Abstract
To combat organ shortage in transplantation medicine, a novel strategy has been proposed to generate human organs from exogenous pluripotent stem cells utilizing the developmental mechanisms of pig embryos/foetuses. Genetically modified pigs missing specific organs are key elements in this strategy. In this study, we demonstrate the feasibility of using a genome-editing approach to generate anephrogenic foetuses in a genetically engineered pig model. SALL1 knockout (KO) was successfully induced by injecting genome-editing molecules into the cytoplasm of pig zygotes, which generated the anephrogenic phenotype. Extinguished SALL1 expression and marked dysgenesis of nephron structures were observed in the rudimentary kidney tissue of SALL1-KO foetuses. Biallelic KO mutations of the target gene induced nephrogenic defects; however, biallelic mutations involving small in-frame deletions did not induce the anephrogenic phenotype. Through production of F1 progeny from mutant founder pigs, we identified mutations that could reliably induce the anephrogenic phenotype and hence established a line of fertile SALL1-mutant pigs. Our study lays important technical groundwork for the realization of human kidney regeneration through the use of an empty developmental niche in pig foetuses.
Similar content being viewed by others
Introduction
Critical shortage of donor organs is a serious challenge in the field of organ transplantation that severely limits opportunities to rescue patients with end-stage organ failure. In recent years, there have been efforts to solve this problem through the generation of functional human tissues and organs using pluripotent stem cells such as induced pluripotent stem (iPS) cells1,2,3. However, generating transplantable solid organs in vitro is considered impractical owing to the complex three-dimensional structure and function of human organs4,5. Under such circumstances, the novel concept of growing human organs in vivo by utilizing the developmental mechanisms of porcine embryos genetically engineered to be unable to form a specific organ has attracted considerable attention [for review, see6,7]. The basic approach underlying this concept, considering the pancreas as an example, is to allow a pancreas to develop from exogenous human pluripotent stem cells in growing porcine embryos that lack endogenous pancreatogenesis8. One of the key technical elements in the application of this strategy for the generation of various human organs is the creation of genetically modified pigs in which normal organ formation is inhibited.
The existence of master regulator genes responsible for organ and tissue formation has previously been reported in mammals9,10,11,12. Previous studies in rodents have demonstrated that knockout (KO) of such genes gives rise to phenotypes lacking specific target organs. For example, knocking out Pdx1, Sall1, and Foxn1 can induce phenotypes associated with hypoplasia or complete loss of the pancreas, kidneys, and thymus, respectively13,14,15. In addition, Kobayashi et al.16 and Usui et al.17 successfully produced xenogeneic pancreases in Pdx1 KO mice and allogeneic kidneys in Sall1 KO mice, respectively, using a blastocyst complementation approach. These studies have paved the way for research on human organogenesis that exploits empty developmental niches, the dedicated in vivo environments that are left vacant when the normal development of endogenous organs is disrupted, in animals.
The genetic engineering strategies used to induce organogenesis-disabled phenotypes in rodents have not been verified in pigs, except with regard to the apancreatic phenotype8,18,19. The aim of this study was to determine whether knocking out SALL1, which is known to play a pivotal role in rodent nephrogenesis, could induce the anephrogenic phenotype in pigs. We report here the results obtained after introducing SALL1 mutations in pigs, including the mutation efficiencies after cytoplasmic injection of transcription activator-like effector nucleases (TALENs) and CRISPR/Cas9 in zygotes, the effects of the mutations on kidney formation in foetuses and progeny, and our success in establishing a line of SALL1-mutant pigs characterized by an anephrogenic phenotype.
Results
Efficiency of mutation induction by cytoplasmic injection of SALL1-targeted Platinum TALEN and CRISPR/Cas9 into parthenogenetic embryos
The mutation induction efficiencies of two genome-editing tools, Platinum TALEN20 and CRISPR/Cas9, designed to target exon 3 of porcine SALL1 (Fig. 1A) were investigated following cytoplasmic injection into porcine parthenogenetic embryos at the pronuclear stage. Mutations were induced in over 80% of blastocysts obtained after injection of 2 or 5 ng/μl Platinum TALEN-encoding mRNA (Table 1). However, the blastocyst formation rate was significantly lower after the 5 ng/μl injection than after the 2 ng/µl injection (17.9% vs. 55.8%, P < 0.05), indicating a detrimental effect of TALEN-encoding mRNA with higher concentration.
