Abstract
Calmodulin-like (CML) proteins are a class of important Ca2+ sensors in plants, which play vital roles in regulating plant growth and development and response to abiotic stress. Tea plant (Camellia sinensis L.) is the most popular non-alcoholic economic beverage crop around the world. However, the potential functions of CMLs in either tea plants growth or in the response to environmental stresses are still unclear. In the present study, five CsCML genes (CsCML16, CsCML18-1, CsCML18-2, CsCML38, and CsCML42) were isolated from tea plant, and functionally characterized. The CsCML genes showed diverse expression patterns in leaves, roots, old stems, immature stems and flowers of tea plants. To investigate the expression changes of the genes under various abiotic stresses and ABA treatment, time-course experiments were also performed, the results indicated that the expression levels of CsCML16, 18-2 and 42 were significantly induced under low temperature and salt condition, while CsCML38 was induced distinctly under drought stress and ABA treatment. Overall, CsCML genes showed diverse function in tea plant under various stimuli. These results will increase our knowledge of the significance of CsCML genes in tea plant in response to abiotic stresses and hormone treatments.
Similar content being viewed by others
Introduction
Plant growth and development is negatively affected by various abiotic and biotic stresses including low temperatures, salinity, drought, heavy metal toxicity, pathogens and insect attacks1. These stresses impede plant’s growth, survival, geographical distribution and agricultural productivity across the world. Plants undergo multiple physiological and biochemical responses to survive, and these responses depend on different gene expressions to combat and overcome stresses by cross talk of stress signals under different stress conditions2. Signal transduction is accomplished predominantly through a Ca2+-induced conformational change. The Ca2+ signals are perceived by various Ca2+ binding proteins or Ca2+ sensors, including calcineurin-B-like (CBL) proteins, calmodulin (CaM), CaM-like (CML) proteins and calcium-dependent protein kinases (CDPKs). These proteins usually include EF-hand motifs with helix-loop-helix (HLH) structure3. The EF-hand motif is reported as a conserved domain identified in Ca2+ binding proteins that are responsible for cooperative binding with Ca2+ ion4. After binding, the Ca2+ sensor proteins induce a molecule structural change and interact with their downstream target proteins, which couple with changes in Ca2+ concentration to regulate biochemical activities in cells5,6.
Plants possess a large family of unique CML proteins that contain the EF hand structure, which is similar to that in CaM7. CMLs contain 1–6 EF-hand motifs, but do not possess other known functional domains. A number of CMLs have been found in many plant species, such as Arabidopsis8, rice9, tomato10 and Chinese cabbage11. The identified CML proteins have been shown to respond to different stresses and hormone stimuli12,13,14. In Arabidopsis, the expression levels of AtCML8 gene were significantly induced by salinity and salicylic acid (SA)15. Additonally, knockout of AtCML9 improved the tolerance of Arabidopsis under salinity and drought treatments by increasing the contents of amino acids16. In rice, OsCML4 could improve drought tolerance through effectively scavenging of reactive oxygen species (ROS)17. Over ShCML44 was variously expressed in all wild tomato tissues and was intensely up-regulated by cold, drought, salinity and plant hormones treatments18. The aforementioned evidence indicated that CML proteins have critical roles in Ca2+ signal transduction in plant development and adaptation to abiotic stresses.
Tea plant (Camellia sinensis L.) is an important economic crop, which provides leaves for producing non-alcoholic beverage19. As a leaf-harvested crop, tea plant is inevitably confronted with various adverse environment stresses throughout the whole life cycle, such as drought20, salt21 and cold22 stresses, which seriously hinders the development of the tea industry. Although CMLs have been isolated and identified in many plant species, CML-mediated abiotic stress adaptation in tea plant has not been previously investigated, even though it is important to tea growth and production. In this study, in order to identify and characterize CML genes in tea plant, we identified five CsCML genes, and analyzed the gene expression patterns under abiotic stresses conditions and ABA treatment. The results of this study would be helpful to further characterize the abiotic stress responses in tea plants, and the breeding work of new tea germplasm resources with improved stress resistance.
