Abstract
Manganese oxide (MnO2) is one of the most promising intercalation cathode materials for zinc ion batteries (ZIBs). Specifically, a layered type delta manganese dioxide (δ-MnO2) allows reversible insertion/extraction of Zn2+ ions and exhibits high storage capacity of Zn2+ ions. However, a poor conductivity of δ-MnO2, as well as other crystallographic forms, limits its potential applications. This study focuses on δ-MnO2 with nanoflower structure supported on graphite flake, namely MNG, for use as an intercalation host material of rechargeable aqueous ZIBs. Pristine δ-MnO2 nanoflowers and MNG were synthesized and examined using X-ray diffraction, electron spectroscopy, and electrochemical techniques. Also, performances of the batteries with the pristine δ-MnO2 nanoflowers and MNG cathodes were studied in CR2032 coin cells. MNG exhibits a fast insertion/extraction of Zn2+ ions with diffusion scheme and pseudocapacitive behavior. The battery using MNG cathode exhibited a high initial discharge capacity of 235 mAh/g at 200 mA/g specific current density compared to 130 mAh/g which is displayed by the pristine δ-MnO2 cathode at the same specific current density. MNG demonstrated superior electrical conductivity compared to the pristine δ-MnO2. The results obtained pave the way for improving the electrical conductivity of MnO2 by using graphite flake support. The graphite flake support significantly improved performances of ZIBs and made them attractive for use in a wide variety of energy applications.
Similar content being viewed by others
Introduction
Manganese dioxide (MnO2) is widely used as a cathode material in battery technologies because of its several advantageous properties such as low-cost, abundant, low toxicity, and environmental friendliness1,2,3. MnO2 were previously studied and applied for a variety of energy storage devices4,5,6,7. Also, it was applied in different metal-ion batteries including Li-ion battery (LIB)8, Mg-ion battery (MIB)9 and Zn-ion battery (ZIB)10,11. MnO2 cathodes are inexpensive and exhibit a high theoretical capacity. Recently, aqueous Zn/MnO2 batteries are considered as promising alternative energy devices due to their high safety and the abundance of both Zn, MnO2 and the electrolyte12,13,14.
However, MnO2 suffers from its poor conductivity that often occurs in high internal resistance of the electrode resulting in poor performance of the battery15. Therefore, to improve the performance of the MnO2 cathode, it is necessary to increase the specific surface area of MnO2 as well as the ion diffusion rate16,17. MnO2 has various crystallographic polymorphs such as α-MnO2, β-MnO2, and δ-MnO2, etc. Among these, δ-MnO2 was reported to be a potential intercalation host material for aqueous ZIBs18 due to a substantial interlayer distance for the reversible insertion/extraction of Zn2+ ions. δ-MnO2 can be prepared by a chemical reduction or hydrothermal process19. The conventional synthesis method is the direct reduction of KMnO4 aqueous solution by dropwise introduction of concentrated HCl. Previously, δ-MnO2 nano-flakes were synthesized and used in aqueous ZIBs18. It led to a significant increase in the power density of the ZIB. Another approach undertaken was to support MnO2 nanostructures on a matrix material with a high surface area5,20,21. It was observed that these nanostructures could accelerate the charge transport during the electrochemical redox process. In this respect, various carbonaceous materials, namely activated carbon, carbon nanotubes (CNTs), carbon nanofibers (CNFs), graphene and graphite, have been integrated with MnO2. MnO2/graphene nanoflowers were synthesized in the form of sandwich-structured nanoflowers which exhibited excellent super capacitive properties effectively making a very conductive electrode material for high-performance super capacitors22. However, it is significant that MnO2 supported on graphite has not been reported previously in ZIBs application. The crystal structure of graphite consists of parallel planes of carbon atoms which is conductive primarily along its planes23,24. In this way, graphite is classified as a semimetal due to its high electrical conductivity25. Therefore, MnO2 supported on graphite is considered as the candidate due to an improvement in electronic conductivity and an increase in the stability of the electrode materials for ZIBs.
The present study reports on δ-MnO2 nanoflower/graphite as a cathode host material for rechargeable aqueous ZIBs. The δ-MnO2 nanoflower supported on graphite not only increases the electrical conductivity and discharge capacity of the battery but also improves the insertion/extraction acceleration by increasing the active area of the δ-MnO2 nanoflower. Accordingly, the electrochemical properties and performances of the batteries which use the MNG as host material cathode are examined and discussed.