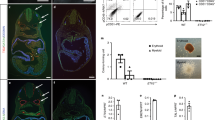
Generation of porcine SALL1 gene knockout foetuses by genome editing. (A) Design of Platinum TALENs and CRISPR/Cas9 targeting the porcine SALL1 gene, which consists of 4 exons, similar to that of humans. The coding and untranslated regions are indicated by black and grey boxes, respectively. A pair of Platinum TALENs (top) and CRISPR/Cas9 (bottom) target the beginning of exon 3 in porcine SALL1. The protospacer adjacent motif (PAM) is a short specific sequence following the target DNA sequence that is essential for cleavage by Cas9 nuclease and is indicated with a white box. (B) Kidney phenotypes of the genome-edited foetuses with mutant SALL1. Foetuses developed from zygotes injected with Platinum TALENs (upper 3 panels) and CRISPR/Cas9 (lower 3 panels) were examined for nephrogenesis on days 36–37 and 39–40 of gestation, respectively. Kidneys with homozygous frameshift mutations (upper and lower middle) exhibited an anephrogenic phenotype. Foetuses harbouring WT SALL1 (13 bp del/WT, upper left) or SALL1 with a small in-frame mutation (6 bp/14 bp del, upper right) developed normal kidneys like those of a WT foetus (lower left). The kidneys of a foetus with complex mosaic mutations (1007 bp/28 bp/12 bp del/WT, lower right) showed mild hypoplasia. Scale bars: 2 mm.
Mutation efficiencies were also compared between Cas9-mRNA-based and Cas9-protein (ribonucleoprotein; RNP)-based CRISPR/Cas9 systems. Blastocyst formation rates and mutation rates were compared for various concentrations of guide RNA (gRNA; 2 to 10 ng/µl) and Cas9 (2 to 20 ng/µl). Blastocyst formation rates were stable (49.4–63.2%) and mutations were consistently detected, except at the lowest concentration (2 ng/µl) of RNP tested (62.0–97.0%; Supplementary Table S1).
These results demonstrated that cytoplasmic injection of the two genome-editing molecules, Platinum TALEN and CRISPR/Cas9, into pronuclear-stage embryos could induce mutations in porcine SALL1 with high efficiency. Subsequent experiments generating mutant foetuses used either 2 ng/µl Platinum TALEN, which achieved a better blastocyst formation rate than the higher concentration, or 20 ng/µl Cas9-RNP, the highest Cas9-RNP concentration and the concentration that achieved the best mutation rate.
Anephrogenic phenotype of SALL1-KO foetuses
SALL1-KO foetuses were generated to test whether SALL1 KO could reliably induce deficiency of nephrogenesis in a porcine model. Platinum TALEN and CRISPR/Cas9 molecules were cytoplasmically injected into in vitro-fertilized embryos at the pronuclear stage.
Platinum TALEN-injected embryos were transplanted into two recipients, yielding 13 viable foetuses after 36–37 days of gestation (Table 2). A wide variety of SALL1 mutations were observed in all foetuses (Supplementary Table S2), and a nephrogenic defect or severe renal hypoplasia was observed in nine (69.2%; Table 2 and Fig. 1B). Genomic DNA analysis revealed homozygous frameshift mutations or large deletions in these individuals (Supplementary Table S2). The tissue of the rudimentary kidneys lacking nephron structures mainly consisted of interstitial cells. Immunohistochemical staining revealed slight yet detectable signals of SALL1 and WT1 (Fig. 2). Together, these results suggested the occurrence of mosaic mutations in founder genome-edited foetuses, indicating that SALL1 gene products, including truncated isoforms, might have been produced in the renal tissue. Rare populations of wild-type (WT) or in-frame mutant cells in the renal tissue could possibly have been left undetected in genomic analysis of the foetal tissues.
Hypoplastic kidneys of SALL1-KO founder foetuses. Foetuses developed from zygotes injected with Platinum TALENs were obtained at day 36–37 of gestation. Left panels: Kidney tissue of a SALL1 heterozygous founder foetus (M231-8; WT/13 bp del). Middle and right panels: Kidney tissue of two homozygous SALL1-KO founder foetuses (M230-1 and M231-6; 5 bp/13 bp del and 1 bp/5 bp del). The homozygous KO foetuses showed severe renal hypoplasia. The signals for SALL1 and WT1 were markedly reduced in the rudimentary kidney tissue of the foetuses with homozygous KO mutations. Scale bars: 500 μm.
