are based on disparate traits between species which allow the population of one species to expand to the detriment of, or at a faster rate than, a co-occurring species, potentially leading to displacement14. It is the balance between these two continuous variables which determines to what extent an INNS coexists with native species, is excluded by native communities, or becomes dominant13.
A coexistence invasion framework can be most easily conceptualised spatially across a stable environment. Following an introduction event, an INNS will spread into a given number of microhabitats and proliferate at varying densities based on its potential niche, the niche diversity across a site and fitness differences between co-occurring species; thus eventually leading to a stable coexisting or dominant climax community14. Often called ‘the storage effect’, coexistence can also be mediated by temporal variation, whereby changes in the abiotic or biotic environment lead to fluctuating fitness differences between species and therefore coexistence16. INNS are often considered ‘passengers of environmental change’, requiring disturbance or degraded ecosystems to establish and spread17. This concept is derivative of the storage effect whereby certain INNS require fluctuations in the abiotic environment in order to coexist or proliferate within native communities18.
The application of coexistence theory has largely been constrained to terrestrial plant invasions15, rarely being considered within marine ecosystems19,20. The widespread invasive kelp Undaria pinnatifida (hereafter referred to as Undaria), native to the northwest Pacific, is now found in parts of the northeast and southwest Atlantic, southwest and east Pacific, and the Tasman Sea21. In many parts of its non-native range, Undaria coexists with native canopy-forming macroalgae on natural substrates (i.e. shallow rocky reefs). However, it is generally recorded at highest abundance where the cover or density of native canopy-forming macroalgae are reduced or absent, often due to abiotic factors such as depth or tidal height22,23, wave exposure24, reduced salinity25 or substrate type, aspect, and stability23,24,26,27,28.
Such niche dissimilarity has been supported by some manipulative experiments where the removal of native canopies did not lead to Undaria recruitment29 or, conversely, when the addition or removal of Undaria had no effect on its native counterparts30,31,32,33. In the majority of cases, however, removal or disturbance of native canopies promotes the recruitment and growth of Undaria31,34,35,36,37. Such promotion suggests that Undaria occupies an overlapping niche with native canopy-formers, but has lower fitness. This theory is further supported by post-disturbance recovery patterns, where declines in Undaria and increases in native species tend to occur over time35,36,37.
There are some site specific-cases, however, where these generalisations do not apply. Where native canopy diversity is naturally low, Undaria may have impacts upon native macroalgal communities or inhibit their recovery on natural rocky substrates e.g. low diversity sites in Argentina38,39. Furthermore, although not conclusive, Undaria may have higher fitness than native canopy-formers on artificial substrates (i.e. man-made structures such as marina pontoons, pilings and port walls), where it can proliferate with or without disturbance to native macroalgae and become the dominant canopy-former24,26,28,40,41,42,43. A better understanding of the drivers of Undaria invasions is needed to predict the potential for ecological impact on native communities and the need for targeted management44.
We monitored and manipulated invaded macroalgal canopies over three years to examine the extent to which Undaria coexists or competes with native canopy-forming species. Spatial (across both sites and depths) and temporal patterns in species distributions, density and biomass were recorded at multiple sites in Plymouth Sound (UK) representing three distinct habitat types: intertidal rocky reef, shallow subtidal rocky reefs and marina pontoons (Fig. 1). Furthermore, primary succession patterns were examined at both a marina and intertidal reef site to better elucidate competitive interactions between species. Our hypothesis was that spatial variation both across and between complex marine habitats will drive niche and fitness differences and determine distribution-abundance patterns of the invasive kelp and co-occurring native canopy forming macroalgae.
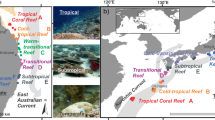
Study sites in Plymouth Sound (location of Plymouth within the UK shown as red point on inset map). Nine monitoring study sites, three each of intertidal rocky reef (green points) subtidal rocky reef (blue points) and marinas (orange points), were selected in March-April 2016. Depth surveys (brown points) were completed in July 2017 at reef monitoring sites and two additional sites (Mount Batten and Jennycliff).
Results
Multi-year monitoring
Considering all three sampling years together, intertidal macroalgal canopies were dominated by Undaria in June, with it contributing on average the highest density and biomass of any species (Fig. 2). There was also a relatively high biomass of H. elongata and L. digitata, and a high density of S. latissima (Fig. 2). The subtidal rocky reef had the most mixed canopy in June, with L. ochroleuca being dominant by biomass but with Undaria, S. latissima and S. polyschides being present in similar densities and only moderately lower in biomass (Fig. 2). There was also a small amount of the non-native S. muticum found at two of the three subtidal sites (Figs 2, S1). Marinas were dominated by Undaria in terms of both biomass and density (Fig. 2), which was interspersed with the native canopy-forming macroalgae S. latissima and S. polyschides, and small amounts of the non-native S. muticum (Fig. 2). There was very low variation in the density and biomass of species between years across the three June sampling events (Fig. 2), although within-year seasonal variability was pronounced (Fig. S1). Across all habitats Undaria and S. polyschides had a clear annual cycle with peak abundance predominantly occurring in June and September, respectively; albeit with some variation between sites. The perennial species S. latissima exhibited a similar annual pattern but with higher variation both between sites and habitats (Fig. S1), whereas Laminaria species on rocky reefs had low variability both seasonally and between sites (Fig. S1). Mean β-diversity (between quadrats) was highest in subtidal habitats and lowest in marinas (Fig. 3). The overall composition of macroalgal canopies did not vary markedly across the three years surveyed within any habitat type (Fig. 3). However, significant between-year variability in assemblage structure (based on count data only) was recorded within intertidal reef habitat, with 2016 being dissimilar to other survey years in 2017 and 2018 (Fig. 3, Tables S1, S2). This was due to lower contributions of Undaria and S. latissima to total density, and higher contributions of L. digitata, H. elongata and S. polyschides (Table S3).
Mean density (A–C) and biomass (D–F) (±standard error between years) of canopy forming macroalgae averaging across the three sites and sampling events in June 2016, 2017 and 2018 for each habitat separately (A,D = intertidal reef; B, E = subtidal reef; C, F = marina pontoons). UP = U. pinnatifida, SL = S. latissima, SP = S. polyschides, LD = L. digitata, HE = H. elongata, LO = L. ochroleuca, SM = S. muticum. Macroalgae drawing courtesy of Graham Epstein and Jack Sewell.
Threshold metric multi-dimensional scaling (tmMDS) plots of bootstrapped average monitoring data within each year of study (blue triangle = 2016, red triangle = 2017, green square = 2018). Biomass (A–C; g 0.25 m−2) and density (D–F; inds. 0.25 m−2) data for each habitat assessed separately (A,D = intertidal reef; B, E = subtidal reef; C, F = marina pontoons). Circular areas indicate the 95% confidence region around the bootstrap average. Asterisks indicate significant difference between years based on PERMANOVA (Table S1). β = proxy of mean beta-diversity for each habitat (±SE) measured by multivariate dispersion.