Experimental
Chemical and materials
Reagent grade chemicals were obtained and used without further purification unless noted otherwise. The graphite powder was purchased from Aldrich Company. Potassium permanganate (KMnO4), manganese sulfate monohydrate (MnSO4.H2O), zinc sulfate (ZnSO4), sulfuric acid (H2SO4), and cellulose acetate were purchased from Ajax Finechem. Nickel foam (0.5 mm thick, 100 PPI) was purchased from Qijing Trading Co., Ltd. Whatman filter paper No.1 was purchased from Sigma-Aldrich. Graphite foil was purchased from Shenzhen 3KS Electronic Material Co. Ltd. Zn sheet (99.99%) was purchased from Sirikul Engineering Ltd., Part.
Preparation of δ-MnO2 nanoflower and δ-MnO2 nanoflower/graphite (MNG)
The pristine δ-MnO2 nanoparticles were synthesized by dissolving KMnO4 (1.98 g) in 60 mL of deionized (DI) water. Then, MnSO4.H2O (0.336 g) was dissolved in 20 mL of DI water. Next, the MnSO4.H2O solution was added dropwise to the KMnO4 solution, and continuous stirring followed for 30 min. Afterward, the mixture was transferred into a 100 mL Teflon autoclave and kept at 160 °C for 24 hr in an oil bath. The product was collected and washed with DI water several times. Then, it was dried at 80 °C for 12 hr. The MNG synthesis was similar to the method reported by Liu et al.22 with some modifications. Graphite (1.0 g) was mixed in DI water (500 mL) with KMnO4 (10.0 g). The mixture of graphite and KMnO4 was stirred for 18 hr. Then, 98% of H2SO4 (5 mL) was added dropwise into the mixture. The solution was continuously stirred for 1 hr and heated to and maintained at 80 °C for 1 hr. After that, the solution was diluted in 1 L of DI water and allowed to stand at room temperature. The solution was filtered using cellulose filter paper (pore size 11 µm). Then, the MNG precipitates were collected and washed by DI water several times until the violet color disappeared. Subsequently, the precipitates were dried at 80 °C overnight.
Characterization and electrochemical measurement
X-ray Diffraction (XRD, Bruker AXS Model D8 Discover) of the powder samples was carried out with Cu Kα radiation at a scanning range of 5–80°. Field Emission Scanning Electron Microscope (FESEM, JEOL JSM-7610F, Tokyo, Japan) was used to take the morphology image and nanoflower size of MNG.
The cathode using MNG was prepared by mixing together 70% wt. of MNG, 20% wt. of carbon black (CB), and 10% wt. of cellulose acetate binder. Alternatively, the cathode using pristine δ-MnO2 was prepared by mixing together 70% wt. of the pristine δ-MnO2, 20% wt. of carbon black (CB), and 10% wt. of cellulose acetate binder. Acetone was used to adjust the viscosity of the slurries. Each mixed slurry was coated on graphite foil using a lab coating machine (AOT-FCM-250, AOT Electronic Technology Co., LTD) and dried at 70 °C under vacuum. The thickness of the cathode material deposited was 25 µm. The zinc anode was prepared by electrodeposition of zinc from ZnSO4 (0.5 M) aqueous solution onto Ni-foam using zinc sheet as a counter electrode at the current density of 60 mA/cm2. The amount of zinc deposited was 20 mg/cm2. Both cathode and anode were punched into a 15 mm diameter disk. The filter paper was punched into a 19 mm disk and used as the separator. Then, 0.3 mL of ZnSO4 (1 M) was added to the cell. The testing cells were fabricated as a coin cell (CR2032).
Electrochemical measurements were carried out using a CR2032 coin cell. Cyclic Voltammetry (CV) was performed by Potentiostat (VersaStat3, Princeton Applied Research) at a scan rate of 0.5 mV/s in the voltage range 1.0–1.8 V versus Zn2+/Zn. A battery testing system (BTS-5V10mA, Neware, China) was used to investigate the performance of the battery. The charge-transfer resistance, as illustrated by the Nyquist plots for the cathode, was carried out using an Electrochemical Impedance Spectroscopy (EIS) technique using an amplitude of 10 mV in the frequency range of 1–100,000 Hz.