Results
Cloning and sequence analysis of CsCML genes
A total of ten expressed sequence tags (ESTs) of CsCMLs were found from the transcriptome data (SRA accession number: SRR5075641). After removing the sequences with length shorter than 200 bp and splice error, five CsCMLs were finally obtained. They encoded five proteins with 136–201 amino acids, molecular weight of 16.06–22.48 KDa and isoelectric point of 4.14–4.72 (Table 1). All CsCMLs except CsCML18-1 mapped to genes in the tea plant genome (www.plantkingdomgdb.com/tea_tree/)23, and no additional CsCML members were identified. With reference to the sequence similarity with homologous genes from other plant species, the five CsCMLs were named as CsCML16, CsCML18-1, CsCML18-2, CsCML38 and CsCML42. CsCML18-1 has one EF-hand conserved domain, while other four CsCMLs have two EF-hand conserved domains, indicating structural differentiation among CsCML genes (Fig. 1).
Conserved motifs of CsCML proteins. Alignment of the deduced amino acid sequences of CsCML proteins. (A) The sequence logo of the conserved CML domain including the Ca2+ binding EF-hand (a helix-loop-helix structure) motif was determined by MEME using amino acid sequences of CsCML. (B) Structure of the EF-hand domain.
Multiple sequence alignment and phylogenetic analysis
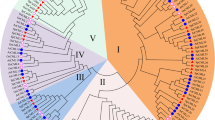
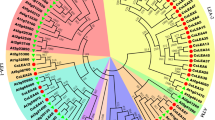
The deduced amino acid sequences of five CsCML were analyzed by multiple sequence alignments. As shown in Fig. 2, CsCML16 and EgCML16 (Erythranthe guttate) clustered in the same branch and showed the highest similarity, followed by ZjCML16 (Ziziphus jujube), PmCML16 (Prunus mume), MdCML16 (Malus domestica) and VvCML16 (Vitis vinifera). CsCML18-1 and CsCML18-2 were classified into the same cluster, indicating that they were paralogous genes. Both CsCML18-1 and CsCML18-2 showed high similarity to CML18 from other plant species. CsCML38 clustered with DcCML38 (Daucus carota subsp. Sativus), followed by RcCML38 (Ricinus communis), PeCML38 (Populus euphratica) and GaCML38 (Gossypium arboretum). For CsCML42, this gene shares highly similarity with ThCML42 (Tarenaya hassleriana), AtCML42 and NcCML42 (Noccaea caerulescens). Phylogenetic tree analysis revealed that the CsCMLs amino acid sequences showed highly homologous to those of other plant species.
Phylogenetic relationship of CsCML proteins with other plant species. Camellia sinensis (CsCML16, CsCML18-1, CsCML18-2, CsCML38, CsCML42), Arachis duranensis (AdCML18, XP_015931565.1), Arabidopsis thaliana (AtCML38, OAP19420.1; AtCML42, NP_193810.1), Capsicum annuum (CaCML18, XP_016560844.1), Daucus carota subsp. Sativus (DcCML38, XP_017246479.1), Erythranthe guttate (EgCML16, XP_012855227.1; EgCML42, XP_010036034.1), Fragaria vesca subsp. vesca (FvCML38, XP_011467704.1), Gossypium arboretum (GaCML38, XP_017621889.1), Ipomoea nil (InCML18, XP_019173083.1; InCML42, XP_019186611.1), Jatropha curcas (JcCML42, XP_012090979.1), Malus domestica (MdCML16, XP_008339727.1), Morus notabilis (MnCML38, XP_010111596.1), Nicotiana attenuata (NaCML18, XP_019257947.1), Noccaea caerulescens (NcCML42, JAU17134.1), Nicotiana tomentosiformis (NtCML18, XP_009628802.1; NtCML38, XP_009596232.2), Nelumbo nucifera (NnCML16, XP_010262279.1; NnCML42, XP_010244597.1), Populus euphratica (PeCML38, XP_011003944.1; PeCML42, XP_011018929.1), Prunus mume (PmCML16, XP_008243136.1; PmCML38, XP_008231145.1; PmCML42, XP_008244386.1), Ricinus communis (RcCML38, XP_002509541.1), Solanum lycopersicum (SlCML16, XP_004237205.1; SlCML18, XP_004233476.1), Sesamum indicum (SiCML18, XP_011099473.1), Theobroma cacao (TcCML42, EOY04379.1), Tarenaya hassleriana (ThCML42, XP_010537462.1), Vigna angularis (VaCML18, XP_017415815.1), Vitis vinifera (VvCML16, XP_003631231.1), Ziziphus jujube (ZjCML16, XP_015895735.1).