There were significant spatial trends between Undaria and co-occurring species in rocky reef habitats. Undaria density and biomass had a significant negative correlation with the Laminaria species in both intertidal and subtidal habitats (Table 1). Undaria biomass was also negatively related to S. latissima in both habitats, although this was only statistically significant for subtidal biomass (Table 1). For all other species in reef habitats the relationships were less well defined. In the subtidal reef habitat there was a positive relationship between the density of Undaria and S. muticum, on intertidal reef H. elongata was negatively related to both Undaria density and biomass, and S. polyschides was positively related to Undaria density and biomass in both habitats; however, only Undaria and S. muticum on subtidal reef was statistically significant (Table 1). In marina habitats the density and biomass of Undaria was not significantly related to any other species, although the positive relationship in biomass between Undaria and S. polyschides was near-significant (Table 1).
Depth-related trends
On rocky reef Undaria was found at depths ranging from + 1 to −4 m CD, with highest density and percent cover in the low intertidal to shallow subtidal zone, and a peak at + 0.5 m CD (Fig. 4). Above + 1 m CD canopy-forming macroalgal assemblages were composed of F. serratus and L. digitata only, with Undaria completely absent (Fig. 4). Within its depth range Undaria co-occurred with seven species of canopy-forming brown macroalgae: L. digitata, H. elongata, S. muticum, S. polyschides, S. latissima, L. ochroleuca, L. hyperborea. The peak in Undaria density/cover at + 0.5 m CD coincided with the lowest density and percentage cover of Laminaria species at any depth (Fig. 4). A weak positive correlation was recorded between S. latissima and Undaria across depth; however, this was only significant for count data (Fig. 4). Undaria had a strong and significant positive correlation with S. polyschides with similar distribution patterns across depth, but a strong negative correlation with L. ochroleuca (Fig. 4). There was also a negative correlation between Undaria and L. digitata, although this was not statistically significant (Fig. 4). When considering the sum of all Laminaria species together, Undaria again exhibited a significant negative correlation across depths (Fig. 4). It should be noted however, that L. hyperborea had a non-significant but positive correlation with Undaria. This reverse relationship is most likely to be due insufficient data as L. hyperborea was recorded at extremely low densities across the entire study area.
Distribution of canopy-forming macroalgae across depth, in reef habitats of Plymouth Sound. Mean percent canopy cover (A–J) and density (K–T) of each species shown ± standard error at each of five sites (orange = Devil’s Point, light blue = Firestone Bay, green = Jennycliff, dark blue = Mount Batten, red = West Hoe). Black lines indicate average across sites. Correlation coefficients with Undaria shown for every co-occurring species as well as for the sum of all Laminaria spp. Significant correlations shown in bold; asterisks indicate significance level (*=<0.05, **=<0.01, ***<0.001). Macroalgae drawing courtesy of Graham Epstein and Jack Sewell.
Primary succession
Following the introduction of new unfouled substrate into an Undaria dominated marina in March 2016, initial colonisation by canopy-forming macroalgae (Oct 16 to Mar 17) was dominated by L. digitata with low levels of S. latissima (Fig. 5). The density and cover of L. digitata were significantly higher compared with control areas on older pontoon substrates, whereas values for S. latissima were comparable between new and control substrates (Fig. 5). The first Undaria recruits were recorded one year following the installation of the new pontoons. The density of Undaria increased rapidly from Mar – Jun 17 and did not differ significantly from the control areas for the remainder of the experiment. The cover of Undaria also increased rapidly but remained marginally lower on new pontoons compared with control areas, although this variability was statistically significant at only one sampling event (Fig. 5). During this period, the density and cover of L. digitata on the new pontoons declined, and during the last four months of monitoring were no longer significantly different to values recorded on the older pontoons (Fig. 5). The density and cover of S. latissima increased on new pontoons during the same period, and was significantly higher than that on older substrates (Fig. 5). However, density and cover subsequently declined and for the last four months of monitoring both metrics were similar between treatments (Fig. 5).
Marina primary succession manipulation - change in density (A–C) and percent cover (D–F) of canopy-forming macroalgae following introduction of new substrate into a marina habitat. Means shown ± standard error. Asterisks indicate pairwise differences between old (red) and new unfouled (blue) substrate at each sampling event (Tables S4 and S5). “nd” indicates where data from old substrates was missing so pairwise tests could not be calculated. Macroalgae drawing courtesy of Graham Epstein and Jack Sewell.
Cleared substrates within the L. digitata dominated intertidal rocky reef habitat were quickly colonised by canopy-forming macroalgae after only two months. This early colonisation (May–Aug 17) was dominated by Undaria, but also S. polyschides, both of which were significantly higher in density and cover compared to the control area of undisturbed substrate (Fig. 6). The density and cover of L. digitata and the cover of H. elongata on cleared substrates remained low during this period, being significantly lower than values recorded on control plots (Fig. 6). For the remainder of the experiment (Oct 17 to July 18) Undaria and S. polyschides remained at low levels within both cleared and control plots, and no significant differences between treatments were recorded at any sampling point (Fig. 6). During this period there was a sustained increase in both L. digitata and H. elongata on new substrates. Even so, density and cover remained significantly lower on cleared compared with undisturbed areas for L. digitata until the last two months of sampling (Fig. 6). S. latissima density and cover varied greatly on both cleared and undisturbed areas across the study period, and exhibited no significant differences between substrate types (Fig. 6).
Intertidal reef primary succession manipulation - change in density (A–D) and percent cover (E–I) of canopy-forming macroalgae following introduction of cleared substrate into an intertidal reef habitat. Means shown ± standard error. Asterisks indicate pairwise differences between old (red) and new (blue) substrate at each sampling event (Tables S6 and S7). Macroalgae drawing courtesy of Graham Epstein and Jack Sewell.
Discussion
Interpreting results from manipulative, trait-based or demographic studies within the framework of contemporary coexistence theory allows for examination of the extent to which INNS may be coexisting or competing with native species in recipient communities45. Given its rapid rate of spread and inconsistencies in its perceived impacts21, Undaria is a useful model INNS to explore coexistence theory within rarely considered marine ecosystems15. Undaria has been present within the current study region for at least 15 years, where it now co-occurs with up to ten different species of canopy-forming macroalgae. Overall, there was very low annual variation and no major changes in macroalgal canopy density, biomass or composition over the three years of monitoring. Although inference is somewhat limited due to the duration of this study, the results suggest that large-scale temporal variability and maintenance of coexistence through the storage effect is not a primary driver of assemblage structure. This also indicates that the invader may have reached a relative equilibrium within the recipient community, as none of the species recorded here exhibited significant increases or decreases in abundance over the study period.