Results and discussion
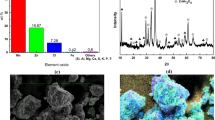
In this study, δ-MnO2 nanoflower/graphite (MNG) was prepared by a modified method of Liu et al.22. In Fig. 1, the XRD patterns of δ-MnO2 and graphite are shown. Thus, it can be seen that both patterns, i.e. δ-MnO2 (JSPDS card no. 80–1098)26,27 and graphite (JSPDS card no. 41–1487)28 match well. In the XRD pattern of MNG. The diffraction peaks (2θ) at 12.2°, 26.5°, 36.6°, and 65.6° correspond to the (001), (002), (−111) and (−312) crystal planes of δ-MnO2, respectively29. The high-intensity diffraction peak of MNG at 26.5° indicates the high crystallinity of graphite. δ-MnO2 possesses a planar layered-structure as illustrated by the inset of Fig. 1. This structure suggests that the interlayer gap is easy to insert/extract foreign cations and thereby can be useful for energy storage applications27. Further, the XRD analysis confirmed that the delta (δ) phase was presented in the pristine δ-MnO2 sample (see Supplementary Information Fig. S1).
In Fig. 2(a), the FESEM image of MNG is shown. It is observed that the numerous MnO2 nanoflowers constructed on the graphite surface reveal a flake-like sample. Figure 2(b) shows the higher magnification image of MNG which indicates that many petals can interconnect forming micropores about 50 nm in diameter size. The MNG having micropores among petals will assist in increasing the contact area between the electrolyte and cathode material as well as ensure fast ion transfer in the charge/discharge process22.
In Fig. 3, the battery configuration in this study which is composed of the MNG cathode, zinc anode, and ZnSO4 aqueous electrolyte is shown. During discharging, anodic zinc is dissolved in the form of Zn2+ ions into an aqueous electrolyte, containing Zn2+ ions and rapidly solvate in the form of solvated Zn2+ ion. Then, they diffuse and pass through the separator to the MNG cathode. The solvated Zn2+ ions are de-solvated in the form of Zn2+ ions and intercalate into δ-MnO2 structure30,31 as illustrated by the inset of Fig. 3. Further, an electron current starts to flow in the electrical loop from the electrical conduction of graphite. These three processes can be reversed by (1) the de-intercalation of Zn2+ ions from MNG cathode; then (2) solvated species are formed and lastly (3) Zn2+ ions are reduced to Zn and deposited back on the zinc anode, respectively. The electrochemical reaction may be expressed as in Eq. (1) anode reaction and Eq. (2) cathode reaction:
During the electrochemical Zn2+ ion insertion13, the layered type δ-MnO2 structure can transform to spinel-type ZnMn2O4 with Mn(III) state and layered type δ-ZnxMnO2 with Mn(II) state.
To investigate the kinetics of the MNG electrode, cyclic voltammograms using sweep rates of 0.5 to 32 mV/s in the voltage range 1.0–1.8 V versus Zn2+/Zn were measured using CR2032 coin cells. As shown in Fig. 4(a), a dominating pair of redox peaks exhibits increasing currents when the sweep rates increase, which do not display rectangular-shape and symmetrical voltammograms even at high scan rates, compared to MnO2/activated carbon composite for supercapacitors32. It is noted that the MNG electrode does not present the capacitive behavior of the electrode33,34. The capacitive effect is characterized by analyzing the cyclic voltammetry data at different sweep rates as in Eq. (3):
where i is the peak specific current, v is a potential sweep rate and a, b are adjustable parameters. The redox reaction is limited by the diffusion-controlled behavior; the peak current i varies as v1/2. Although the capacitance contribution suggests that the peak current i varies as v35,36. Equation (3) can be taken with logarithm and can be expressed according to Eq. (4):
The b value denotes the slope of the plot of lni versus lnv. When b value is close to 1, the system is mainly controlled by capacitance; when b value is close to 0.5, the Zn2+ ion insertion process dominates. Figure 4(b) shows the lni versus lnv plots at oxidation and reduction process of the cyclic voltammogram. The bo (oxidation process) and br (reduction process) of the MNG cathode are 0.58 and 0.62, respectively. Since the average b values are close to 0.5, it may imply that the redox reactions on the MNG cathode are controlled by the diffusion process. MnO2 is a transition metal oxide that typically displays the pseudocapacitance behavior37. The capacitive-controlled process occurs only on the surface. However, in the case of MNG cathode, the characteristic of Zn2+ ions insertion/extraction deviates from capacitive-controlled process towards the diffusion-controlled process. That is, the insertion/extraction of Zn2+ ions occur not only on the surface but also the pores inside. The result shows good agreement of a fast Zn2+ ion insertion/extraction or high rate property for the battery35.
