Abstract
The aim of the study was to investigate the antibacterial effect of arginine (Arg) in NaF toothpaste. 24-h mono-/3-species biofilm cultures of S. mutans, S. sanguis and S. gordonii inoculated sHA discs were subjected to treatment with toothpaste supernatants prepared as - [1]:2% Arg –NaF (0.147% F), [2]:4% Arg–NaF (0.144% F), [3]:8% Arg – NaF (0.138% F), [4]:NaF (0.15%) and [5]:deionized water. After 24-h incubation, the mono-species biofilms were subjected to viability assay using WST-8, SEM and confocal imaging (CLSM). The 3-species biofilm were quantified for bacterial composition by PCR analysis, SEM, CLSM, and RNA isolation with reverse-transcription PCR analysis. Increasing arginine concentrations in NaF toothpaste had no effect on microbial viability. The mono-/3-species biofilm imaging depicted that the 2% Arg-NaF and 4% Arg-NaF had a biofilm disrupting effect. The 3-species biofilm bacterial composition indicated that the 2% Arg-NaF group maintained an ecological homeostasis by inhibiting S. mutans growth and enriching the growth of S. sanguis and S. gordonii. The 2% Arg-NaF group significantly downregulated the expression of virulent gtfB gene and upregulated the expression of sagP with relative dominance of arcA. Incorporation of 2% arginine in NaF toothpaste might enrich the alkali-producing bacteria and provide enhanced counter mechanisms against cariogenic pathogen when compared to NaF toothpaste.
Similar content being viewed by others
Introduction
Considering the global burden of untreated dental caries, especially in the high risk population, the alarming need for caries prevention is evident1. The biofilm-mediated acidogenic/aciduric microbial shift is a proposed factor for the initiation of the caries process. The ecological shift reciprocally suppresses the oral commensals that prevails in the microflora in healthy conditions2. The commensals constitute S. sanguis and S. gordonii (alkali-producing bacteria) that neutralize pH by utilizing salivary/plaque arginine through the arginine deiminase system (ADS)3,4. Hence, caries preventive measures directed towards the enrichment of alkali-producing bacteria with concurrent suppression of cariogenic bacteria (e.g. S. mutans) is potentially beneficial3,4,5.
Fluoride is a known anti-caries agent; however, it has little sustained effect on cariogenic biofilms5,6. A limited antimicrobial effect of sodium fluoride (NaF) at ≥1200 ppm fluoride on S. mutans biofilms has been reported7,8. The effect of fluoride on bacterial metabolism is theorized by inducing cytoplasmic acidification and glycolytic enzymes inhibition9. However, a recent study advocate that the presence of biofilms influences the outcome of anti-caries agent10. Studies have also shown that the presence of biofilms negatively influences NaF anti-caries treatment outcomes8,10. Additionally, long-term use of fluorides has led to the evolution of fluoride-resistance S. mutans11,12. Therefore, augmenting the antibacterial effect of fluorides with biofilm modifiers may help to counter the concerns without reducing its remineralization potential13.
The use of arginine, a prebiotic amino acid, has been shown to affect the oral biofilms ecology14,15,16. Primarily, S. gordonii and S. sanguis metabolize arginine into ornithine, citrulline, ammonia, carbon dioxide and ATP through ADS. These oral bacteria which are associated with a biofilm compatible with health utilize the metabolic byproduct (ATP) for their survival; thereby promoting ecological homeostasis3. Furthermore, presence of these bacteria and their metabolites inhibits the growth of cariogenic bacteria due to a non-conducive alkalogenic environment17. Several studies have found a significant association between saliva/plaque ADS activity and dental caries which suggests that an increase in ADS activity leads to a decrease in dental caries18,19,20,21. In addition, external supplementation of arginine will increase ADS activity. Thus, upregulation of ADS activity by arginine as a biofilm modifier appears promising.
So far, studies have investigated the effect of arginine in low concentration (≤500 ppm F) NaF solution reporting its synergistic effect in maintaining a healthy oral microbial equilibrium22,23. No studies have analyzed the antimicrobial effect of arginine in sodium fluoride solutions ≥1000-ppm, concentrations commonly present in commercial toothpastes. Although the commercially available arginine-fluoride toothpaste contains 1.5% arginine bicarbonate in Na-MFP at a fluoride concentration of 1450 ppm24,25,26,27,28, the optimum concentration of arginine in NaF toothpaste is yet unexplored. Albeit the evidence contemplates the prebiotic effect of arginine to counter dental caries; a previous study examining the remineralization potential of arginine in 1100-ppm fluoride toothpaste concluded that 2% arginine in NaF toothpaste significantly increased the remineralization of artificial incipient enamel lesion compared to the control toothpaste containing NaF alone29. Given the prebiotic effect of arginine, the microbial effect of arginine-NaF blend on multi-species ecology is worth further investigation. Thus, this study aimed to evaluate the effect of different concentrations of arginine in NaF toothpaste on mono-species/3(multi)-species biofilm, simulating environment in high caries-risk patients. The null hypothesis in this study was that incorporating arginine in NaF toothpaste does not have an additional antibacterial effect as compared to NaF alone.