We recorded high variation in macroalgal assemblage structure between habitats. In complex rocky reef habitats, Undaria was predominantly found in areas that supported lower density and cover of perennial species, and reached relatively low abundances in areas where these species were experimentally removed. Conversely, on uniform marina pontoons, Undaria was the dominant species, exhibited no significant spatial relationships with native species and became established as the dominant species following succession on newly introduced substrate. Overall, when considering potential drivers of assemblage structure at the site level, based on these results we suggest that niche diversity primarily promoted coexistence of species on rocky reef, whereas fitness differences governed competition within marinas. It must be noted, however, that at the level of micro-habitats there are complex species-specific patterns which cannot be generalised across a given site. Using coexistence theory to contextualise observational or correlative findings, as shown here, allows for a clearer understanding of INNS distribution patterns and potential community level impacts. These factors should be of primary concern for INNS research, as they will directly contribute to management and conservation priorities.
On natural rocky reef substrates, Undaria exhibited a significant negative spatial relationship with the two dominant perennial species in both intertidal (i.e. Laminaria digitata) and subtidal habitats (i.e. Laminaria ochroleuca). The depth surveys also indicated that Undaria predominantly occupies a depth/elevation zone in which Laminaria species are found in lower abundance and cover, which may suggest niche differences as the primary driver leading to differing abundance-distribution patterns.
One factor which may drive niche differences and spatial separation between Undaria and Laminaria spp. is the ability of Undaria to grow successfully on a wide range of types of substrate, aspects and stabilities23,24,27. This generalist life strategy may allow it to fill a niche that is not occupied by the perennial Laminarians which generally require stable rocky substrates in order to successfully grow and mature46. In many cases, however, these species interact on stable rocky substrates, suggesting other drivers influence abundance-distribution patterns.
The primary succession experiment showed that clearance of L. digitata dominated areas leads to an opportunistic pulse of Undaria recruitment, confirming an overlapping niche. This was followed by recovery of L. digitata and a concurrent decline in Undaria, indicating higher fitness in L. digitata. However, Undaria recruited in relatively low densities and cover following the removal of L. digitata, and it was not able to recruit in the second year after clearances even when there was still significantly lower Laminaria density and cover compared to controls. This would suggest that Undaria has an overlapping but distinct niche when compared to L. digitata, and where they do overlap Undaria has lower fitness.
Due to differences in tolerance to desiccation and temperature stress, the two Laminaria species within this study region form two relatively distinct zones on rocky reefs; L. digitata on intertidal reef and L. ochroleuca on subtidal reef47. Although there is evidence of relatively high desiccation tolerance in Undaria sporophylls and gametophytes, information is lacking on the tolerance of growing blade tissue48. Due to its inability to successfully proliferate in cleared areas of the L. digitata zone, we suggest that Undaria has lower resistance to desiccation and temperature stress than L. digitata. However, Undaria is found at highest density and biomass at elevations above the L. ochroleuca zone, potentially indicating an intermediate tolerance between the two Laminaria species and allowing it to occupy a depth niche between the two Laminarians.
This does not, however, explain why L. digitata is not outcompeting Undaria and dominating on stable rocky substrate in the low intertidal to shallow subtidal fringe. In some regions L. digitata can be found to depths of up to 15 m, dependent on competition, wave exposure, light, temperature and grazing pressure49,50. While it is feasible that any of these factors could influence observed abundance-distribution patterns in the current study, L. digitata can be present in a wide range of wave exposures50. ‘Top-down’ grazing pressure is also not thought to be of major importance to kelp population structure along most of the UK coastline49,51, except at its deeper extent52. L. digitata is, however, known to require relatively high light levels to reproduce and grow53,54, whereas Undaria is able to persist under a wide range of light climates21,55. In the relatively turbid conditions of Plymouth Sound, it may be that L. digitata becomes light limited in the deeper intertidal-subtidal fringe (which is generally immersed in 1–5 m of turbid seawater due to the tidal range in the region), allowing Undaria to occupy this vacant space. Further manipulations of assemblages within the intertidal fringe would be needed to fully determine the niche versus fitness differences between these species.
It is also necessary to consider why Undaria is not proliferating in the lower subtidal areas which are currently L. ochroleuca dominated. One potential driver could be the comparatively low rate of nutrient uptake and nitrate storage in Undaria when compared to Laminarians21,56. Increased water motion enhances nutrient uptake in kelps57 and can enhance the growth rate of Undaria58. As tidal water flow is likely to be higher in the subtidal fringe when compared to the lower subtidal zone, Undaria may have lower fitness than L. ochroleuca in lower velocity subtidal waters. Although not possible as part of this study due to logistical challenges in experimental design, providing cleared or clean substrate in lower subtidal areas and assessing the colonisation and succession of these two species would allow identification of potential niche versus fitness differences between Undaria and L. ochroleuca.
Overall, these results support studies from other regions which suggest that Undaria has lower competitive ability when compared to native canopy dominant perennials21,59, referencestherein. The results also highlight that the persistence of Undaria in many settings is likely to be due to its relatively broad niche, allowing it to occupy underused resources or vacant space to coexist with native perennials at the site level.
On rocky reef, both Undaria and the native annual canopy-forming species S. polyschides were strongly correlated across depth and showed similar responses in the primary succession experiments. This would suggest that these species occupy a similar niche throughout rocky reefs in the study region. Both species are annual, relatively opportunistic and are found at highest abundance and cover in the subtidal fringe22,24,60,61. They also recruit, reach maximum biomass and senesce at similar times of year60,61,62. Therefore, it has previously been suggested that these two species occupy a similar niche and may directly compete for space or resources22,24,50,60. Species which occupy the same or highly similar niche while having little to no fitness dissimilarities would be expected to have strong positive spatial relationships across a given site. However, in this study, neither the density nor biomass of S. polyschides showed a significant spatial relationship with Undaria in either intertidal or subtidal reef habitats. This would suggest that there are more complex competitive interactions occurring which does not allow full coexistence between these species.
If Undaria was found to be outcompeting and potentially displacing S. polyschides, it would be pertinent to consider how this may alter wider ecosystem functioning. Previous studies have shown that these two species harbour a similar epifaunal and epifloral assemblages and therefore substitution of the species may have minimal community-level impacts63. Moreover, at the wider regional scale Undaria is far less tolerant of wave action than S. polyschides, which can dominate under wave-exposed conditions24,64, suggesting that competitive exclusion could only occur at wave sheltered sites and that regional displacement of S. polyschides is highly unlikely. However, further research would be needed to determine wider ecological consequences such as trophic provision to grazers, habitat provision to mobile species, primary production and carbon cycling49.