In order to compare the improved electrochemical properties of MNG as the cathode for ZIB, the pristine δ-MnO2 is used as a comparable cathode. Figure 5 (a) shows the CV profiles of the pristine δ-MnO2 and MNG electrodes at a scan rate of 0.5 mV/s over the potential range 1.0–1.8 V for the initial three cycles. During the first cycle, two distinct peaks are observed at 1.20 and 1.57 V for the pristine δ-MnO2 electrode and at 1.18 and 1.54 V for MNG. The peaks in the low potential region appear at 1.20 and 1.18 V which can be attributed to Zn2+ ion insertion into the δ-MnO2 host structure. In the higher potential region, two oxidation peaks can be seen at 1.57 and 1.54 V for δ-MnO2 and MNG, respectively, which correspond to the extraction of Zn2+ ions from the δ-MnO2 host structure. The results suggest that, the oxidation state of Mn is reduced to Mn3+ states and is oxidized back to Mn4+ states, during Zn2+ ion insertion and extraction, respectively13,14,18,38. On subsequent cycling, two distinct peaks appear at 1.37 and 1.21 V for Zn2+ ion insertion into δ-MnO2 whereas the peaks at 1.38 and 1.20 V were observed for MNG in the low voltage region. In the high voltage region, the peak at 1.57 and shoulder at 1.62 V can be clearly seen for the pristine δ-MnO2. Likewise, the peak at 1.53 V and shoulder at 1.60 V can be clearly seen for the MNG electrodes. The cyclic voltammogram, having two peaks during discharge and having a peak with shoulder during charge, exhibits typical characteristics of the electrochemical insertion/extraction of Zn2+ ions in MnO2 structure12,13,39,40,41. These results with two peaks during discharge may be more clearly described by the two-step reaction of Zn2+ ion insertion in electrochemical reaction12,38 and spinel-type ZnMn2O4 transformation33. In the following scan cycles, the peaks at 1.37 V for δ-MnO2 and at 1.38 for MNG increase gradually during discharge indicating an activation process38. The CV curve of MNG exhibits a higher peak intensity and a larger enclosed area when compared with the pristine δ-MnO2 indicating improved electrochemical performance and fast Zn2+ ion insertion/extraction42.
Figure 5(b) shows the first and second discharge/charge profiles of the pristine δ-MnO2 and MNG cathode in a coin cell battery when cycled at a specific current density of 200 mA/g in the potential range of 1.0–1.8 V. The battery fabricated with the pristine δ-MnO2 and MNG host cathode material under open air condition displays an open-circuit voltage (OCV) of about 1.4 V. The first discharge capacity for MNG is 235 mAh/g whereas the pristine δ-MnO2 registers only 130 mAh/g. Compared to todorokite-type MnO214 and δ-MnO2 nano-flake13, the initial discharge capacity can deliver only 98 mAh/g (at 50 mA/g) and 122 mAh/g (at 83 mA/g), respectively. It is clear that δ-MnO2 supported on graphite samples can accommodate more numbers of Zn2+ ions than the unsupported δ-MnO2 (pristine δ-MnO2). It appears that the nanoflower δ-MnO2 in the structure of MNG tend to enhance the electrode/electrolyte contact area, thereby favoring Zn2+ ion insertion41. In addition, MNG shows a longer horizontal discharge curve than that of δ-MnO2, suggesting a more stable Zn2+ ion insertion into the MNG than in the pristine δ-MnO2. On the subsequent cycle, during the continuous discharge, the voltage profiles present two distinct plateaus at 1.45 and 1.25 V for MNG and 1.4 and 1.2 V for δ-MnO2. These characteristics were also observed for MnO2 electrodes in aqueous ZIB systems14,43. It can be implied that Zn2+ ions can insert into the layered δ-MnO239 which is in agreement with the two distinct peaks during discharge, as shown in Fig. 5(a).