Results
Test agent characterization
Figure 1 shows representative mean UV-visible absorption spectra of different test agents characterized at different time-points in the entire experimental process. The characteristic peak for all the test agents fall within the UV spectra (200–400 nm). The presence of peaks in the UV spectra from all test agents suggested that the color change observed during the preparation of dentifrice slurries from control was non-contributory, since none of the characteristic peaks were measured in the visible spectra. Therefore, the test solution characterization advocated that incorporating arginine in NaF toothpaste might not cause any aesthetic-based deleterious effect to hard tissues.
Mono-species biofilm microbial cell-viability
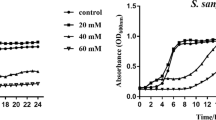
Microbial cell-viability of mono-species biofilm treated with different test agents is presented in Fig. 2. Kruskal-Wallis one-way ANOVA identified a statistically significant difference among the different test and control groups in individual mono-species biofilm (p < 0.001). All the dentifrice slurries had a significantly greater inhibitory effect on S. mutans and S. sanguis when compared to deionized water (DIW) (p < 0.05), with no significant difference among the different Arg-NaF dentifrice slurries and NaF (p > 0.05). Conversely, NaF has a significantly higher inhibitory effect on S. gordonii than DIW (p < 0.05). No significant difference in S. gordonii viability was observed among the different Arg-NaF dentifrice slurries and NaF (p > 0.05). Increasing the concentrations of arginine in NaF toothpaste has no effect on the viability of S. mutans, S. sanguis and S. gordonii in mono-species biofilm.
Microbial cell viability using WST-8 assay for mono-species - S.mutans, S.sanguis, S.gordonii biofilm subjected to different treatments (2%, 4%, 8% Arg-NaF, NaF, and DIW). The lowercase letters (a,b)/uppercase letters (A,B)/Greek alphabets (αβ) represent differences between different test groups as analysed by Kruskal-Wallis 1-way ANOVA followed by Dunn’s post-hoc test.
Mono-/3-species biofilm imaging (SEM & CLSM)
The SEM analysis showed that both 2% Arg-NaF and 4% Arg-NaF had a biofilm disrupting effect on S. mutans mono-species and 3-species biofilms (Figs 3 and 4). The biofilm surface topography for S. mutans mono-species and 3-species appeared similar for biofilms treated with 8% Arg-NaF, NaF, and DIW, revealing that these test solutions had minimal effect on cariogenic biofilms (Figs 3 and 4). The SEM images at 6000-x depicted that NaF inhibited the formation of S. sanguis and S. gordonii biofilms; whereas arginine-incorporated NaF upregulated the formation of S. sanguis and S. gordonii biofilms (Fig. 3).
Figures 4 and 5 present confocal imaging (100-x magnification) of treated 3-species and mono-species biofilms, respectively. The live/dead bacteria ratio determined using the confocal images is shown in Fig. 6. One-way ANOVA revealed statistically significant difference in the log-transformed live/dead bacterial vitality ratio of the mono- and 3-species biofilms treated with different test agents (p < 0.001). For S. mutans biofilm, the live/dead bacteria ratio was significantly lower in the 2% Arg-NaF group than the other groups (p < 0.05). The S. sanguis biofilms treated with 2% Arg-NaF and DIW presented with significantly higher live/dead ratio when compared to 4% Arg-NaF, 8% Arg-NaF and NaF (p < 0.05). Conversely, the live/dead ratio for S. gordonii biofilms treated with Arg-NaF and DIW were significantly higher when compared to NaF (alone).
Live/dead bacteria ratio analyzed using confocal imaging (100x) for mono-/3-species biofilm with S.mutans, S.sanguis, S.gordonii. The difference between test agents (2%, 4%, 8% Arg-NaF, NaF, and DIW) treated biofilms for data analyzed with 1-way ANOVA followed by SNK test is represented by lowercase letters (a,b,c), uppercase letters (A,B), Greek alphabets i.e. α,β/Greek alphabets with signs i.e. +, δ, θ.
Overall, the mono- and 3-species biofilm imaging indicated that 2% Arg-NaF had a biofilm disrupting effect by inhibiting the growth of cariogenic S. mutans and it subsequently enriched the growth of S. sanguis and S. gordonii. Although both 4% Arg-NaF and 8% Arg-NaF enriched the growth of S. sanguis and S. gordonii, their inhibitory effect on S. mutans was lowered. Moreover, in the 3-species biofilm, the live/dead bacterial vitality ratio of 2% Arg-NaF is similar to that of NaF, suggesting comparable vitality of biofilm enriched bacteria as that of control.