In contrast, the remaining four individuals in which nephrogenic defects were not induced had either heterozygous or homozygous mutations involving small in-frame deletions (Fig. 1B and Supplementary Table S2). Truncated SALL1s translated from DNA with only small in-frame mutations seemed to have activity comparable to that of the WT protein.
CRISPR/Cas9-injected embryos were transferred to two recipients, yielding 12 viable foetuses after 39–40 days of gestation (Table 2). Various SALL1 mutations were detected in seven foetuses (58.3%; Supplementary Table S3), and the anephrogenic phenotype was expressed in three (42.9%; Fig. 1B). Mild renal hypoplasia was observed in one mosaic individual (#1705-5) possessing various mutant and WT cells.
Taken together, the above data indicated that SALL1 KO could induce an anephrogenic phenotype or severe renal hypoplasia in pigs, just as it does in mice14.
Additionally, we determined whether any off-target mutation had occurred in the SALL1-KO pig foetuses obtained using Platinum TALEN. No mutations were detected in any of the three off-target sites with the highest target-sequence similarity with the Platinum TALEN used (Supplementary Table S4).
Genotypic and phenotypic variations in founder SALL1-mutant pigs
After confirming the anephrogenic phenotype in the foetuses, we examined whether SALL1 KO resulted in viable offspring with nephrogenic defects. Platinum TALEN was exclusively used for these experiments, considering that its yield of mutant SALL1 foetuses was greater than that of CRISPR/Cas9 in the present study, as described in the previous section. Platinum TALEN-injected embryos were transferred to three recipients, yielding 16 offspring in total (Table 3). Genetic analysis detected mutant SALL1 sequences in 13 offspring (81.3%; Supplementary Table S5); two of these (M253-3 and M253-5) were biallelic mutants with a small in-frame deletion. These founder SALL1 mutants developed normally. In addition, two of the SALL1mutant (mut)/WT piglets (M243-3 and M253-2) had normally developed kidneys. No viable SALL1-KO piglets were born with an anephrogenic or renal hypoplasia phenotype.
Founder animals engineered via cytoplasmic injection of genome-editing molecules in zygotes are prone to mosaic mutations and are characterized by a number of coexisting populations of mutant cells21,22,23,24,25; indeed, over half of the mutant piglets carried mosaic mutations (Supplementary Table S5).
To compare mosaicism rates across different tissues, we explored the variations in SALL1 mutations in tail, kidney, and gonad/germ cells (ovarian tissue or epididymal sperm) from five mosaic offspring (1 female and 4 males). The mutations detected did not differ greatly across the three tissues; however, two mutations were found only in the tail and not in gonads or kidneys (Supplementary Table S6). This finding implies that the mutations detected in tail samples may not be included in germ cells of the mosaic individuals and may therefore not be able to be transmitted to subsequent generations.
Genotypic and phenotypic features of F1 progeny carrying SALL1 mutations
Although we could generate founder SALL1-mutant offspring with high efficiency via cytoplasmic injection of SALL1-targeted Platinum TALEN into in vitro-fertilized pronuclear embryos, not a single SALL1−/− piglet was obtained. To determine whether the SALL1−/− genotype is prenatally lethal in pigs, we investigated whether viable homozygous SALL1-KO offspring could be obtained by crossing two mutant founders carrying frameshift mutations. A total of 11 F1 progeny were delivered by two sows in three litters. All F1 offspring were heterozygous mutants or WT piglets; not a single one had the SALL1−/− genotype (Table 4).
To further investigate the outcome of SALL1-KO mutations, we obtained viable foetuses produced by crossing founder SALL1 mutants (M253-4♂ × M244-1♀, Supplementary Tables S5 and S7) at day 40 of gestation. Twelve F1 foetuses were collected, of which five were homozygous for SALL1 KO (Supplementary Table S7). These five individuals exhibited an anephrogenic or severe renal hypoplasia phenotype, including disorganized formation of a few glomeruli and tubules (Figs 3 and 4A).
Anephrogenic phenotype in F1 progeny carrying SALL1 mutations. F1 foetuses on day 40 of gestation were obtained by mating founder SALL1 mutant pigs. All foetuses with homozygous mutations (lower panels; W307-6, W307-7, W307-8, W307-9, and W307-11) exhibited various phenotypes of renal hypoplasia. Foetuses with heterozygous mutations (upper panels; W307-1, W307-2, W307-4, and W307-10) showed normally formed kidneys like those of a WT foetus (W307-3). Scale bars (white): 2 mm.