Expression analysis of CsCMLs genes in different tissues
Tissue-specific gene expression profile might be associated with the specific physiological and developmental functions in plants. The expression levels of CsCML family gene in different tissues, including leaves, roots, old stems, immature stems and flowers, were examined by qRT-PCR. As shown in Fig. 3, all CsCMLs were expressed in different tissues of tea plant with the expression level of tea roots as control. The expression levels of CsCML16 and CsCML18-1 were remarkably higher in flowers than in other tissues. CsCML16 also had a higher expression level in leaves than as expressed by other genes. However, their expression levels were induced slightly in other tissues, indicating that CsCMLs display tissue-specific expression in tea plant. The expression levels of CsCMLs in different tissues suggest that CsCML genes may have different functional variation in tea plant and this remains to be further investigated.
Expression analysis of CsCMLs genes in tea plant under various abiotic stresses
To explore the expression profiles of the CsCMLs genes under various abiotic stresses, time-course experiments of 5 CsCML genes in two-year-old seedlings treated with low temperature (10 °C), drought (PEG 6000), salinity (NaCl), and hormone (ABA) we performed. Generally, the 5 CsCML genes displayed distinctively expression patterns at different time points after stress treatments (Fig. 4). The expression levels of CsCML16, CsCML18-2 and CsCML42 were induced significantly by cold stress, and these genes showed highest expression levels at 24 h after cold stress. In contrast, the expression level of CsCML18-1 was suppressed by cold stress. The reverse expression patterns between CsCML18-1 and CsCML18-2 revealed a diverse function in paralogs of CML genes in plants. In addition, CsCML38 showed relative stable expressions under cold stress, which indicate that CsCML38 is insensitive to cold stress.
Under drought stress caused by 20% PEG 6000 treatment, the expression of CsCML38 was induced distinctly. However, expressions of CsCML16 and CsCML18-1 were reduced significantly after drought stress. The expression level of CsCML18-2 had a little fluctuation between 0–12 h, but decreased significantly at 24 h, and then showed highest expression levels at 36 h. CsCML42 was obviously inhibited at 4 h, followed by an increase until 24 h, and a decline at 36 h. The results indicate that CsCML genes showed different expression patterns under drought stress. In addition, the expression patterns of CsCMLs under drought stress were significantly different from that under cold and salt stress, suggesting that different response mechanisms were activated under different abiotic stresses (Fig. 5).
ABA, an important plant hormone, plays vital roles in the regulation of plant adaptation to surrounding environment. With the induction of ABA treatment, the expression of CsCML38 showed the highest change, indicating that CsCML38 was very sensitive to ABA treatment. The expression level of CsCML16 significantly increased and reached maximum at 12 h, followed by an acute decrease at 24 h. The expression level of CsCML18-1 rapidly decreased at 4 h, and the induction of CsCML18-1 steadily increased until 24 h. The expression level of CsCML18-2 increased significantly at 4 h, then decreased gradually. The expression level of CsCML42 declined at 2 h, followed by an increase at 12 h, and a decrease at 24 h (Fig. 6).