3-species biofilm bacterial composition
The total bacterial composition in 3-species biofilm treated with different test agents determined by DNA extraction and real-time PCR analysis is shown in Fig. 7. The values obtained through PCR analysis were converted to CFU/ml. Figure 7 presents the mean proportional total bacterial composition of biofilms treated with different test agents at disparate time-points. The data was log transformed to satisfy the assumption of normality. Two-way ANOVA identified significant differences in the different groups (p < 0.01), bacteria (p < 0.001) and their interaction (p < 0.001). A statistically significant difference in the S. mutans composition was observed across the different test and control groups (p < 0.05). The 2% Arg-NaF and 4% Arg-NaF showed significantly higher inhibition of S. mutans growth when compared to 8% Arg-NaF and NaF (p < 0.05). The proportion of S. sanguis in the biofilms treated with the Arg-NaF and NaF slurries was similar (p > 0.05) and significantly higher than DIW (p < 0.05). The S. gordonii proportional composition was significantly lower for biofilms treated with DIW than all the dentifrice slurries (p < 0.05). Overall, the results of the 3-species biofilm bacterial composition treated with different test solutions indicated that 2% Arg-NaF maintained an ecological homeostasis by inhibiting S. mutans growth and enriching growth of S. sanguis and S. gordonii.
Effect on total bacterial composition in 3-species biofilm (S.mutans, S.sanguis, S.gordonii) treated with different test agents (2%, 4%, 8% Arg-NaF, NaF, and DIW) represented as proportions per treatment based on mean from 3 repeated time-points. The lowercase letters (a–c), numerals (I,II), Greek alphabets (α,β,δ) represent differences between different test groups as analyzed by 2-way ANOVA with Bonferroni’s correction.
Gene expression profile
Figure 8 presents the relative expression of genes – gtfB, sagP and arcA. Friedman’s non-parametric ANOVA showed a statistically significant difference in the treatment groups (p < 0.001) and genes (p = 0.002). Considering the relative genes expression, the gtfB expression for 2% Arg-NaF treated biofilms was significantly lower than 4% Arg-NaF (p = 0.014) but similar to NaF; while sagP expression for 2% Arg-NaF, 4% Arg-NaF, NaF was significantly higher than DIW-treated biofilms (p = 0.001). There was no significant difference in arcA expression of biofilms treated with different test solutions (p = 0.101).
Target gene expression in 3-species biofilm (S.mutans, S.sanguis, S.gordonii) treated with different test agents (2%, 4%, 8% Arg-NaF, NaF, and DIW) represented as proportions of relative gene expression for (gtfB, sagP, arcA). The lowercase letters (a,b), uppercase letters (A,B), numerals (I, II), Greek alphabets (α,β), signs (+) represent differences between different gene expressions analyzed by Kruskal-Wallis H test with Dunn’s post-hoc test. The signs i.e. ^,^^, * represent differences between different test groups. Statistical differences for genes: gtfB - 2% Arg-NaF < 4% Arg-NaF; sagP – 2% Arg-NaF, 4% Arg-NaF, NaF > DIW. The factors analysis was done using Friedman’s ANOVA with Dunn’s post-hoc test (Factor 1: Groups; Factor 2: Genes).
The forbye analysis further identified that for both 2% Arg-NaF and 4% Arg-NaF-treated biofilms, sagP expression was significantly higher when compared to gtfB and arcA (p < 0.001). The relative gene expression profile of treated biofilms indicated that 2% Arg-NaF downregulated the expression of virulent gtfB gene and significantly upregulated the expression of sagP with relative dominance of arcA, thereby demonstrating a promising anti-caries approach to counter virulent cariogenic biofilms and possible maintenance of ecological homeostasis.
Discussion
Based on the findings, the null hypothesis in the present study was rejected. It was observed that 2% Arg-NaF exhibited an enhanced antimicrobial effect on cariogenic S. mutans biofilms (mono-/3-species) with concurrent enrichment of S. sanguis and S. gordonii – ADS positive bacteria and an additional biofilm disrupting effect compared to the control – NaF toothpaste (alone). The observation was supported by the microbial cell viability using WST-8 assay, biofilm imaging (SEM and CLSM) and DNA/RNA extraction with subsequent PCR analysis. In this study, a mono-/3-species biofilm model simulating biofilms covering white spot carious lesions in sucrose-exposure conditions was adopted. Being non-mutans streptococci and ADS-positive bacteria, S. sanguis and S. gordonii are early colonizers that still form major bacterial group in white spot carious lesions and hence, were used in this study. S. mutans, a virulent cariogenic bacteria, was included in the study since its proportion in plaque covering early enamel carious lesions is higher as compared to healthy tooth sites2.
In the present study, WST-8 assay showed no significant difference in microbial viability among the 2% Arg-NaF, 4% Arg – NaF, 8% Arg – NaF and NaF groups suggesting that increasing the concentrations of arginine in NaF toothpaste had no effect on microbial viability of mono-species biofilm. It is quite possible that the microbial viability will be affected in multi-species biofilm as arginine is known to enrich alkali-producing bacteria by utilizing ADS system with concurrent suppression of S. mutans as found in previous studies3,15,30.