In comparison to reef habitats, the environment within marinas is relatively homogenous and as such niche diversity is reduced. The relative homogeneity in community structure was highlighted by the low β-diversity recorded on marina pontoons when compared to reef habitats. The pontoons available for sampling in this study were all of uniform substrate, depth and exposure, and were located adjacent to one another, with similar exposure, light availability, water flow and temperature. It is likely, therefore, that relationships in distribution-abundance patterns between species across a given site will be strongly influenced by fitness differences and competitive interactions. In all three marinas Undaria was the dominant species in terms of both density and biomass (Fig. S1); it also exhibited no spatial or temporal correlations with co-occurring species. This may indicate that Undaria is able to reach similar population size or density at varying levels of interspecific competition from co-occurring species, however further evidence would be needed to confirm this. The primary succession experiment which showed that newly-available substrate introduced into Undaria dominated marinas can lead to a significantly higher recruitment of native canopy-forming species (L. digitata and S. latissima), both of which were eventually replaced by Undaria, also suggests fitness differences as the primary driver.
Marinas are generally located in areas of intense human activity with modified abiotic conditions. Marina environments are often characterised by low salinity and high turbidity, pollution and nutrient levels, which generally support distinct and often depauperate epifaunal and epifloral communities that lack long-lived native species compared to adjacent natural habitats65. The higher fitness of Undaria when compared to native canopy-formers in marinas may in part be due to its ability to tolerate more extremes in environmental variables4,40,41,62. Perhaps the largest abiotic dissimilarity between marina pontoons and rocky reef habitats is the constant shallow depth in which floating pontoons are maintained when compared to tidal rocky habitats. One consequence of this is that macroalgae are subjected to constant high light intensities, which often leads to substantial biofouling and eventual detachment or smothering of blade tissue66,67. Here, Undaria plants were observed to support significantly less epiphytic loading compared with native species, potentially due to its fast growth rate and annual senescence, or perhaps the presence of antifouling compounds66,67. This may be a further mechanism underpinning the higher fitness of Undaria over native perennials within marinas. This study adds further support to the importance of artificial habitats in the invasion pathway of Undaria both across regions and locally into adjacent natural habitats24,42,68. The optimal habitat for Undaria within this study region appears to be floating pontoons in marinas – a habitat that has no natural analogue69.
Undaria has been reported to have relatively low ecological impact in many locations to which it has been introduced, particularly where it occurs within dense native canopies21,59. Although this study largely supports these conclusions, it highlights that there can be many context-specific species interactions which should also be considered before clear conclusions can be drawn. Within this study region, and perhaps across the northeast Atlantic, Undaria may exert low community-level impacts within natural habitats, due to its relatively low fitness when compared to dominant native perennial canopy-formers. Undaria could, however, influence co-occurring macroalgae within its invaded niche, potentially displacing functionally similar native species. Further targeted research would be needed to better quantify both lethal and sub-lethal effects of Undaria on species within its niche.
The distribution, proliferation and potential impact of INNS is highly dependent on complex niche and fitness differences between individual species, which themselves can vary across habitats. The quantification of INNS impact is therefore wholly dependent on the response metrics recorded and the spatial scale to which conclusions are drawn20,70. This is particularly evident within complex marine habitats dominated by, for example, macroalgal canopy-formers, which interact across multiple spatial and temporal scales, occupying distinct yet overlapping microhabitats and niches. For management purposes INNS are often ranked in terms of their broad-scale impact on the natural environment, which may occur at a continental or even global scale71,72. More consideration must be given to the context-specific nature of INNS impacts prior to wider scale management decisions.
Methods
Site selection
Undaria was first recorded in Plymouth Sound (southwest UK) in 2003. Since then it has successfully colonised both artificial and natural substrates in intertidal and shallow subtidal habitats24,28,34,63. Here it interacts with a range of native canopy forming macroalgae, including both perennial and annual species, although its impacts on native assemblages are poorly understood21,50.
Within Plymouth Sound, study sites were chosen based on the presence of: 1) available safe access points; 2) approval for scientific work; 3) widespread occurrence of Undaria (based on previous information or in situ sightings); 4) similar substrate within habitat types; 5) extensive suitable substrate. Nine monitoring sites were selected randomly from a larger pool between 10th March and 5th April 2016. Searches of the low intertidal zone were made across the Plymouth waterfront on low spring tides, subtidal searches were made at seven sites across the same area, and site visits were made at four marina sites (Fig. 1). Three marina and reef sites were selected, with subtidal sites deeper and adjacent to intertidal sites (Fig. 1). All marinas were within sheltered, non-drying harbours, with similarly constructed concrete pontoons. The intertidal and subtidal reef sites were all sheltered to moderately-sheltered from wave action, being characterised by extensive bedrock platforms interspersed with areas of larger boulders and compacted cobbles. Two additional reef sites were selected at the eastern end of the Plymouth waterfront to increase replication for depth profile surveys (Fig. 1). Manipulative primary succession experiments were established at one marina (Marina 1) and one intertidal site (Devil’s Point), adjacent to two of the monitoring sites.
Multi-year monitoring
Macroalgal canopies were surveyed in June 2016, 2017 and 2018. All nine monitoring sites (Marinas: 1–3; Intertidal rocky-reef: Devil’s Point, Firestone Bay, West Hoe; Subtidal rocky-reef: Devil’s Point, Firestone Bay, West Hoe) were sampled over a two week period at each sampling event. Although Undaria can be found throughout the year in the UK69 it has an annual life-history, predominantly being found in late spring to early summer26,60,63,69 when sampling intensity was concentrated. Sampling in September and December 2016 and March 2017 explored seasonal patterns in density and biomass. This was not used in formal analyses and is used for qualitative comparisons only.
As Undaria is predominantly found in low intertidal to shallow subtidal habitats24,28,63, subtidal sites were restricted to depths of 0.5–1.2 m below chart datum (CD) and intertidal sites to 0.3–1 m above CD. On each survey, ten replicate 0.5 × 0.5 m quadrats (stratified to rocky substrate) were placed haphazardly (each separated by at least 2 m) within an area of approximately 100 m2 around a permanent marker at each site. All subtidal sites were sampled using SCUBA, intertidal sites were sampled either with SCUBA or on low-spring tides.
Surveys within marinas were conducted on the immersed vertical sides of floating pontoons at a depth of 0–0.5 m below the surface. Ten replicate 0.5 × 0.5 m quadrats were positioned haphazardly against the pontoon surface. Based on substrate suitability, interactions with vessels and human disturbance, sampling was restricted to approximately 40 m2 of pontoon in the outer section of the marina. Due to the relatively limited area available for sampling, the position of each quadrat was noted to avoid overlapping quadrat samples during the study.