Figure 5(c) displays the cycling behavior and corresponding coulombic efficiency of the pristine δ-MnO2 and MNG electrodes, under the specific current density of 400 mA/g. At the 100th cycle, the discharge and charge capacities registered by δ-MnO2 were 63.3 and 63.8 mAh/g, respectively while that of MNG were 113.4 and 114.2 mAh/g, respectively. The lower capacity retention of δ-MnO2 may result from low intrinsic electronic conductivity because of the appearance of unstable Mn3+ states during zinc-ion insertion10. The higher capacity retention of MNG may indicate that the electronic conductivity of MNG is improved. In the initial cycles, gradual capacity fade was observed for the pristine δ-MnO2 electrode, but fast capacity fade was observed for the MNG electrode. The formation of ZnMn2O4 with the Jahn-Teller Mn3+ ion may contribute to electrode degradation and hence lead to the fast capacity fade13. However, MNG demonstrates higher capacity than the pristine δ-MnO2. Over long-term cycling, the coulombic efficiency of both host material electrodes is maintained at around 100%. Thus, this clearly demonstrates that no irreversible capacity losses occurred41.
In Fig. 5(d), the rate performances of the pristine δ-MnO2 and MNG host material cathodes are shown. Cycling takes place at various specific current densities of 200, 400, 800 and 1600 mA/g, namely 5 times for each rate. The rate performance of MNG is significantly higher than those of the pristine δ-MnO2. It is indicated that nanoscale morphology of δ-MnO2 nanoflowers on graphite increases the contact area between the electrode and the electrolyte and provides more electrochemically active sites for ion-insertion13. Graphite not only improves the electronic conductivity of the MNG electrode but also tends to disperse the δ-MnO2 nanoflower sites. The MNG cathode can be charged and discharged at different rates; a high rate of 1600 mA/g leads to a discharge and charge capacity of 76 and 64 mAh/g, respectively. When cycled at a specific current density of 200 mA/g, the MNG cathode can deliver a discharge and charge capacity of 181 and 179 mAh/g, respectively. This behavior indicates that the MNG cathode can well be considered for the Zn2+ ion storage material10. It is clear therefore that MNG can improve not only the cycling performance but also the rate performance for ZIBs.
As displayed in Fig. 6(a), the difference in electrochemical conductivity before cycling between the pristine δ-MnO2 and MNG host cathodes was examined using EIS. The curves of the pristine δ-MnO2 and MNG host cathodes consist of depressed semicircles and diffusion drift which can be perfectly fitted using Randles equivalent circuit (see Inset Fig. 6(a)). Hence, in the equivalent circuit, Rs is the solution resistance, Rct is the charge-transfer resistance at the interfaces and Zw is the Warburg impedance related to the diffusion of Zn2+ ions. The Rct value for the pristine δ-MnO2 is 5.9 Ω and the value reduces to 4.8 Ω when supported on graphite (MNG). The result indicates that the electrical conductivity of the MNG sample is improved by constructing a conductive support using the graphite. The relationship between real impedance (Z′) and angular frequency (ω) in the low frequency region can be expressed accordingly by Eq. (5)44:
where σ is the Warburg factor which is relative to Z′- ω obtained from the slope of the lines in Fig. 6(b). The diffusion coefficient of zinc ion can be calculated as in Eq. (6)45:
where R is the gas constant, T is the absolute temperature, n is the number of electrons per molecule oxidized, A is the surface area, F is Faraday’s constant, C is the concentration and D is the diffusion coefficient.
EIS results of the batteries: (a) Nyquist plot of EIS spectra, (b) relationship between real part of impedance versus ω−0.5 for the batteries using MNG and the pristine δ-MnO2, (c) schematic illustration for the Zn2+ ion insertion into the MNG electrode, and (d) Nyquist plot of EIS spectra of MNG at various charge/discharge cycles.
As shown in Fig. 6(b), the slope which is the σ value of MNG (3.94) host electrode is lower than that of the pristine δ-MnO2 (9.01) indicating that the diffusion coefficient of MNG is higher than that of the pristine δ-MnO2, as in Eq. (6). It is clear that the MNG host material electrode can enhance the diffusion coefficient of Zn2+ ion, highlighting the electrical conductivity improvement44,46.
In Fig. 6(c), the MNG cathode which is a δ-MnO2 structure supported on graphite is drawn and illustrated. It is highly possible that Zn2+ ions can insert into the δ-MnO2 nanoflower structure having short diffusion length. Thereby, electrochemical performance can be improved during cycling at high rate47.
In Fig. 6(d), after charge/discharge cycling, the EIS measurements of MNG are shown. After the 1st, 10th, 20th, and 30th cycles, the MNG host cathode exhibits the Rct values, namely 6.51, 10.9, 13.7 and 18.2 Ω, respectively. After the first cycle, the charge-transfer resistance increases which indicates that the intercalation of Zn2+ ions into the δ-MnO2 structure becomes more difficult. The conduction of ions before intercalation depends not only on the cathode material but also on the electrolyte access into the cathode. The porosity of the cathode material is an important factor which can affect the electrolyte access. The SEM image of MNG and the pristine δ-MnO2 compound is displayed in the Supplementary Information Fig. S2.