Hypoplastic kidneys of F1 progeny carrying SALL1 mutations. Left panels: Kidney tissue of a WT foetus (W307-3). Middle and right panels: Kidney tissue of homozygous SALL1-KO F1 progeny (W307-8; 1 bp ins/13 bp del and W307-11; 1 bp ins/660 bp del) at day 40 of gestation. (A) Histological examination of foetal kidneys by haematoxylin-eosin staining. Homozygous KO foetuses showed kidneys that were disorganized and severely hypoplastic kidneys compared to those of WT foetuses. Black scale bars: 200 µm. (B) Immunofluorescence analysis of kidneys of homozygous SALL1-KO F1 progeny. Upper panels: SALL1, which was expressed in nephron progenitors (*) and distal nephrons (white arrow) in WT foetuses, was absent in homozygous KO foetuses (middle and right panels). WT1 was expressed in proximal nephrons (yellow arrow) and glomeruli (white arrowheads) in WT foetuses, whereas residual glomeruli (white arrowheads) were detected in KO foetuses. Lower panels: SIX2, which was expressed in nephron progenitors (*) in WT foetuses, was absent in homozygous KO foetuses. Ureteric buds (yellow arrowheads), which expressed CDH1 (E-cadherin), were detected only residually in KO foetuses. Yellow scale bars: 100 µm.
Renal tissue from SALL1-KO foetuses and a control WT foetus was subjected to immunohistochemical analysis. In WT tissue, SALL1 was expressed in nephron progenitor cells and distally in immature nephrons, whereas WT1 was expressed in nephron progenitor cells, proximally in immature nephrons, and in glomeruli (Fig. 4B).
In contrast, SALL1 expression was not apparent in the hypoplastic kidney tissue of SALL1−/− foetuses (Fig. 4B). SIX2, which is typically expressed in nephron progenitor cells, was absent from the renal tissue of SALL1−/− foetuses (Fig. 4B). In addition, formation of nascent renal tubules and ureteric buds, indicated by positive signals for CDH1, was limited (Fig. 4B). Analysis of hypoplastic renal tissue of a SALL1660 bp del/1 bp ins foetus (biallelic mutant, 660 bp del/1 bp ins) showed a small number of WT1-positive glomeruli, as well as structures resembling nascent renal tubules or ureteric buds (Fig. 4B). Immunostaining with an antibody that recognizes the C-terminus of SALL1 was performed on rudimentary renal tissue from a different foetus with the same mutation profile (660 bp del/1 bp ins); SALL1 expression could still not be confirmed (Supplementary Fig. S1).
Discussion
The present study demonstrated that SALL1−/− in pigs gave rise to anephrogenic phenotype as shown in mice previously14. Creating a pig line exhibiting the anephrogenic trait is a prerequisite for kidney regeneration via the blastocyst complementation strategy.
Sall1 is an essential gene for kidney formation, and its KO induces nephrogenic defects in mice14. However, when a Sall1 mutation does not lead to loss of function and instead produces a truncated protein, the dominant negative effects of the isoform may cause kidney malformation, as in Townes-Brocks syndrome26,27. To induce an anephrogenic phenotype, therefore, a null mutation of this gene is desirable. For this reason, we designed Platinum TALENs and CRISPR/Cas9 with target sites in the upper N-terminal region of SALL1.
We opted to use cytoplasmic injection in zygotes to generate SALL1-mutant pigs. While this approach has been used successfully to generate gene KO rodents and pigs28,29,30,31,32,33, it has several limitations, including the production of offspring with undesirable mutations and of mosaic individuals harbouring multiple mutations21,22,23,24,25. Indeed, we observed a high incidence of mosaicism in the founder foetuses and offspring obtained in this study. Mosaicism cannot necessarily be detected by DNA analysis of a tissue sample such as a tail snip34,35,36. In fact, some of our founder foetuses that were identified as SALL1−/− during tissue DNA analysis, showed faintly positive signs of SALL1 expression in the rudimentary kidney upon immunohistochemistry, thereby suggesting the presence of a small population of cells carrying an undetected mutation or the WT sequence. The prolonged activity of genome-editing molecules after the first cleavage of a zygote is considered to be responsible for the development of mosaicism37,38. To inhibit the development of mosaicism, an attempt to advance the time of introduction of genome editing molecules into zygotes39 and use of destabilized molecules with shorter reactivity have been reported40. A method for the introduction of Cas9 protein instead of mRNA has also been proposed41,42, even though our preliminary study did not reveal any discernible effect of inhibiting the development of mosaicism. At present, to our knowledge, there is no reliable measure for preventing the development of mosaicism and further studies are awaited.