The biofilm imaging results indicated that both 2% Arg-NaF and 4% Arg-NaF had a biofilm disrupting effect on mono-species S. mutans and 3-species biofilm. The disrupting effect in 3-species biofilm is mainly seen in S. mutans biofilms. This is beneficial from the remineralization aspect, as presence of biofilm can reduce the effect of anti-caries agent10. Additionally, it has been shown in a previous study that 2% Arg-NaF enhanced the remineralization effect of NaF toothpaste29. Therefore, it appears that 2% Arg-NaF might have a potential anti-caries effect considering its enhanced antibacterial effect on cariogenic biofilms.
The biofilm disrupting effect that 2% Arg-NaF and 4% Arg-NaF has demonstrated in the present study could be due to either the formation of arginine-fluoride complex, which further enhanced the cytoplasmic acidification of S. mutans by fluorides or the ecological pressure exerted by the abundance of S. sanguis and S. gordonii with the supplementation of arginine. It has been shown that L-arginine supplementation can modify extracellular polymeric substances (EPS) with a reduction in the number of S. mutans in mixed-species biofilm due to an increase in the number of S. gordonii31. The thwarting effect on S. mutans outgrowth by L-arginine as explained by the study could be true in this study since a 3-species-biofilm model comprising S. mutans, S. sanguis and S. gordini was adopted. However, the increased ecological pressure encountered could be due to S. sanguis as opposed to S. gordini because the bacterial dominance of S. sanguis was higher compared to S. gordonii. Similarly, the gene expression of sagP was significantly higher as compared to arcA in 2% Arg-NaF-treated multi-species biofilm. Also for 2% Arg-NaF, this explanation is obvious as reflected by the live/dead bacteria ratio computed from CLSM of mono-species biofilm, whereby 2% Arg-NaF demonstrated a significant reduction in S. mutans and a predominant enhancement of S. sanguis and mild but contributory enrichment of S. gordonii.
The total bacterial composition analyzed quantitatively using real-time PCR indicates that 2% Arg-NaF might provide a substantial ecological homeostasis by inhibiting the growth of S. mutans with simultaneous increase in the proportion of S. sanguis and S. gordonii. These results are in concordance with those of a previous study which showed that NaF/Arginine suppressed S. mutans and subsequent EPS production23. However, on close analysis, it is quite evident that S. mutans was still dominant in the composition, irrespective of the treatment, which the biofilms had received. This could be due to the perpetual source of sucrose provided to the biofilms, as the growth of S. mutans increased in the presence of sucrose32. The model used for the present study simulated conditions in high caries-risk patients and thus justifies the use of 2% Arg-NaF for better ecological homeostasis when compared to the control NaF toothpaste. The enhanced inhibitory effect on S. mutans by 2% Arg-NaF and the subsequent enrichment of S. sanguis and S. gordonii, as explained by the evaluations in mono-species biofilm microbial viability and biofilm imaging, could be the potential reasons.
Moreover, it has been shown that in the presence of arginolytic commensal streptococci, the growth and virulence of cariogenic pathogens are inhibited, which can be an additional explanation of the observed effect of 2% Arg-NaF on the studied multi-species biofilm12. It is also noteworthy, that 4% Arg-NaF had similar effect on total bacterial composition in the biofilm as that of 2% Arg-NaF. This shows that 4% Arg- NaF had similar antimicrobial effect as 2% Arg-NaF in a multi-species biofilm. However, in a previous study evaluating the combined remineralization effect of Arg-NaF toothpaste, 4% Arg-NaF did not demonstrate statistically significant remineralization effect when compared to NaF toothpaste29. Thus, the combined results of this and the previous study suggest that 2% Arg-NaF has a potential anti-caries effect. The results of the present study further support that 2% Arg-NaF might substantially contribute to ecological homeostasis and biofilm disruption in high-risk patients.
S. mutans is identified as cariogenic mainly due to its acidogenic and aciduric properties. During dental caries development, sucrose (fermentable carbohydrates) is used for the production of exopolysaccharides (mostly insoluble glucans) through the bacterial (S. mutans) exo-enzyme glucosyltransferases (gtfs). S. mutans activity with gtfB is known to increase during cariogenic shift of the biofilm33. The sagP (S. sanguinis) and arcA (S. gordonii) are ADS-positive genes involved in the arginine metabolism through ADS activity19,21. It has been shown that arginine supplementation can increase the expression of arcA gene when compared to no supplementation to biofilms31. Moreover, the arcA and sagP expressions are increased in caries-free subjects when compared to caries-affected subjects19,21. Hence, the increased expression of arcA and sagP genes with subsequent suppression of gftB is desirable for effective caries management. In the present study, 2% Arg-NaF significantly inhibited the expression of gtfB gene with concurrent significantly enhanced expression of sagP gene. The effect on arcA expression was statistically similar to that of gtfB but was relatively dominant. The gene expression in the 2% Arg-NaF-treated biofilms signified that the treatment had a direct effect on gtfs, which might inhibit the production of insoluble glucans32, modifying the EPS matrix considerably. This could also be the factor responsible for the observed biofilm disrupting effect of 2% Arg-NaF. Also, the gene expression in the 2% Arg-NaF-treated biofilms provided new insights that supplementation of arginine at 2% by wt. enhanced expression of sagP, but did not induce considerable arcA expression as found in a previous study31. Therefore, in a multi-species biofilm, the effect of arginine on S. sanguis (sagP) might be predominant when compared to S. gordonii (arcA).