All canopy-forming macroalgae (U. pinnatifida, Saccharina latissima, Laminaria ochroleuca, Laminaria digitata, Laminaria hyperborea, Fucus serratus, Fucus vesiculosus, Ascophyllum nodosum, Saccorhiza polyschides, Himanthalia elongata, Sargassum muticum) were destructively sampled from each quadrat by gently prising the holdfast from the substrate, and placed into collection bags for further analysis. The density (inds. 0.25 m−2) and biomass (g 0.25 m−2) of each species was recorded for each quadrat separately. To concentrate further analyses on those species that have the potential to influence distribution-abundance patterns of Undaria, species which contributed less than 1% of the total biomass of the native macroalgal canopies in each habitat type were excluded.
Depth profiles
In July 2017 depth-related patterns in the density and cover of canopy-forming macroalgae were examined at five reef sites (Fig. 1). At each site four replicate transects (each separated by approximately 8 m) were surveyed from + 1.5 m to −4 m CD by SCUBA. Based on previous experience and preliminary surveys in Plymouth Sound, depths below −4 m CD are dominated by gravel-sandy substrates lacking canopy-forming macroalgae. Therefore greater depths were not included within this survey; however, in some areas canopy-forming macroalgae may be present at such depths, albeit at low density and cover. For each transect the same two surveyors swam 2–3 m apart along a fixed compass bearing, perpendicular to the depth contour. Each surveyor haphazardly placed a 0.5 × 0.5 m quadrat (stratified to rocky substrate) at eight depths across the survey range ( + 1.5, + 1.0, + 0.5, 0, −0.5, −1, −2. −4 CD). These were located using a digital depth meter corrected by estimated tidal height from the POLTIPS-3 software at the median time of the survey. Sampling was non-destructive; within each quadrat both density and cover of canopy-forming macroalgae (species as above) was visually quantified as counts of individual plants and percent canopy-cover per 0.25 m2. If no suitable rocky substrate could be found at a given depth along a transect, data (counts and cover) was recorded as missing rather than zero and replication at that depth was reduced. Prior to analysis the paired quadrats were averaged.
Primary succession
In March 2016 five new unfouled sections of marina pontoon were replaced at Marina 1 (Fig. 1). This novel substrate provided an opportunity to monitor colonisation and primary succession of canopy-forming macroalgae over time. From monthly observations, new recruits were noted in August 2016 and could be identified to species by October 2016. The canopy-forming macroalgae were non-destructively sampled monthly from October 2016 – January 2018 (except for November 2016). At each sampling event, three 0.5 × 0.5 m quadrats were placed haphazardly against the outer side of each of the five pontoon sections. Within each quadrat, the density and cover of each canopy-forming macroalgae species was estimated visually by a single observer. New pontoons were compared to five adjacent pontoon sections which had not been replaced, therefore containing established macroalgal assemblages. These older pontoons (which had been in situ for > 10 years) were sampled using the same method from March 2016 – January 2018, with sampling intensity initially at three month intervals, but monthly from March 2017.
A similar manipulation was also conducted at Devil’s Point to compare primary succession between natural and artificial habitats (Fig. 1). Eight circular treatment plots, 2 m in diameter (each separated by at least 2 m), were established within Laminaria digitata dominated reef habitat at a tidal height of + 0.8 to + 1.2 m CD. Each plot was randomly assigned as either ‘control’ or ‘clearance’ treatments. A permanent marker was placed in the centre of each plot using a stainless steel screw and coloured labels. In March 2017 all biota were removed from clearance plots in three stages: 1) manual removal by hand, 2) heat treatment of substrate using a Sheen x300 weed control flame gun, 3) secondary heat treatment using the same method 12 days later. Following the clearances, canopy-forming macroalgae were non-destructively sampled (monthly where possible but at least bimonthly) from March 2017 to July 2018. At each sampling event, three 0.5 × 0.5 m quadrats were haphazardly placed in each plot. Counts were made of each canopy-forming macroalgae (except for H. elongata ‘buttons’ due to time constraints) and the percentage canopy cover of each species was estimated visually by a single observer. For both the marina and intertidal reef, data were averaged within each pontoon/plot at each sampling event before further analysis.
Data analysis
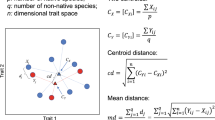
Using the monitoring data, interannual variability in macroalgal canopy composition was examined for each habitat-type separately using multivariate techniques. Raw data were first converted to proportional values and square root transformed (to down weight the importance of dominant species) prior to analysis. Resemblance matrices were constructed based on Bray-Curtis similarity and visualised using threshold metric multidimensional scaling (tmMDS) on bootstrap averages with their 95% confidence regions. Statistical differences in multivariate canopy structure between years was assessed using PERMANOVA with ‘year’ (three levels, fixed factor), and ‘site’ (three levels, random factor) as factors. Where differences between years were identified, post-hoc tests using pair-wise PERMANOVA, and similarity percentage breakdowns (SIMPER) were used to determine the principal contributors to the observed dissimilarity within significant pairwise contrasts. As a proxy for beta-diversity (β), the PERMDISP routine was used to calculate mean multivariate dispersion between quadrats for each habitat separately. All PERMANOVAs were run with 9999 permutations of residuals under a reduced model with Type 3 (partial) sums of squares. tmMDS plots were visualised using 50 restarts and a minimum stress of 0.01. Bootstrap averages were calculated with 100 bootstraps per group, with automatic selection of dimensions based on ρ > 0.99.
Spatial trends between Undaria and co-occurring macroalgae were assessed using linear mixed models (LMM) for biomass data (log transformed due to strong right-skewness and heterogeneity of variances) and negative binomial generalized linear mixed models (nbGLMM) for count data (due to overdispersion from the Poisson distribution). Data from the multi-year monitoring survey were analysed for each habitat separately. In all cases Undaria was modelled as a function of the biomass/count of each co-occurring species (treated as additive fixed continuous factors). Both ‘site’ (categorical; three levels) and ‘year’ (categorical; three levels) were also included as random factors in order to discern overall spatial trends across sites and years. Validation of all models was graphical, using diagnostic quantile-quantile plots and predicted versus residual plots. Where transformations or random error structures failed to fully reduce residual structuring or heterogeneity of variances, test statistics were interpreted at a conservative level of ɑ = 0.01 to decrease the probability of Type 1 errors. LMMs and nbGLMMs were fitted using the lmer and glmer.nb commands respectively, both from the lme4 package in R73.
To assess depth-related distribution trends between Undaria and co-occurring species, Spearman rank correlation tests were calculated between Undaria and each co-occurring species from the depth distribution survey. Data were first averaged across transects within each survey site and only those depths in the range of Undaria occurrence were used to calculate correlations. All Spearman correlations and significance tests were calculated with Holm adjusted p-values to account for multiple testing using the corr.test function from the psych package in R74.