Renal hypoplasia and nephrogenic defects varied across the SALL1−/− foetuses obtained in the present study. Impaired nephrogenesis in Sall1-deficient mice is not uniform in nature; it can present as missing kidneys and ureters or as unilateral or bilateral renal hypoplasia, with each of the three phenotypes occurring at roughly the same frequency14. This phenotypic variation could be explained by the involvement of multiple genes besides Sall1 in kidney development.
Kidneys form through a mutual inductive interaction between the ureteric bud, which emerges from the mesonephric duct, and the metanephric mesenchyme14. GDNF (glial-cell derived neurotropic factor) is released from the metanephric mesenchyme43,44,45. GDNF is an important humoural factor that guides the elongation of the ureteric bud into the metanephric mesenchyme. Since SALL1 is a transcription factor that upregulates GDNF, reduction or KO of its expression could downregulate GDNF production, thus impeding proper renal development. GDNF is upregulated by other genes besides Sall1, such as Wt1 and Pax246,47,48. The involvement of affiliated genes in the phenotypic variation in renal hypoplasia following SALL1 knockout has yet to be determined. Genetic heterogeneity of the non-inbred domestic pig used in this study might be one of the causes of the phenotypic diversity.
Homozygous Sall1-KO mice can develop to full term, although the KO is neonatally lethal14; however, we failed to obtain any viable SALL1−/− piglets among either mutant founders or F1 progeny generated by crossing two mutant founders. Notably, our F1 litter size per sow was apparently smaller than normal. However, SALL1−/− foetuses were alive in the uterus at day 40 of gestation. Accordingly, we presume that homozygous SALL1 KO is prenatally lethal in pigs during the second trimester of pregnancy or later. This supposition is corroborated by our findings from a separate analysis of cloned SALL1−/− foetuses produced via somatic cell nuclear transfer (in submission).
Difference in prenatal lethality of SALL1-KO mice and pigs may be ascribed to the role of SALL1 during foetal development in both the species. Further studies are needed to elucidate the influence of the loss-of-function mutation in SALL1, which is normally also expressed in non-renal tissues, on the entire embryogenesis process in pigs49,50,51,52. A relatively immature character of mouse foetuses compared to pig foetuses may also be involved in the survival of SALL1-KO mouse to full term.
In conclusion, our current study demonstrates that homozygous SALL1 KO in pigs induces a nephrogenesis-deficient phenotype in foetuses. We identified mutations that could reliably induce the anephrogenic phenotype and established lines of fertile individuals harbouring these mutations. Our study has laid important technical groundwork for the realization of human kidney regeneration through the use of an empty developmental niche in developing pig foetuses.
Methods
Animal care and chemicals
All of the animal experiments including genetic modifications performed in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Meiji University (IACUC-12-0008, IACUC-17-0004). All recombinant DNA experiments performed in this study were approved by the Gene Recombination Experiment Safety Committee (GRESC) of Meiji University (GRESC-13-7, GRESC-18-5). All experiments were performed in accordance with the relevant guidelines and regulations. All chemicals were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) unless otherwise indicated.
Design and preparation of TALENs and CRISPR/Cas9
Both Platinum TALENs and CRISPR/Cas9 (i.e., gRNA) were designed to target the sequence of exon 3 in the pig SALL1 gene (Fig. 1A). The SALL1-targeted Platinum TALEN plasmids were constructed using the Platinum Gate TALEN Kit (Addgene Kit #1000000043) developed by Sakuma et al.53. The Platinum TALEN plasmids to be used as templates for in vitro transcription (IVT) were linearized, and then capped and poly (A)-tailed Platinum TALEN mRNAs were synthesized by using an mMESSAGE mMACHINE T7 Ultra kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. For the CRISPR/Cas9 system, four nucleotide spacers (GCGT) and the T7 promoter were added to the gRNA template by polymerase chain reaction (PCR) amplification using the following primers: 5′-GCGTTAATACGACTCACTATAGGGCCGCCCAGGCCGGCAACC and 5′-AAAAGCACCGACTCGGTGCC. The amplified template was gel-purified and used as the template for IVT using a MEGAshortscript T7 kit (Thermo Fisher Scientific). Cas9 mRNA was synthesized by the methods described above using pCAG-hCas9 (Addgene #51142) as a template for IVT. Cas9 protein was obtained from Takara Bio (Shiga, Japan). The Platinum TALEN-encoding mRNAs, gRNA and Cas9 mRNA were purified using a MEGAclear kit and eluted into RNase-free water. These genome-editing molecules were stored at −80 °C until cytoplasmic injection.