Further studies are required to understand how 2% Arg-NaF might behave in a long-term biofilm model, specifically highlighting its effect on biofilm matrix and bacterial interaction. Additionally, clinical exploration or bioreactor simulations with more complex inoculum of the tested formulations in the current study might provide different insights, since the study is in vitro and has inherent limitations. Considering the availability of arginine-fluoride combination in a commercial dentifrice, increasing arginine concentrations by weight eventually had reduced F concentrations. The preparation technique was based on our previous study which examined the remineralization potential of the combinations examined in the present study29. Despite the limitations, the present study shows how arginine-NaF blend can further enhance the anti-caries effect of NaF toothpaste (alone) with an optimum (2% by wt.) concentration of arginine as opposed to 8% arginine that might further impair the existing effect of NaF. Also, this study confirms that NaF toothpaste alone might not have substantial antimicrobial effect on multi-species biofilm, especially in high caries-risk patients and thus demands supplementation with a biofilm modifier whereby arginine can play a pivotal role.
Methods
Preparation and characterization of test agents
A NaF toothpaste (Colgate Triple Action, Colgate-Palmolive Company, USA), milled arginine (L-arginine monohydrochloride, Sigma-Aldrich, St. Louis, USA) and sterilized deionized water (DIW) were used to prepare fresh dentifrice slurry for biofilm treatment as per concentrations listed in Table 1. A standard ratio of 1:3 (dentifrice with/without arginine:DIW) was vortexed at 60 s at room temperature to prepare homogenized aqueous solution. The homogenized solution was then centrifuged at 4000 rpm for 20 mins (Hitachi high-speed centrifuge CR22N, Japan) at 25 °C. The sediment was discarded and the supernatant was transferred to a sterile tube for biofilm treatment and characterization34.
The freshly prepared test arginine-NaF solutions were analyzed for UV-visible characteristics because a notable change in color (as compared to NaF alone) was identified after incorporating the arginine. Randomly selected test solutions during different experimental phases were transferred to 96-well microplate to measure absorbance for UV-visible spectra (200–900 nm) at every 5 nm step increment using microplate reader35. The values thus obtained for each group-specific toothpaste supernatant was then summarized as mean to assess spectrum.
Bacterial strains and mono-species/3-species biofilm model
S. mutans UA159 (ATCC 700610), S. sanguis DSS-10 (ATCC 10556), and S. gordonii DL1 (ATCC 35105) were cultured at 37 °C under anaerobic condition (85% N2, 10% H2, 5% CO2) in brain heart infusion broth (BHI). Cell pellet of S. mutans, S. sanguinis, S. gordonii was adjusted to a concentration of 107 CFU/ml in BHI for biofilm growth.
Artificial saliva-coated hydroxyapaptite (sHA) discs (5 mm in diameter × 2 mm thickness; Clarkson Chromatography Products, Inc., South Williamsport, PA, USA) were used for biofilm growth. The disc dimensions were matched to estimate clinical dimensions of early enamel carious lesions. The manufacturer delivered HA discs were autoclaved and inserted in sterilized (autoclaved) artificial saliva (Phenol red, 4% NaOH, CaCl2, MgCl2.H2O, KH2PO4, KCl, HEPES, NaN3 - 1 ml/disc) for 1 h in incubator at 37 °C to simulate salivary pellicle formation. Mono-species and 3-species biofilm (inoculum ratio - 1:1:1 of S. mutans: S. sanguis: S. gordonii) were inoculated on sHA discs with BHI containing 1% sucrose (adjusted to pH 7) in anaerobic chamber (85% N2, 10% H2, 5% CO2) for 24 h. The biofilms were then dip-washed in sterile PBS (1 ml/disc) before receiving treatment with the test solutions. The treatment regimen for group-restricted test solutions (toothpaste supernatant) was: Group 1: 2% arginine – NaF (0.147% F) (2% Arg-NaF), Group 2: 4% arginine – NaF (0.144% F) (4% Arg-NaF), Group 3: 8% arginine – NaF (0.138% F) (8% Arg-NaF), Group 4 (toothpaste supernatant control): NaF (0.15% F) and Group 5: sterilized DIW. The biofilms were then transferred to the respective treatment solutions (180 µl/disc) for 1 min with constant agitation on a shaking incubator (80 rpm, 37 °C). The biofilms were then dip-washed in 1 ml/disc PBS for further transfer to wells containing BHI with 1% sucrose for 24 h anaerobic incubation (85% N2, 10% H2, 5% CO2). After 24 h, the biofilms were subjected to different characterizations. The group-specific mono-species biofilm were subjected to microbial cell viability assay, scanning electron microscopy (SEM) and confocal imaging. The 3-species biofilm were quantified for bacterial composition by DNA extraction and real-time polymerase chain reaction (PCR) analysis, SEM, confocal laser scanning microscopy, and RNA isolation for quantitative reverse-transcription real-time PCR analysis.