Salt stress is a common threat which restricts growth and development of plants. After NaCl treatment, the expression levels of CsCML16, CsCML18-2 and CsCML42 were increased significantly with the highest expression levels during 4–24 h after stress. However, the expression of CsCML38 decreased after salt stress, indicating that salt stress could suppress the expression of CsCML38. The expression patterns of CsCML16, CsCML18-2 and CsCML42 under salt stress were similar to that under cold stress, suggesting that these genes may be involved in similar signal pathway in response to salt and cold stresses (Fig. 7).
Discussion
Ca2+ ion is a critical second messenger and could induce various physiological responses in plants in response to diverse stimuli. CML, as a Ca2+ sensor, mediates interpretation of Ca2+ signals in plant cell and also plays an important role in plants respond to various environmental stresses. In the present study, five CML genes namely CsCML16, CsCML18-1, CsCML18-2, CsCML38 and CsCML42 were identified and cloned from tea plant leaves. These genes encode small proteins containing 136–201 amino acids. Apart from CML18-1 protein which contains only one EF-hand motif, four proteins were found to have two EF-hand motifs. The CsCML gene family in tea plants contains EF-hand motifs with no other identifiable functional domains. These findings are in accordance with previously reports in Arabidopsis, rice and tomato10,17,24. Phylogenetic analysis revealed that amino acid sequences of putative CsCMLs from tea plant have high similarity with CMLs from other plant species. These results indicated that the close evolutionary relationship existed in plant CML proteins.
Furthermore, the expression profiles of CsCML in different tissues indicated that CsCML genes were differentially expressed in tea plant. Expression levels of CsCML16 and CsCML18-1 in flowers were significantly higher than in other tissues. Interestingly, the expression levels of CsCML16 and CsCML18 were lower in flowers of Arabidopsis24, whereas AtCML24 and AtCML25 strongly affected the pollen germination and and pollen tube growth25,26. It can; therefore, be suggested that CsCML16 and CsCML18-1 expressed differently to adapt different species. Conversely, expression levels of CsCML18-2, CsCML38 and CsCML42 in roots were significantly higher than in leaves, stem and flower organs, suggesting that different CML gene members from different species have distinct expression levels in various tissues, and may function in different physiological processes. The diversified expression of these CsCML genes revealed that they might play significant role at different plant developmental stages.
It is known that CML genes are involved in response to different environmental stresses and hormones. ABA plays an important role in cellular signal transduction in response to abiotic stresses. It could regulate the expression of several ABA-responsive genes through cellular Ca2+ ion changes27. Over expression of Oryza sativa multi-stress-responsive gene 2 (OsMSR2), which was a novel CML gene, improved the drought and salt tolerance of plants via ABA-mediated pathways28. Subsequently, another novel CML gene named OsDSR1 (Oryza sativa drought stress response-1) showed greater sensitivity to ABA during the development of plant29. OsCML4 could improve drought tolerance by effectively scavenging of ROS in rice17. In Arabidopsis, some studies have reported that AtCML24 was induced by cold and drought stress, suggest that AtCML24 might be involved in cold-related Ca2+ signals transduction30. AtCML37, AtCML39 and AtCML38 were also in response to several stimulus-induced and developmental signalling pathways, including salt, drought, and ABA31, while AtCML42 may function to increase resistance to pathogens32. MtCML40 in Medicago truncatula was reported to be involved in salt, cold tolerance as well as ABA treatment33. Altogether, accumulating evidence has demonstrated that CML proteins play important roles in Ca2+ signal transduction during plant growth and adaptation to abiotic stress.
The transcriptional regulation analysis under abiotic stress and hormones showed that the expression level of CsCMLs was affected in tea plants. The results revealed that the expression levels of CsCML16, CsCML18-2 and CsCML42 were induced significantly under low temperature condition, suggesting that these genes were involved in cold tolerance. Previous investigations have confirmed that CMLs are involved in plant responses to cold stress. For example, cold treatment could induce the expression levels of AtCML24 and OsMSR2, which might participate in cold-induced Ca2+ signal transduction28,30.