For the primary succession manipulation, variability in the biomass/count of each species between the cleared (or new) substrate and control areas were examined with two-way analysis of variance (ANOVA). Prior to statistical analysis all count data were log transformed (log[x + 1]) and percent cover data were arcsin transformed (asin√x/100). Values of density (inds. 0.25 m−2), and cover for each species were modelled as a function of “date” (categorical; 15 levels for marina, 10 levels for reef) and “substrate” (categorical; two levels: cleared/new, control), with their interaction. The approach to model validation was the same as described above. Significant pairwise differences between substrates at each sampling point were tested using post hoc F-tests with Holm adjusted p-values. ANOVAs were constructed using the lm function from base R75 and pairwise tests were implemented using the testInteractions function from the phia package in R76. All univariate statistics were implemented in R 3.4.375, multivariate statistics in PRIMER-e version 777, data manipulation used the dplyr package78, graphs were created using ggplot279 and maps (Fig. 1) were made within ArcMap 10.3.1.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Darwin, C. On the Origin of the Species by Means of Natural Selection: or, the Preservation of Favoured Races in the Struggle for Life (J. Murray, 1859).
Elton, C. S. The ecology of invasions by animals and plants. (Springer, 1958).
Hutchinson, G. E. Homage to Santa Rosalia or Why Are There So Many Kinds of Animals? The American Naturalist 93, 145–159, https://doi.org/10.1086/282070 (1959).
Herbold, B. & Moyle, P. B. Introduced Species and Vacant Niches. The American Naturalist 128, 751–760, https://doi.org/10.1086/284600 (1986).
Levine, J. M. & D’Antonio, C. M. Elton revisited: A review of evidence linking diversity and invasibility. Oikos 87, 15–26 (1999).
Shea, K. & Chesson, P. Community ecology theory as a framework for biological invasions. Trends in Ecology &. Evolution 17, 170–176 (2002).
Dunstan, P. K. & Johnson, C. R. Mechanisms of invasions: Can the recipient community influence invasion rates? Botanica Marina 50, 361–372, https://doi.org/10.1515/bot.2007.041 (2007).
Stachowicz, J. J., Fried, H., Osman, R. W. & Whitlatch, R. B. Biodiversity, invasion resistance, and marine ecosystem function: Reconciling pattern and process. Ecology 83, 2575–2590 (2002).
Lodge, D. M. Biological invasions: Lessons for ecology. Trends in Ecology & Evolution 8, 133–137, https://doi.org/10.1016/0169-5347(93)90025-K (1993).
Ricciardi, A. & Cohen, J. The invasiveness of an introduced species does not predict its impact. Biological Invasions 9, 309–315, https://doi.org/10.1007/s10530-006-9034-4 (2007).
Ricciardi, A., Hoopes, M. F., Marchetti, M. P. & Lockwood, J. L. Progress toward understanding the ecological impacts of nonnative species. Ecological Monographs 83, 263–282, https://doi.org/10.1890/13-0183.1 (2013).
Valéry, L., Fritz, H., Lefeuvre, J.-C. & Simberloff, D. In search of a real definition of the biological invasion phenomenon itself. Biological Invasions 10, 1345–1351, https://doi.org/10.1007/s10530-007-9209-7 (2008).
MacDougall, A. S., Gilbert, B. & Levine, J. M. Plant invasions and the niche. Journal of Ecology 97, 609–615, https://doi.org/10.1111/j.1365-2745.2009.01514.x (2009).
Chesson, P. General theory of competitive coexistence in spatially-varying environments. Theoretical population biology 58, 211–237, https://doi.org/10.1006/tpbi.2000.1486 (2000).
Barabas, G., Andrea, R. D. & Stump, S. M. Chesson’s coexistence theory. Ecological Monographs 88, 277–303 (2018).
Chesson, P. L. & Warner, R. R. Environmental Variability Promotes Coexistence in Lottery Competitive Systems. The American Naturalist 117, 923–943, https://doi.org/10.1086/283778 (1981).
MacDougall, A. S. & Turkington, R. Are invasive species drivers or passengers of change in degraded ecosystems. Ecology 86, 42–55 (2005).
Britton-Simmons, K. H. & Abbott, K. C. Short- and long-term effects of disturbance and propagule pressure on a biological invasion. Journal of Ecology 96, 68–77, https://doi.org/10.1111/j.1365-2745.2007.01319.x (2008).
Hart, S. P. & Marshall, D. J. Environmental stress, facilitation, competition, and coexistence. Ecology 94, 2719–2731, https://doi.org/10.1890/12-0804.1 (2013).
Hannam, M. P. & Wyllie-Echeverria, S. Microtopography promotes coexistence of an invasive seagrass and its native congener. Biological Invasions 17, 381–395, https://doi.org/10.1007/s10530-014-0736-8 (2014).
Epstein, G. & Smale, D. A. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecology and evolution 7, 8624–8642, https://doi.org/10.1002/ece3.3430 (2017).
Castric-Fey, A., Girard, A. & Lhardyhalos, M. T. The Distribution of Undaria pinnatifida (Phaeophyceae, Laminariales) on the Coast of St. Malo (Brittany, France). Botanica Marina 36, 351–358, https://doi.org/10.1515/botm.1993.36.4.351 (1993).
Russell, L. K., Hepburn, C. D., Hurd, C. L. & Stuart, M. D. The expanding range of Undaria pinnatifida in southern New Zealand: distribution, dispersal mechanisms and the invasion of wave-exposed environments. Biological Invasions 10, 103–115, https://doi.org/10.1007/s10530-007-9113-1 (2008).
Epstein, G. & Smale, D. A. Environmental and ecological factors influencing the spillover of the non-native kelp, Undaria pinnatifida, from marinas into natural rocky reef communities. Biological Invasions 20, 1049–1072, https://doi.org/10.1007/s10530-017-1610-2 (2017).
Bollen, M., Pilditch, C. A., Battershill, C. N. & Bischof, K. Salinity and temperature tolerance of the invasive alga Undaria pinnatifida and native New Zealand kelps: Implications for competition. Marine Biology 163, 194, https://doi.org/10.1007/s00227-016-2954-3 (2016).
Cremades, J., Freire, O. & Peteiro, C. Biología, distribución e integración del alga alóctona Undaria pinnatifida (Laminariales, Phaeophyta) en las comunidades bentónicas de las costas de Galicia (NW de la Península Ibérica). Anales del Jardín Botánico de Madrid 63, 169–187 (2006).
Sliwa, C., Johnson, C. R. & Hewitt, C. L. Mesoscale dispersal of the introduced kelp Undaria pinnatifida attached to unstable substrata. Botanica Marina 49, 396–405, https://doi.org/10.1515/bot.2006.051 (2006).
Heiser, S., Hall-Spencer, J. M. & Hiscock, K. Assessing the extent of establishment of Undaria pinnatifida in Plymouth Sound Special Area of Conservation, UK. Marine Biodiversity Records 7, e93, https://doi.org/10.1017/s1755267214000608 (2014).