Microbial cell viability in mono-species biofilm
For each test group, inoculated treated biofilms on three sHA discs were transferred to a sterile tube with 1 ml PBS further vortexed for 60 s to receive biofilm suspensions. Aliquots of suspension were used for colorimetric microbial viability detection using a microbial viability assay kit (Dojindo Laboratories, Japan) – water-soluble tetrazolium salt (WST-8). Prior to assay, the coloring reagent was freshly prepared by mixing 9 parts of WST-8 solution with 1 part of diluted electron mediator reagent (mPMS) with dimethyl sulfoxide (DMSO). A 190-µl suspension aliquot was transferred to each well of a 96-well microplate followed by addition of 10-µl coloring reagent. The microplate was incubated for 2 h at 37 °C. After incubation, the absorbance was measured at 450 nm using microplate reader. The assay was performed in duplicate per biofilm suspension with repetitions at three time-points.
Scanning electron microscopy
Surface topographic biofilm (mono-species/3-species) assessment was done using SEM (SU1510, Hitachi, Japan) at 15 kV, 6000x. The biofilms were fixed with 2.5% glutaraldehyde in PBS (1 ml/disc) overnight. The biofilms were then serially dehydrated with graded series of ethanol concentrations (70%, 85%, 95%, 100%) at every ½ h. The biofilms were then air-dried at room temperature for 1 h, following which were subjected to sputtering (MSP-2S, IXRF systems, USA) with palladium and platinum (120 secs/disc). The sHA discs with inoculated biofilms were mounted on stubs for further analysis. Images subjected to qualitative assessment were captured at different randomly selected areas within the confines of the discs at 6000-x magnification.
Confocal imaging of biofilms
Confocal laser scanning microscopy (CLSM) of biofilms was done with CLS Biological Microscope (Olympus, FLUOVIEW FV1000, USA) – a two-photon laser scanning microscope. Prior to initializing scan, the treated biofilms were stained with LIVE/DEAD® BacLight™ Bacterial Viability Kit (SYTO 9/propidium iodide – 1:1 solution in DMSO) L7012 (Invitrogen detection technologies, USA) for 15 mins incubated at room temperature in dark. The biofilms on the sHA discs were transferred to circular coverslips mounted on the fluorescence microscope. Images were taken at 100-x magnification with three randomly selected view fields per biofilm. The images were then examined with Leica QWin v. 2.6. (Leica Microsystems Imaging Solutions, Germany) to determine the percent area with live and dead cells. A ratio of live/dead cells was calculated. The image examination to identify live/dead cells was done in triplicate per biofilm.
Total bacterial composition in 3-species biofilm
Biofilms were transferred to 1 ml PBS sterile tube to receive 60 s vortexed biofilm suspensions. The biofilm suspension was centrifuged at 7600 g for 10 mins. Then, the supernatant was decanted. The pellet was resuspended in 50 mM EDTA at pH 8.0 with 20 mg/ml lyzoyme and kept in water bath at 37 °C for 1 h. Then, the QIAamp Mini DNA kit (Qiagen, Hilden, Germany) was used to perform the DNA extraction as per manufacturer instructions. DNA extracted from S. mutans (ATCC 700610), S. sanguis (ATCC 10556) and S. gordonii (ATCC 35105) were used as the positive controls. The primers used for real-time PCR in the present study are listed in Table 2. Oligoneucleotide primers (Table 2) and probes (S. mutans P is 5′-FAM-TGGAAATGACGGTCGCCGTTATGAA-TAMRA-3′) were used (Applied Biosystems, USA). For each real-time PCR reaction (S. mutans), 20 µl of a mixture containing 5 µl of biofilm suspension, 1x Taqman Universal PCR Mix (Applied Biosystems, USA), 200 nM of forward (F) and reverse (R) primers and 250 nM Taqman Probe was prepared. The reaction was performed using Step One Plus (Applied Biosystems, USA). The cycle condition was: 50 °C/2 mins; 95 °C/10 mins; 50 cycles of 95 °C/15 sec and 58 °C/1 min. The standard curve was performed with the DNA of control ATCC S. mutans strain containing 5 × 103 – 1 × 107 CFU/ml. For each S. sanguis and S. gordonii real-time PCR reaction, 20 µl of a mixture containing 5 µl of biofilm suspension, 1x SYBR Green Master Mix (Applied Biosystems, USA) and 200 nM of F/R primers were used. The reaction was performed using Step One Plus (Applied Biosystems, USA). The cycle condition was: 95 °C/20 s; 50 cycles of 95 °C/3 s and 58 °C/30 s; and following melt-curve: 95 °C/15 s; 60 °C/1 min. The standard curve was performed with the DNA of control ATCC S. sanguis and S. gordonii strain containing 1 × 104 – 1 × 109 CFU/ml.