Under drought stress, the expression levels of CsCML16 and CsCML18-1 decreased. This result is in contrast with the study by Jung et al.34 who reported that OsCML16 was involved in drought tolerance through enhanced root growth. CML38 is a likely homolog of an endogenous suppressor of antiviral silencing35,36. In the present study, CsCML38 was responsive to all treatments except cold stress. However, the molecular mechanism of CsCML38 in response to abiotic stresses remains unclear. Whether it is similar to other CsCML homologs needs to be verified in the future study. The result reveals that CsCML genes have diverse functions in response to different stimuli. Meanwhile, the role of CsCML genes may be activated by different signals.
Both CsCML18-2 and CsCML42 were up-regulated under low temperature and ABA treatments. This result indicates that CMLs are links of signal pathways which involved in different stimuli. It is in accordance with the role of a CDPK in ABA-dependent cold acclimation37. The up-regulated CsCML16, CsCML18-2 and CsCML42 in the present study suggest that these genes are involved in high salinity tolerance. The function of these salt responsive CML genes in the regulation of salt tolerance may be due to targeting high affinity K+ transporter-dependent Na+ accumulation38.
In summary, our results indicated that CsCMLs possibly function as stress-responsive genes to improve stress tolerance in tea plant. Although the study of CsCML family genes is still largely unexplored; our results, to some extent, advanced the understanding of the biological function of CsCML genes in tea plant and supply theory foundation for breeding stress-resistant tea cultivars.
Materials and Methods
Plant materials and stress treatment
Two-year-old tea plant seedlings (Camellia sinensis cv. Longjing Changye) were used in this study. The tea seedlings were selected and cultured in a growth chamber maintained at 24/22 °C day/night temperature with 75 ± 5% humidity and a photoperiod of 12 h light (200 μmol m−2 s−1) and 12 h dark. To evaluate the expression patterns of CsCML genes in different tissues, the leaves, roots, old stems, immature stems and flowers from the tea plant were collected. To determine the expression changes of the CsCML genes under various abiotic stresses, time-course experiments were carried out. After one week, the plants were treated with low temperature (10 °C), drought [PEG-6000, 20% (w/v)], abscisic acid (ABA, 200 mg/L) and NaCl (12 g/L), respectively. The third leaves from the top of tea plant were picked at 0, 2, 4, 8, 12, 24, 36 and 48 h after treatments. The time point of 0 h was set as control. The picked samples were frozen in liquid nitrogen immediately and stored at −80 °C for RNA extraction. Each sample has three biological replicates.
RNA extraction and cDNA synthesis
Total RNA was isolated using EASYspin Plant RNA Kit (Aidlab, Beijing, China) following manufacturer’s instruction. RNA quality was checked on Agilent 2100 Bioanalyzer (Agilent, USA). First strand cDNA was produced using Revert Aid™ First Strand cDNA Synthesis Kit (Thermo, America) according to manufacturer’s instruction, and stored at −20 °C.
Amplification and cloning of CsCML genes from tea plant
In order to find the CsCML family genes of tea plants, CsCML21 nucleic acid sequence (GenBank accession: JQ999983) and the transcriptome data (SRA accession number: SRR5075641) were used for homogenous alignment by using BLAST program. The ORF regions of CsCML genes were generated by RT-PCR using primers in Table 2. PCR amplification was conducted by the following program: 94 °C for 2 min, 35 cycles of amplification at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min, followed by final extension at 72 °C for 10 min. The PCR products were loaded on 1.2% agarose gels for electrophoretic analysis and purified by a DNA purification kit (Qiagen, Valencia, CA). The purified DNA products was ligated into TOPO-TA vector and transformed into component cells (E. coli DH5α). Three independent clones from each isolate were sequenced by GenScript company (Nanjing, China).