Conclusion
In summary, δ-MnO2 nanoflower supported on graphite flake was synthesized and used as an intercalation host material for a rechargeable ZIB. While the XRD studies confirmed the crystallographic structure, the FESEM studies revealed that the sample showed a nanoflower-like morphology having micropores about 50 nm in diameter. This study demonstrates that a diffusion process controlled the electrochemical reactions of the MNG cathode. When tested in ZIB, the MNG sample registered a prominent discharge capacity of 235 mAh/g, which was higher than the discharge capacity of pristine δ-MnO2 (130 mAh/g). After the 100th cycle, the discharge capacity registered by the δ-MnO2 was only 63.3 mAh/g whereas the MNG delivered a discharge capacity of 113.4 mAh/g. Moreover, the conductivity of the supporting graphite improved the conductivity by reducing the charge transfer resistance of the cathode materials. The present results concluded that the supporting graphite not only improved the electrical conductivity but also enhanced the specific capacity and the cycling performance of the pristine δ-MnO2. Thus, MNG is one of promising candidate cathode materials for ZIBs.
Data Availability
The authors declare that all relevant data are within the paper Competing interests: The authors declare no potential conflict of interest.
References
Misnon, I. I. et al. High performance MnO2 nanoflower electrode and the relationship between solvated ion size and specific capacitance in highly conductive electrolytes. Mater. Res. Bull. 57, 221–230, https://doi.org/10.1016/j.materresbull.2014.05.044 (2014).
Lao-atiman, W., Julaphatachote, T., Boonmongkolras, P. & Kheawhom, S. Printed transparent thin film Zn-MnO2 battery. J. Electrochem. Soc. 164, A859–A863, https://doi.org/10.1149/2.1511704jes (2017).
Suren, S. & Kheawhom, S. Development of a high energy density flexible zinc-air battery. J. Electrochem. Soc. 163, A846–A850, https://doi.org/10.1149/2.0361606jes (2016).
Hosseini, S. et al. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 8, 14909, https://doi.org/10.1038/s41598-018-32806-3 (2018).
Wang, J.-W., Chen, Y. & Chen, B.-Z. Synthesis and control of high-performance MnO2/carbon nanotubes nanocomposites for supercapacitors. J. Alloy Compd. 688, 184–197, https://doi.org/10.1016/j.jallcom.2016.07.005 (2016).
Hosseini, S., Han, S. J., Arpornwichanop, A., Yonezawa, T. & Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 8, 11273, https://doi.org/10.1038/s41598-018-29630-0 (2018).
Lao-atiman, W. et al. Model-Based Analysis of an Integrated Zinc-Air Flow Battery/Zinc Electrolyzer System. Frontiers in Energy Research 7, https://doi.org/10.3389/fenrg.2019.00015 (2019).
Chen, J. et al. Electrochemical properties of MnO2 nanorods as anode materials for lithium ion batteries. Electrochim. Acta 142, 152–156, https://doi.org/10.1016/j.electacta.2014.07.089 (2014).
Kim, J.-S. et al. High-capacity nanostructured manganese dioxide cathode for rechargeable magnesium ion batteries. J. Power Sources 273, 210–215, https://doi.org/10.1016/j.jpowsour.2014.07.162 (2015).
Alfaruqi, M. H. et al. A high surface area tunnel-type α-MnO2 nanorod cathode by a simple solvent-free synthesis for rechargeable aqueous zinc-ion batteries. Chem. Phys. Lett. 650, 64–68, https://doi.org/10.1016/j.cplett.2016.02.067 (2016).
Kao-ian, W., Pornprasertsuk, R., Thamyongkit, P., Maiyalagan, T. & Kheawhom, S. Rechargeable Zinc-Ion Battery Based on Choline Chloride-Urea Deep Eutectic Solvent. Journal of The Electrochemical Society 166, A1063–A1069, https://doi.org/10.1149/2.0641906jes (2019).
Alfaruqi, M. H. et al. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 288, 320–327, https://doi.org/10.1016/j.jpowsour.2015.04.140 (2015).
Alfaruqi, M. H. et al. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem commun. 60, 121–125, https://doi.org/10.1016/j.elecom.2015.08.019 (2015).