Relative target gene expression
Three sHA discs per test group inoculated biofilms were vortexed for 60 s in 1 ml of PBS to receive suspensions. The suspension was centrifuged at 7600 g for 10 mins. Then, the supernatant was decanted. The pellet was re-suspended in freshly-prepared TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.0) containing 0.4 mg/ml lyzoyme and kept in water bath at room temperature for 15 mins. Then, the SV Total RNA Isolation kit (Promega, Madison, USA) was used to extract the RNA as per manufacturer instructions. The purity of RNA was verified by absorbance measurement at A260/280 and run by gel electrophoresis. cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen detection technologies, USA) as per manufacturer instructions. Oligoneucleotide primers (Applied Biosystems, USA) as listed in Table 2 were used to quantify relative gene expression31. The relative quantification of gtfB, sagP, and arcA genes was performed using 16S rRNA as a reference gene; an internal control for quantification. For each real-time PCR reaction, 20 µl of a mixture containing 5 µl of suspension, 1x SYBR Green Master Mix (Applied Biosystems, USA) and 200 nM of F/R primers were used. The reaction was performed using Step One Plus (Applied Biosystems, USA). The cycle condition was: 95 °C/20 s; 50 cycles of 95 °C/3 s and 58 °C/30 s; and following melt-curve: 95 °C/15 s; 60 °C/1 min including appropriate negative and positive controls.
Statistical analysis
All experiments were repeated at least three times at disparate points. The statistical analysis of obtained data collocated in MS Office Excel 2016 (Microsoft Office Professional Plus 2016, Microsoft, USA) was done using SPSS v. 24 (IBM SPSS® Statistics Inc, USA). Data was assessed for normality and homogeneity of variances using Shapiro-Wilk test and Levene’s test, respectively. The statistical differences amongst the test groups for microbial cell viability was determined using Kruskal-Wallis One-way ANOVA with Dunn’s post-hoc test. The data for live/dead bacteria ratio was log transformed for homogeneity of variances and conversion to achieve the vital (live) cells existence subtracted by dead bacteria. The log-transformed data was further analyzed using one-way ANOVA followed by Student-Newman-Keuls (SNK) test for multiple comparisons. DNA and RNA quantified by real-time PCR in 3-species biofilm was subjected to parametric (factors: treatment and bacteria) and non-parametric (factors: treatment and gene) two-way ANOVA with post-hoc tests, respectively. The level of statistical significance for all tests was at 2-tailed p-value < 0.05.
Conclusion
Within the limitations of the in vitro study, the following conclusions are drawn:
-
1.
The incorporation of 2% arginine in NaF toothpaste significantly enhances the antimicrobial effect against caries-generating bacteria (S. mutans) when compared to NaF (alone), while 4% and 8% arginine in NaF toothpaste were ineffective in enhancing the antimicrobial effect of NaF.
-
2.
The 2% Arg-NaF toothpaste might maintain better ecological homeostasis by upregulating the non-mutans streptococci (S. sanguis and S. gordonii).
-
3.
The 2% Arg-NaF toothpaste significantly downregulates the expression of virulent gtfB gene and upregulates the expression of sagP with relative dominance of arcA.
References
Kassebaum, N. J. et al. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 94, 650–658 (2015).
Takahashi, N. & Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 90, 294–303 (2011).
Burne, R. A. & Marquis, R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193, 1–6 (2000).
Liu, Y.-L., Nascimento, M. & Burne, R. A. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int. J. Oral Sci. 4, 135–140 (2012).
Philip, N., Suneja, B. & Walsh, L. J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 52, 153–165 (2018).
Dang, M. H., Jung, J. E., Lee, D. W., Song, K. Y. & Jeon, J. G. Recovery of Acid Production in Streptococcus mutans Biofilms after Short-Term Fluoride Treatment. Caries Res. 50, 363–371 (2016).
Liu, J., Ling, J. Q., Zhang, K., Huo, L. J. & Ning, Y. Effect of sodium fluoride, ampicillin, and chlorhexidine on Streptococcus mutans biofilm detachment. Antimicrob. Agents Chemother. 56, 4532–4535 (2012).
Fernández, C. E. et al. Effect of Fluoride-Containing Toothpastes on Enamel Demineralization and Streptococcus mutans Biofilm Architecture. Caries Res. 50, 151–158 (2016).
Marquis, R. E. Antimicrobial actions of fluoride for oral bacteria. Can. J Microbiol. 964, 955–964 (1995).
Zhang, M. et al. Biofilm layers affect the treatment outcomes of NaF and nano-hydroxyapatite. J. Dent. Res. 94, 602–607 (2015).
Liao, Y. et al. Fluoride resistance in Streptococcus mutans: a mini review. J. Oral Microbiol. 9, 1344509 (2017).