Preparation of pronuclear-stage embryos for cytoplasmic injection
In vitro maturation (IVM) of porcine oocytes was performed as described elsewhere54. Induction of parthenogenesis and in vitro fertilization (IVF) of the IVM oocytes were also performed as reported previously55. For parthenogenetic activation, oocytes were washed twice in an activation solution composed of 280 mM mannitol (Nacalai Tesque, Inc., Kyoto, Japan), 0.05 mM CaCl2, 0.1 mM MgSO4 and 0.01% (w/v) polyvinyl alcohol (PVA). The oocytes were then aligned between two wire electrodes (1.0 mm apart) in a drop of activation solution on a fusion chamber slide (CUY500G1, Nepa Gene, Chiba, Japan). A single direct current pulse of 150 V/mm was applied for 100 µsec using an electrical pulsing machine (LF201; Nepa Gene). Activated oocytes were treated with 5 µg/ml cytochalasin B for 3 h to suppress extrusion of the second polar body.
For IVF, frozen epididymal sperm (1.0 × 109/300 µl) from a WT boar were suspended in 5 ml of Dulbecco’s phosphate-buffered saline (DPBS; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 0.1% bovine serum albumin (BSA; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and washed three times by centrifugation at 1,000 × g for 4 min. After washing, the precipitated sperm pellet was resuspended in porcine fertilization medium (PFM; Research Institute for the Functional Peptides, Yamagata, Japan) at a concentration of 1 × 107 cells/ml56. For insemination, 20 cumulus-oocyte complexes that had been matured in vitro were placed in a 100 µl drop of PFM containing spermatozoa (5.0 × 104–1.0 × 105 cells/ml); the oocytes and sperm were incubated for 8 h at 38.5 °C in a humidified atmosphere containing 5% CO2, 5% O2, and 90% N2. After insemination, the eggs were transferred to HEPES-TL-polyvinylpyrrolidone (PVP); cumulus cells and excess sperm were removed by gentle pipetting. Eggs (i.e., IVM/IVF-derived zygotes) that showed release of polar bodies with normal cytoplasmic morphology were selected for cytoplasmic injection.
Both the parthenogenetic and in vitro-fertilized embryos were confirmed in the preliminary experiments to be at the pronuclear stage.
Cytoplasmic injection
Genome-editing molecules were injected into the cytoplasm of parthenogenetic or IVM/IVF-derived embryos. Cytoplasmic injection was performed by using an inverted microscope equipped with a micromanipulator (MD-102, Narishige, Tokyo, Japan) and an air injector (IG-2, S. Co., Ltd., Tokyo, Japan) at the pronuclear stage, i.e., 4.5–6 h after electrical activation for the parthenotes or 9–10 h after insemination for the IVM/IVF-derived zygotes. Injected embryos were cultured in porcine zygote medium (PZM)-5 medium for 4 days and then cultured until day 7 in PZM-5 supplemented with 10% foetal bovine serum (FBS). After 7 days of culture, the injected embryos were harvested for mutation analysis. For production of foetuses and offspring, injected embryos were transferred at the 1- to 8-cell or blastocyst stage to recipient gilts.
Embryo transfer
Crossbred (Large White/Landrace × Duroc) prepubertal gilts weighing between 100 and 105 kg were used as recipients for the injected embryos. The gilts were each given a single intramuscular injection of 1,000 IU of equine chorionic gonadotropin (eCG, ASKA Pharmaceutical, Tokyo, Japan) to induce oestrus. Ovulation was induced by an intramuscular injection of 1,500 IU of human chorionic gonadotropin (hCG, Kyoritsu Seiyaku, Tokyo, Japan) 66 h after the injection of eCG. The injected embryos cultured for 1–3 days or 5–6 days were surgically transferred into the oviducts of recipient gilts 52 h (day 1–3 embryos) or 149 h (day 5–6 embryos) after hCG injection under general anaesthesia.