Lee, J., Ju, J. B., Cho, W. I., Cho, B. W. & Oh, S. H. Todorokite-type MnO2 as a zinc-ion intercalating material. Electrochim. Acta 112, 138–143, https://doi.org/10.1016/j.electacta.2013.08.136 (2013).
Toupin, M., Brousse, T. & Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 16, 3184–3190, https://doi.org/10.1021/cm049649j (2004).
Feng, M. et al. Manganese oxide electrode with excellent electrochemical performance for sodium ion batteries by pre-intercalation of K and Na ions. Sci. Rep. 7, 2219, https://doi.org/10.1038/s41598-017-02028-0 (2017).
Song, J., Kim, J., Kang, T. & Kim, D. Design of a porous cathode for ultrahigh performance of a Li-ion battery: An overlooked pore distribution. Sci. Rep. 7, 42521, https://doi.org/10.1038/srep42521 (2017).
Wei, C., Xu, C., Li, B., Du, H. & Kang, F. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage. J. Phys. Chem. Solids 73, 1487–1491, https://doi.org/10.1016/j.jpcs.2011.11.038 (2012).
Renuka, R. & Ramamurthy, S. An investigation on layered birnessite type manganese oxides for battery applications. J. Power Sources 87, 144–152 (2000).
Zhu, C. et al. One-step electrochemical approach to the synthesis of Graphene/MnO2 nanowall hybrids. Nano Res. 4, 648–657, https://doi.org/10.1007/s12274-011-0120-2 (2011).
Zhou, J. et al. Novel synthesis of birnessite-type MnO2 nanostructure for water treatment and electrochemical capacitor. Ind. Eng. Chem. Res. 52, 9586–9593, https://doi.org/10.1021/ie400577a (2013).
Liu, J. et al. In situ chemical synthesis of sandwich-structured MnO2/graphene nanoflowers and their supercapacitive behavior. Electrochim. Acta 173, 148–155, https://doi.org/10.1016/j.electacta.2015.05.040 (2015).
Phillips, C., Al-Ahmadi, A., Potts, S.-J., Claypole, T. & Deganello, D. The effect of graphite and carbon black ratios on conductive ink performance. J. Mater. Sci. 52, 9520–9530, https://doi.org/10.1007/s10853-017-1114-6 (2017).
Wongrujipairoj, K., Poolnapol, L., Arpornwichanop, A., Suren, S. & Kheawhom, S. Suppression of zinc anode corrosion for printed flexible zinc-air battery. Phys. Status Solidi B 254, https://doi.org/10.1002/pssb.201600442 (2017).
Chernozatonskii, L. A., Sorokin, P. B., Belova, E. É., Brüning, J. & Fedorov, A. S. Metal-semiconductor (semimetal) superlattices on a graphite sheet with vacancies. JETP Lett. 84, 115–118, https://doi.org/10.1134/s0021364006150033 (2006).
Li, F. et al. MnO2 nanostructures with three-dimensional (3D) morphology replicated from diatoms for high-performance supercapacitors. J. Mater. Chem. A 3, 7855–7861, https://doi.org/10.1039/c5ta00634a (2015).
Thapa, A. K. et al. Synthesis of mesoporous birnessite-MnO2 composite as a cathode electrode for lithium battery. Electrochim. Acta 116, 188–193, https://doi.org/10.1016/j.electacta.2013.11.032 (2014).
Rajarao, R. & Bhat, B. R. Large scale synthesis of carbon nanofibres on sodium chloride support. Nanomater. Nanotechno. 2, 5, https://doi.org/10.5772/50306 (2012).
Zhang, J., Li, Y., Wang, L., Zhang, C. & He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 5, 2305–2313, https://doi.org/10.1039/c4cy01461h (2015).
Kundu, D. et al. Aqueous vs. nonaqueous Zn-ion batteries: consequences of the desolvation penalty at the interface. Energy Environ. Sci. 11, 881–892, https://doi.org/10.1039/c8ee00378e (2018).
Hayes, A. C., Kruus, P. & Adams, W. A. Raman spectroscopic study of aqueous (NH4)2SO4 and ZnSO4 solutions. J. Solution Chem. 13, 61–75, https://doi.org/10.1007/BF00648592 (1984).
Wang, J.-W., Chen, Y. & Chen, B.-Z. A synthesis method of MnO2/activated carbon composite for electrochemical supercapacitors. J. Electrochem. Soc. 162, A1654–A1661, https://doi.org/10.1149/2.0031509jes (2015).