Cai, Y. et al. The fitness cost of fluoride resistance for different Streptococcus mutans strains in biofilms. Front. Microbiol. 8, 1–8 (2017).
González-Cabezas, C. & Fernández, C. E. Recent Advances in Remineralization Therapies for Caries Lesions. Adv. Dent. Res. 29, 55–59 (2018).
Nascimento, M. M. Potential Uses of Arginine in Dentistry. Adv. Dent. Res. 29, 98–103 (2018).
Zheng, X. et al. Ecological Effect of Arginine on Oral Microbiota. Sci. Rep. 7, 1–10 (2017).
Ledder, R., Mistry, H., Sreenivasan, P., Humphreys, G. & McBain, A. Arginine Exposure Decreases Acidogenesis in Long-Term Oral Biofilm Microcosms. mSphere 2, 1–11 (2017).
Huang, X., Exterkate, R. A. & Ten Cate, J. M. Factors associated with alkali production from arginine in dental biofilms. J. Dent. Res. 91, 1130–1134 (2012).
Bijle, M. N. A., Yiu, C. K. Y. & Ekambaram, M. Can oral ADS activity or arginine levels be a caries risk indicator? A systematic review and meta-analysis. Clin. Oral Investig. 22, 583–596 (2018).
Gordan, V. V. et al. Could alkali production be considered an approach for caries control? Caries Res. 44, 547–554 (2010).
Reyes, E. et al. Caries-free subjects have high levels of urease and arginine deiminase activity. J. Appl. ORAL Sci. 22, 235–240 (2014).
Nascimento, M. M., Gordan, V. V., Garvan, C. W., Browngardt, C. M. & Burne, R. A. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol. Immunol. 24, 89–95 (2009).
Cheng, X. et al. Arginine promotes fluoride uptake into artificial carious lesions in vitro. Aust. Dent. J. 60, 104–111 (2015).
Zheng, X. et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 94, 344–53 (2015).
Ten Cate, J. M. & Cummins, D. Fluoride toothpaste containing 1.5% arginine and insoluble calcium as a new standard of care in caries prevention. J. Clin. Dent. 24, 79–87 (2013).
Li, J., Huang, Z., Mei, L., Li, G. & Li, H. Anti-Caries Effect of Arginine-Containing Formulations in vivo: A Systematic Review and Meta-Analysis. Caries Res. 49, 606–617 (2015).
Srisilapanan, P. et al. Comparing the efficacy of a dentifrice containing 1.5% arginine and 1450ppm fluoride to a dentifrice containing 1450ppm fluoride alone in the management of early coronal caries as assessed using Quantitaive Light-induced Fluorescence. J. Dent. 41(Suppl 2), S29–S34 (2013).
Souza, M. L. et al. Comparing the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of primary root caries. J. Dent. 41(Suppl 2), S35–41 (2013).
Kraivaphan, P. et al. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 47, 582–590 (2013).
Bijle, M. N. A., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 76, 75–82 (2018).
Huang, X. et al. Effect of arginine on the growth and biofilm formation of oral bacteria. Arch. Oral Biol. 82, 256–262 (2017).
He, J. et al. L-arginine modifies the exopolysaccharides matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 198, JB.00021–16 (2016).
Bowen, W. H. & Koo, H. Biology of streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86 (2011).
Hajishengallis, E., Parsaei, Y., Klein, M. I. & Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 32, 1–11 (2016).
Kumar, V. L., Itthagarun, A. & King, N. M. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust. Dent. J. 53, 34–40 (2008).
Targino, A. G. et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J. Mater. Sci. Mater. Med. 25, 2041–2047 (2014).
Acknowledgements
This study was supported by The University of Hong Kong seed fund for basic research 201611159314. The funders have no role in this research.
Author information
Authors and Affiliations
Contributions
Bijle M.N.A.: Contributed to the planning and conduct of the study, collection of the data, statistical analysis, writing all drafts of the manuscript, and approving the final manuscript. Ekambaram M: Co-contributed to the planning and conduct of the study, critically reading, and approving the final manuscript. E.C.M. Lo, C.K.Y. Yiu: Co-contributed to the planning and conduct of the study, analysis, critically reading, and approving the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bijle, M.N.A., Ekambaram, M., Lo, E.C.M. et al. The combined antimicrobial effect of arginine and fluoride toothpaste. Sci Rep 9, 8405 (2019). https://doi.org/10.1038/s41598-019-44612-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44612-6
This article is cited by
-
Evaluation of glycyl-arginine and lysyl-aspartic acid dipeptides for their antimicrobial, antibiofilm, and anticancer potentials
Archives of Microbiology (2023)
-
Combined effect of arginine and fluoride on the growth of Lactobacillus rhamnosus GG
Scientific Reports (2021)
-
Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppression of oxidative stress and stimulation of nitric oxide production in rats
Toxicology and Environmental Health Sciences (2021)
-
Effect of a novel synbiotic on Streptococcus mutans
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.