Nucleotide and amino acid sequences analysis
Phylogenetic analysis of the CML gene sequences was carried out by using BLAST program in the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST). The phylogenetic tree was carried out by MEGA 7.0 using the neighbor-joining method. The predictions of possible open reading frame (ORF), theoretical isoelectric point (pI), and the molecular mass (Mw) were performed using online software (https://web.expasy.org/protparam/). Multiple sequence alignments of selected amino acids were conducted using ClustalW software. The MEME Suite software (http://meme-suite.org/tools/meme) was used for identifying sequence of EF-hand motif. The SWISS MODEL server (http://swissmodel.expasy.org/interactive) was used to predict and generate 3D structures of the CML members.
Quantitative real-time PCR (qRT-PCR) analysis
qRT-PCR was performed to investigate the expression profiles of CsCML genes in different tissues and treatments. The cDNA from all tissues and treatments were used as template. The GAPDH (Accession No. GE651107) of tea plants was selected as the reference gene. The primer pairs are listed in Table 3. The qRT-PCR was conducted on a LightCycler®480 software (Roche) using FastStart Universal SYBR Green Master (Rox, Shanghai, China) in a 25 μL reaction mixture, containing 2 μL sample cDNA, 12.5 μL SYBR Green Master mixture, 0.5 μL each specific primer and 9.5 μL nuclease-free water. The qRT-PCR program included 95 °C for 5 min followed by 40 cycles at 95 °C for 10 s, 55 °C for 20 s and 72 °C for 30 s. At the end of experiment, the relative gene expression levels were calculated using the 2−ΔΔCT method39.
Statistical analysis
The statistical analyses were conducted by Microsoft EXCEL 2016 and SPSS 22.0 (https://www.ibm.com/products/spss-statistics). Difference significance analysis was performed using One-way ANOVA analysis and P < 0.05 was considered as significant difference.
References
Zeng, H. et al. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 6, 600 (2015).
Mahajan, S. & Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 444, 139–158 (2005).
Luan, S. et al. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 14, S389–S400 (2002).
Perochon, A., Aldon, D., Galaud, J. P. & Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie. 93, 2048–2053 (2011).
Zielinski, R. E. Characterization of three new members of the Arabidopsis thaliana calmodulin gene family: Conserved and highly diverged members of the gene family functionally complement a yeast calmodulin null. Planta 214, 446–455 (2002).
Gifford, J. L., Walsh, M. P. & Vogel, H. J. Structures and metal-ion-binding properties of the Ca2+-binding helix–loop–helix EF-hand motifs. Biochem. J. 405, 199–221 (2007).
Popescu, S. C. et al. Differential binding of calmodulin-related proteins to their targets revealed through high-density Arabidopsis protein microarrays. PNAS. 104, 4730–4735 (2007).
McCormack, E. & Braam, J. Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 159, 585–598 (2003).
Boonburapong, B. & Buaboocha, T. Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol. 7, 4 (2007).
Munir, S. et al. Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol. Biochem. 102, 167–179 (2016).
Nie, S., Zhang, M. & Zhang, L. G. Genome-wide identification and expression analysis of calmodulin-like (CML) genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genomics 18, 842 (2017).
Ali, G. S. et al. Differential expression of genes encoding calmodulin-binding proteins in response to bacterial pathogens and inducers of defense responses. Plant Mol. Biol. 51, 803–815 (2003).
Liu, H. T. et al. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 132, 1186–1195 (2003).
Wu, X. et al. CML20, an Arabidopsis calmodulin-like protein, negatively regulates guard cell ABA signaling and drought stress tolerance. Front. Plant Sci. 8, 824 (2017).
Park, H. C. et al. AtCML8, a calmodulin-like protein, differentially activating CaM-dependent enzymes in Arabidopsis thaliana. Plant Cell Rep. 29, 1297–1304 (2010).
Magnan, F. et al. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 56, 575–589 (2008).
Yin, X. M. et al. OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 58, 68–73 (2015).
Munir, S. et al. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 6, 31772 (2016).
Koech, R. et al. Identification of novel QTL for black tea quality traits and drought tolerance in tea plants (Camellia sinensis). Tree Genet Genomes 14, 9 (2018).