Ji, C., Ren, H. & Yang, S. Control of manganese dioxide crystallographic structure in the redox reaction between graphene and permanganate ions and their electrochemical performance. RSC Adv. 5, 21978–21987, https://doi.org/10.1039/c5ra01455g (2015).
Yang, Y.-j et al. Nanostructured MnO2/exfoliated graphite composite electrode as supercapacitors. J. Alloy Compd. 487, 564–567, https://doi.org/10.1016/j.jallcom.2009.08.008 (2009).
Li, J. et al. Improved Li-ion diffusion process in TiO2/rGO anode for lithium-ion battery. J. Alloy Compd. 727, 998–1005, https://doi.org/10.1016/j.jallcom.2017.08.121 (2017).
Simon, P., Gogotsi, Y. & Dunn, B. Where do batteries end and supercapacitors begin. Science 343, 1210–1211 (2014).
Chen, D. et al. Probing the charge storage mechanism of a pseudocapacitive MnO2 electrode using in operando raman spectroscopy. Chem. Mater. 27, 6608–6619, https://doi.org/10.1021/acs.chemmater.5b03118 (2015).
Guo, X. et al. A hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries. Nanomaterials 8, https://doi.org/10.3390/nano8050301 (2018).
Alfaruqi, M. H. et al. Structural transformation and electrochemical study of layered MnO2 in rechargeable aqueous zinc-ion battery. Electrochim. Acta 276, 1–11, https://doi.org/10.1016/j.electacta.2018.04.139 (2018).
Qiu, W. et al. High-performance flexible quasi-solid-state Zn–MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 5, 14838–14846, https://doi.org/10.1039/c7ta03274a (2017).
Alfaruqi, M. H. et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-Ion battery system. Chem. Mater. 27, 3609–3620, https://doi.org/10.1021/cm504717p (2015).
Islam, S. et al. Carbon-coated manganese dioxide nanoparticles and their enhanced electrochemical properties for zinc-ion battery applications. J. Energy Chem. 26, 815–819, https://doi.org/10.1016/j.jechem.2017.04.002 (2017).
Xu, C., Li, B., Du, H. & Kang, F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Ed. Engl. 51, 933–935, https://doi.org/10.1002/anie.201106307 (2012).
Liu, H. et al. Kinetic study on LiFePO4/C nanocomposites synthesized by solid state technique. J. Power Sources 159, 717–720, https://doi.org/10.1016/j.jpowsour.2005.10.098 (2006).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods. 2 edn, 211 (JOHN WILEY & SONS, INC. 2001).
Li, H. et al. Dual-carbon confined SnO2 as ultralong-life anode for Li-ion batteries. Ceram. Int. 45, 7830–7838, https://doi.org/10.1016/j.ceramint.2019.01.090 (2019).
Cao, Q. et al. A novel carbon-coated LiCoO2 as cathode material for lithium ion battery. Electrochem commun. 9, 1228–1232, https://doi.org/10.1016/j.elecom.2007.01.017 (2007).
Acknowledgements
SKS acknowledged the scholarship from “The 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship” as well as “The 90th Anniversary Chulalongkorn University, Ratchadapisek Sompote Fund”. Also, SKH acknowledged Thailand Research Fund (RSA6180008) and Ratchadapisek Sompote Fund, Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
SKH conceived the experiments, SKS conducted the experiments. Data analyses were done by SKS with consultation with SKH The paper was written by SKS and SKH, and all authors contributed to the subsequent drafts and revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khamsanga, S., Pornprasertsuk, R., Yonezawa, T. et al. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci Rep 9, 8441 (2019). https://doi.org/10.1038/s41598-019-44915-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44915-8
This article is cited by
-
Inkjet-printed flexible planar Zn-MnO2 battery on paper substrate
Scientific Reports (2024)
-
Rational Design of Nanostructured MnO2 Cathode for High-performance Aqueous Zinc Ion Batteries
Chemical Research in Chinese Universities (2023)
-
Tuning crystal water of α-MnO2 with enhanced diffusion kinetics for zinc-ion batteries
Journal of Solid State Electrochemistry (2023)
-
Recent advances of Na3V2(PO4)3 as cathode for rechargeable zinc-based batteries
Carbon Letters (2023)
-
In situ synthesis of Bi3+-doped δ-MnO2 cathode to enhance the cycle stability for aqueous zinc-ion batteries
Journal of Solid State Electrochemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.