Analysis of platinum TALEN- and CRISPR/Cas9-induced mutations in blastocysts, foetuses, and piglets
The target regions of SALL1-targeted TALENs and CRISPR/Cas9 were amplified by direct PCR from blastocysts using MightyAmp DNA Polymerase Ver.2 (Takara Bio). The primer sequences for Platinum TALENs were 5′-ATTAGGCACCAATGTCGGCAG-3′ and 5′-TGCAGAGCTAAGAGCTGCTCC-3′. The primer sequences for CRISPR/Cas9 were 5′-TGATAGGAAGTCCTCCAACAG-3′ and 5′-AAGAACGCCTCGTCGGAAGC-3′. Nested PCR was then performed using PrimeSTAR GXL DNA Polymerase (Takara Bio), and the appropriate primers were 5′-TGATAGGAAGTCCTCCAACAG-3′ and 5′-TCTCGATGATGACGTTGCTG-3′ for TALENs and 5′-TCGAAGTCACAGGTGGCTCC-3′ and 5′-AAGAACGCCTCGTCGGAAGC-3′ for CRISPR/Cas9. Subsequently, the PCR fragments including the target region were examined using a BigDye Terminator Cycle Sequencing Kit and an ABI PRISM 3130xl Genetic Analyzer (Thermo Fisher Scientific). The sequencing primers were 5′-TCGAAGTCACAGGTGGCTCC-3′ for TALEN and 5′-AGCCAGTCTGCCAGCATCAG-3′ for CRISPR/Cas9.
For analysis of mutations in the foetuses and piglets, genomic DNA was extracted from tail biopsies using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), and DNA sequencing was then performed as described above.
To determine the incidence of each mutation type in founder SALL1 mutant piglets, genomic DNA was extracted from tail biopsies, gonads (ovaries or epididymal sperm), and kidneys, and the target regions were amplified as described above. PCR products were cloned and sequenced using a Zero Blunt TOPO PCR Cloning Kit for Sequencing (Thermo Fisher Scientific). The 19 to 33 clones for each piglet were analysed.
Off-target analysis
Off-target sites of SALL1-targeted Platinum TALENs in the pig genome were identified by using the online tool PROGNOS (Predicted Report Of Genome-wide Nuclease Off-target Sites; http://bao.rice.edu/Research/BioinformaticTools/prognos.html)57. Three of the off-target candidate sites with the highest scores (TALEN scores) were analysed (Supplementary Table S4). Genomic DNA was extracted from the founder foetuses derived from zygotes injected with Platinum TALENs, the region including the off-target candidate sites was amplified by PCR using the appropriate primers, and then the amplicons were sequenced (Supplementary Table S4).
Immunohistological analysis
Kidney tissues were fixed in a 4% paraformaldehyde solution (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), embedded in paraffin, sectioned, and stained with haematoxylin-eosin using standard methods. Immunostaining was carried out using a BlueMap Kit and automated Discovery System (Roche) or manually for immunofluorescence staining.
The following primary antibodies were used: anti-SALL1 (Perseus Proteomics, Tokyo, Japan), anti-WT1 (Santa Cruz Biotechnology), anti-WT1 (Abcam), anti-cytokeratin (Sigma-Aldrich), anti-E-cadherin (BD Sciences), anti-SIX2 (Proteintech, Rosemont, IL), and anti-SALL1 (Abcam), which recognizes the C-terminus of SALL1. Secondary antibodies were conjugated with Alexa 488 or 568 (Thermo Fisher Scientific), and the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired using an LSM780 confocal microscope (Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 20.0 software (IBM Corporation, NY, USA). Differences in proportional data between two groups were analysed with the χ2 test or Fisher’s exact test. For comparisons among three groups or more, the data were subjected to arcsine transformation and evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons tests. Differences in blastocyst cell numbers between groups were analysed with ANOVA followed by Tukey’s multiple comparisons tests. The level of significance was set at P < 0.05.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mae, S. I. et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 4, 1367 (2013).
Sharmin, S. et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am Soc. Nephrol. 27, 1778–1791 (2016).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Wu, J. et al. Generation of human organs in pigs via interspecies blastocyst complementation. Reprod. Domest. Anim. 51(Suppl 2), 18–24 (2016).
Wu, J. et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 168, 473–486 (2017).
Rashid, T., Kobayashi, T. & Nakauchi, H. Revisiting the flight of Icarus: making human organs from PSCs with large animal chimeras. Cell Stem Cell 15, 406–409 (2014).
Nagashima, H. & Matsunari, H. Growing human organs in pigs-A dream or reality? Theriogenology 86, 422–426 (2016).
Matsunari, H. et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc. Natl. Acad. Sci. USA 110, 4557–4562 (2013).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995).