Morelissen, B., Dudley, B. D. & Phillips, N. E. Recruitment of the invasive kelp Undaria pinnatifida does not always benefit from disturbance to native algal communities in low-intertidal habitats. Marine Biology 163, 241, https://doi.org/10.1007/s00227-016-3014-8 (2016).
South, P. M. et al. Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Marine and Freshwater Research 67, 103–112, https://doi.org/10.1071/mf14211 (2015).
South, P. M. & Thomsen, M. S. The ecological role of invading Undaria pinnatifida: an experimental test of the driver–passenger models. Marine Biology 163, 175, https://doi.org/10.1007/s00227-016-2948-1 (2016).
Valentine, J. P. & Johnson, C. R. Persistence of the exotic kelp Undaria pinnatifida does not depend on sea urchin grazing. Marine Ecology Progress Series 285, 43–55 (2005).
Forrest, B. M. & Taylor, M. D. Assessing invasion impact: survey design considerations and implications for management of an invasive marine plant. Biological Invasions 4, 375–386 (2002).
De Leij, R., Epstein, G., Brown, M. P. & Smale, D. A. The influence of native macroalgal canopies on the distribution and abundance of the non-native kelp Undaria pinnatifida in natural reef habitats. Marine Biology 164, https://doi.org/10.1007/s00227-017-3183-0 (2017).
Edgar, G. J., Barrett, N. S., Morton, A. J. & Samson, C. R. Effects of algal canopy clearance on plant, fish and macroinvertebrate communities on eastern Tasmanian reefs. Journal of Experimental Marine Biology and Ecology 312, 67–87, https://doi.org/10.1016/j.jembe.2004.06.005 (2004).
Thompson, G. A. & Schiel, D. R. Resistance and facilitation by native algal communities in the invasion success of Undaria pinnatifida. Marine Ecology Progress Series 468, 95–105, https://doi.org/10.3354/meps09995 (2012).
Valentine, J. P. & Johnson, C. R. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. Journal of Experimental Marine Biology and Ecology 295, 63–90, https://doi.org/10.1016/s0022-0981(03)00272-7 (2003).
Casas, G., Scrosati, R. & Piriz, M. L. The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biological Invasions 6, 411–416 (2004).
Irigoyen, A. J., Trobbiani, G., Sgarlatta, M. P. & Raffo, M. P. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biological Invasions 13, 1521–1532, https://doi.org/10.1007/s10530-010-9910-9 (2011).
Curiel, D., Guidetti, P., Bellemo, G., Scattolin, M. & Marzocchi, M. The introduced alga Undaria pinnatifida (Laminariales, Alariaceae) in the lagoon of Venice. Hydrobiologia 477, 209–219, https://doi.org/10.1023/a:1021094008569 (2001).
Farrell, P. & Fletcher, R. L. An investigation of dispersal of the introduced brown alga Undaria pinnatifida (Harvey) Suringar and its competition with some species on the man-made structures of Torquay Marina (Devon, UK). Journal of Experimental Marine Biology and Ecology 334, 236–243, https://doi.org/10.1016/j.jembe.2006.02.006 (2006).
James, K. & Shears, N. T. Proliferation of the invasive kelp Undaria pinnatifida at aquaculture sites promotes spread to coastal reefs. Marine Biology 163, 1–12, https://doi.org/10.1007/s00227-015-2811-9 (2016).
Veiga, P., Torres, A. C., Rubal, M., Troncoso, J. & Sousa-Pinto, I. The invasive kelp Undaria pinnatifida (Laminariales, Ochrophyta) along the north coast of Portugal: Distribution model versus field observations. Marine Pollution Bulletin 84, 363–365, https://doi.org/10.1016/j.marpolbul.2014.05.038 (2014).
Epstein, G., Hawkins, S. J. & Smale, D. A. Removal treatments alter the recruitment dynamics of a global marine invader - Implications for management feasibility. Marine Environmental Research 140, 322–331, https://doi.org/10.1016/j.marenvres.2018.06.022 (2018).
HilleRisLambers, J., Adler, P. B., Harpole, W. S., Levine, J. M. & Mayfield, M. M. Rethinking Community Assembly through the Lens of Coexistence Theory. Annual Review of Ecology, Evolution, and Systematics 43, 227–248, https://doi.org/10.1146/annurev-ecolsys-110411-160411 (2012).
Kain, J. M. Aspects Of the biology of Laminaria hyperborea I. vertical distribution. Journal of the Marine Biological Association of the United Kingdom 42, 377–385, https://doi.org/10.1017/s0025315400001363 (1962).
King, N. G. et al. Cumulative stress restricts niche filling potential of habitat-forming kelps in a future climate. Functional Ecology, https://doi.org/10.1111/1365-2435.12977 (2017).
Bollen, M., Battershill, C. N., Pilditch, C. A. & Bischof, K. Desiccation tolerance of different life stages of the invasive marine kelp Undaria pinnatifida: Potential for overland transport as invasion vector. Journal of Experimental Marine Biology and Ecology 496, 1–8, https://doi.org/10.1016/j.jembe.2017.07.005 (2017).
Smale, D. A., Burrows, M. T., Moore, P., O’Connor, N. & Hawkins, S. J. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and evolution 3, 4016–4038, https://doi.org/10.1002/ece3.774 (2013).
Yesson, C., Bush, L. E., Davies, A. J., Maggs, C. A. & Brodie, J. The distribution and environmental requirements of large brown seaweeds in the British Isles. Journal of the Marine Biological Association of the United Kingdom 95, 669–680, https://doi.org/10.1017/s0025315414001453 (2015).
Hereward, H. F. R., Foggo, A., Hinckley, S. L., Greenwood, J. & Smale, D. A. Seasonal variability in the population structure of a habitat-forming kelp and a conspicuous gastropod grazer: Do blue-rayed limpets (Patella pellucida) exert top-down pressure on Laminaria digitata populations? Journal of Experimental Marine Biology and Ecology 506, 171–181, https://doi.org/10.1016/j.jembe.2018.06.011 (2018).
Jones, N. S. & Kain, J. M. Subtidal algal colonization following the removal of Echinus. Helgoländer wissenschaftliche Meeresuntersuchungen 15, 460–466, https://doi.org/10.1007/bf01618642 (1967).
Han, T. & Kain, J. M. Effect of photon irradiance and photoperiod on young sporophytes of four species of the Laminariales. European Journal of Phycology 31, 233–240, https://doi.org/10.1080/09670269600651431 (1996).
Delebecq, G. et al. Influence of local environmental conditions on the seasonal acclimation process and the daily integrated production rates of Laminaria digitata (Phaeophyta) in the English Channel. Marine Biology 160, 503–517, https://doi.org/10.1007/s00227-012-2106-3 (2013).