Xie, H. et al. Global ubiquitome profiling revealed the roles of ubiquitinated proteins in metabolic pathways of tea leaves in responding to drought stress. Sci. Rep. 9(1), 4286 (2019).
Wan, S. Q. et al. Transcriptomic analysis reveals the molecular mechanisms of Camellia sinensis in response to salt stress. Plant Growth Regul. 84(3), 481–492 (2018).
Li, N. N. et al. Transcriptome sequencing dissection of the mechanisms underlying differential cold sensitivity in young and mature leaves of the tea plant (Camellia sinensis). J Plant Physiol. 224, 144–155 (2018).
Xia, E. H. et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol. Plant. 10, 866–877 (2017).
McCormack, E., Tsai, Y. C. & Braam, J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 10, 383–389 (2005).
Wang, S. S. et al. Arabidopsis thaliana CML25 mediates the Ca(2+) regulation of K(+) transmembranetrafficking during pollen germination and tube elongation. Plant Cell Environ. 38(11), 2372 (2015).
Yang, X. et al. Arabidopsis thaliana, calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration. Plant Mol Biol. 86(3), 225–236 (2014).
Kaplan, B. et al. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. The Plant Cell 18, 2733–2748 (2006).
Xu, G. Y. et al. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234, 47–59 (2011).
Yin, X. M. et al. Rice gene OsDSR-1 promotes lateral root development in Arabidopsis under high-potassium conditions. J Plant Biol. 54, 180–189 (2011).
Delk, N. A., Johnson, K. A., Chowdhury, N. I. & Braam, J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 139, 240–253 (2005).
Vanderbeld, B. & Snedden, W. A. Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol Biol. 64, 683–97 (2007).
Vadassery, J. et al. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 159, 1159–1175 (2012).
Zhang, X. et al. Calmodulin-like gene MtCML40 is involved in salt tolerance by regulating MtHKTs transporters in Medicago truncatula. Environ Exp Bot. 157, 79–90 (2019).
Jung, H. et al. Overexpression of OsERF 48 causes regulation of OsCML 16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotech. J. 15, 1295–1308 (2017).
Li, F., Huang, C., Li, Z. & Zhou, X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 10, e1003921 (2014).
Lokdarshi, A. et al. Arabidopsis CML38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol. 170, 1046–1059 (2016).
Li, H. et al. The role of calcium-dependent protein kinase in hydrogen peroxide, nitric oxide and ABA-dependent cold acclimation. J. Exp. Bot. 69, 4127–4139 (2018).
Zhang, X. et al. Calmodulin-like gene MtCML40 is involved in salt tolerance by regulating MtHKTs transporters in Medicago truncatula. Environ. Exp. Bot. 157, 79–90 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31470690), the Earmarked Fund for Modern Agro-industry Technology Research System of China (CARS-19), the National program for student innovation through research and training (20181037024) and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Q.M., Q.Z., C.C., Q.C. and X.L. conceived the experiments. Q.M., Q.C., Y.Z. and K.W. conducted the experiments. E.A., X.C. and K.S. analyzed the results and wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Q., Zhou, Q., Chen, C. et al. Isolation and expression analysis of CsCML genes in response to abiotic stresses in the tea plant (Camellia sinensis). Sci Rep 9, 8211 (2019). https://doi.org/10.1038/s41598-019-44681-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44681-7
This article is cited by
-
Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) family genes in tea plant
BMC Genomics (2022)
-
The effect of stress hormones, UV-C, and stilbene precursors on calmodulin (CaM) and calmodulin-like gene (CML) expression in Vitis amurensis Rupr
Plant Cell, Tissue and Organ Culture (PCTOC) (2021)
-
Transcriptome analysis reveals underlying immune response mechanism of fungal (Penicillium oxalicum) disease in Gastrodia elata Bl. f. glauca S. chow (Orchidaceae)
BMC Plant Biology (2020)
-
Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses
Plant Growth Regulation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.