Morelissen, B., Dudley, B. D., Geange, S. W. & Phillips, N. E. Gametophyte reproduction and development of Undaria pinnatifida under varied nutrient and irradiance conditions. Journal of Experimental Marine Biology and Ecology 448, 197–206, https://doi.org/10.1016/j.jembe.2013.07.009 (2013).
Dean, P. R. & Hurd, C. L. Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (heterokontophyta) in southern New Zealand. Journal of Phycology 43, 1138–1148 (2007).
Gerard, V. A. In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera. Marine Biology 69, 51–54, https://doi.org/10.1007/bf00396960 (1982).
Nanba, N. et al. Effect of water flow velocity on growth and morphology of cultured Undaria pinnatifida sporophytes (Laminariales, Phaeophyceae) in Okirai Bay on the Sanriku coast, Northeast Japan. Journal of Applied Phycology 23, 1023–1030, https://doi.org/10.1007/s10811-010-9635-2 (2011).
South, P. M., Floerl, O., Forrest, B. M. & Thomsen, M. S. A review of three decades of research on the invasive kelp Undaria pinnatifida in Australasia: An assessment of its success, impacts and status as one of the world’s worst invaders. Marine Environmental Research 131, 243–257, https://doi.org/10.1016/j.marenvres.2017.09.015 (2017).
Fletcher, R. L. & Farrell, P. Introduced brown algae in the North East Atlantic, with particular respect to Undaria pinnatifida (Harvey) Suringar. Helgolander Meeresuntersuchungen 52, 259–275, https://doi.org/10.1007/bf02908901 (1999).
Norton, T. & Burrows, E. Studies on marine algae of the British Isles. 7. Saccorhiza polyschides (Lightf.) Batt. British Phycological Journal 4, 19–53 (1969).
Floc’h, J. Y., Pajot, R. & Wallentinus, I. The Japanese brown alga Undaria pinnatifida on the coast of France and its possible establishment in European waters. ICES Journal of Marine Science 47, 379–390, https://doi.org/10.1093/icesjms/47.3.379 (1991).
Arnold, M., Teagle, H., Brown, M. P. & Smale, D. A. The structure and diversity of epibiotic assemblages associated with the invasive kelp Undaria pinnatifida in comparison to native habitat-forming macroalgae on a subtidal temperate reef. Biological Invasions 18, 661–676, https://doi.org/10.1007/s10530-015-1037-6 (2016).
Burrows, M. T. Influences of wave fetch, tidal flow and ocean colour on subtidal rocky communities. Marine Ecology Progress Series 445, 193–207, https://doi.org/10.3354/meps09422 (2012).
Connell, S. D. Urban structures as marine habitats: an experimental comparison of the composition and abundance of subtidal epibiota among pilings, pontoons and rocky reef. Marine Environmental Research 52, 115–125 (2001).
Marzinelli, E. M. Artificial structures influence fouling on habitat-forming kelps. Biofouling 28, 339–349, https://doi.org/10.1080/08927014.2012.674518 (2012).
Sogn Andersen, G., Steen, H., Christie, H., Fredriksen, S. & Moy, F. E. Seasonal Patterns of Sporophyte Growth, Fertility, Fouling, and Mortality of Saccharina latissima in Skagerrak, Norway: Implications for Forest Recovery. Journal of Marine Biology 2011, 8, https://doi.org/10.1155/2011/690375 (2011).
Guzinski, J., Ballenghien, M., Daguin-Thiébaut, C., Lévêque, L. & Viard, F. Population genomics of the introduced and cultivated Pacific kelp Undaria pinnatifida: marinas - not farms - drive regional connectivity and establishment in natural rocky reefs. Evolutionary Applications, https://doi.org/10.1111/eva.12647 (2018).
Epstein, G. & Smale, D. A. Between-habitat variability in the population dynamics of a global marine invader may drive management uncertainty. Marine Pollution Bulletin 137, 488–500, https://doi.org/10.1016/j.marpolbul.2018.10.055 (2018).
Chase, J. M. Spatial scale resolves the niche versus neutral theory debate. Journal of Vegetation Science 25, 319–322 (2014).
Carboneras, C. et al. A prioritised list of invasive alien species to assist the effective implementation of EU legislation. Journal of Applied Ecology 55, 539–547, https://doi.org/10.1111/1365-2664.12997 (2018).
Lowe, S., Browne, M., Boudjekas, S. & De Poorter, M. 100 of the World’s Worst Invasive Alien Species. 11 pp (The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 2000).
Bates, D., Machler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67, 1–48, https://doi.org/10.18637/jss.v067.i01 (2015).
Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research. (Northwestern University, 2017).
R Core Team. R: A Language and Environment for Statistical Computing. (Vienna, Austria, 2017).
De Rosario-Martinez, H. phia: Post-Hoc Interaction Analysis (2015).
Clarke, K. R., Gorley, R. N., Somerfield, P. J. & Warwick, R. M. Change in marine communities: an approach to statistical analysis and interpretation, 3rd Edition (2014).
Wickham, H. & Francois, R. dplyr: A Grammar of Data Manipulation (2015).
Wickham, H. gplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York, 2009).
Acknowledgements
We would like to thank Harry Teagle, Albert Pessarrodona, Andy Cameron, Matt Arnold, Franki Perry and Chris Johnson for their support during various parts of this study. Macroalgae drawings shown in some figures are courtesy of Jack Sewell, habitat symbols courtesy of the Integration and Application Network (ian.umces.edu/symbols/). G.E. is funded through the Natural Environmental Research Council (NERC) Doctoral Training Partnership SPITFIRE (NE/L002531/1), led by Southampton University. D.A.S. is supported by an Independent Research Fellowship awarded by the Natural Environment Research Council of the UK (NE/K008439/1). S.J.H. is supported by a Lankester Fellowship of the Marine Biological Association and was supported by sabbatical leave from the Faculty of Natural and Environmental Sciences of the University of Southampton. This work was also supported by a British Ecological Society (BES) small grant awarded to D.A.S.
Author information
Authors and Affiliations
Contributions
G.E. conducted the majority of the research and manuscript preparation. D.A.S. was involved in conception, design and review of the research methods, and significantly contributed to manuscript composition and critical review. S.J.H. provided extensive guidance throughout the project, as well as with writing and critical review of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Epstein, G., Hawkins, S.J. & Smale, D.A. Identifying niche and fitness dissimilarities in invaded marine macroalgal canopies within the context of contemporary coexistence theory. Sci Rep 9, 8816 (2019). https://doi.org/10.1038/s41598-019-45388-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45388-5
This article is cited by
-
Habitat formed by the invasive macroalga Caulerpa filiformis (Suhr) Hering (Caulerpales, Chlorophyta) alters benthic macroinvertebrate assemblages in Peru
Biological Invasions (2022)
-
Spill-back events followed by self-sustainment explain the fast colonization of a newly built marina by a notorious invasive seaweed
Biological Invasions (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.