Romano, R. et al. FOXN1: A Master Regulator Gene of Thymic Epithelial Development Program. Front Immunol. 4, 187 (2013).
Marcos, S. et al. Meis1 coordinates a network of genes implicated in eye development and microphthalmia. Development 142, 3009–3020 (2015).
Caprioli, A. et al. Wnt4 is essential to normal mammalian lung development. Dev. Biol. 406, 222–234 (2015).
Offileld, M. F. et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983–995 (1996).
Nishinakamura, R. et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128, 3105–3115 (2001).
Goto, T. et al. Hypomorphic phenotype of Foxn1 gene-modified rats by CRISPR/Cas9 system. Transgenic Res. 25, 533–544 (2016).
Kobayashi, T. et al. Generation of Rat Pancreas in Mouse by Interspecific Blastocyst Injection of Pluripotent Stem Cells. Cell 142, 787–799 (2010).
Usui, J. et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am. J. Pathol. 180, 2417–2426 (2012).
Wu, J. et al. CRISPR-Cas9 mediated one-step disabling of pancreatogenesis in pigs. Sci. Rep. 7, 10487 (2017).
Kang, J. D. et al. Arancreatic pigs cloned using Pdx1-disrupted fibroblasts created via TALEN-mediated mutagenesis. Oncotarget 8, 115480–115489 (2017).
Sakuma, T. et al. Repeating pattern of non-RVD variations in DNA-binding modules enhances TALEN activity. Sci. Rep. 3, 3379 (2013).
Li, D. et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31, 681–683 (2013).
Sung, Y. H. et al. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 24, 125–131 (2014).
Sung, Y. H. et al. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 31, 23–24 (2013).
Nakagawa, Y. et al. Screening methods to identify TALEN-mediated knockout mice. Exp. Anim. 63, 79–84 (2014).
Yen, S. T. et al. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev. Biol. 393, 3–9 (2014).
Kohlhase, J. et al. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat. Genet. 18, 81–83 (1998).
Kiefer, S. M. et al. Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum. Mol. Genet. 12, 2221–2227 (2003).
Geurts, A. M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433 (2009).
Tesson, L. et al. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 29, 695–696 (2011).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Shen, B. et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723 (2013).
Carlson, D. F. et al. Efficient TALEN-mediated gene knockout in livestock. Proc. Natl. Acad. Sci. USA 109, 17382–17387 (2012).
Tan, W. et al. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc. Natl. Acad. Sci. USA 110, 16526–16531 (2013).
Fujii, W., Kawasaki, K., Sugiura, K. & Naito, K. Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res. 41, e187 (2013).
Li, C. et al. Simultaneous gene editing by injection of mRNAs encoding transcription activator-like effector nucleases into mouse zygotes. Mol. Cell Biol. 34, 1649–1658 (2014).
Qiu, Z. et al. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic Acids Res. 41, e120 (2013).
Niu, Y. et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156, 836–843 (2014).
Chen, Y. et al. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum. Mol. Genet. 24, 3764–3774 (2015).
Kim, S. et al. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Tu, Z. et al. Promoting Cas9 degradation reduces mosaic mutations in non-human primate embryos. Sci. Rep. 7, 42081 (2017).
Hashimoto, M., Yamashita, Y. & Takemoto, T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 418, 1–9 (2016).
Mehravar, M., Shirazi, A., Nazari, M. & Banan, M. Mosaicism in CRISPR/Cas9-mediated genome editing. Dev. Biol. 445, 156–162 (2019).
Hellmich, H. L. et al. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech. Dev. 54, 95–105 (1996).
Sainio, K. et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124, 4077–4087 (1997).
Plisov, S. Y. et al. TGFb2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128, 1045–1057 (2001).
Schedl, A. Renal abnormalities and their developmental origin. Nat. Rev Genet. 8, 791–802 (2007).
Kreidberg, J. A. et al. WT-1 is required for early kidney development. Cell 74, 679–691 (1993).
Brophy, P. D. et al. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128, 4747–4756 (2001).
Sakaki-Yumoto, M. et al. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development 133, 3005–3013 (2006).
Buttgereit, A. et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397–1406 (2016).
Vodopiutz, J. et al. Homozygous SALL1 mutation causes a novel multiple congenital anomaly-mental retardation syndrome. J. Pediatr. 162, 612–617 (2013).
Morita, Y. et al. Sall1 transiently marks undifferentiated heart precursors and regulates their fate. J. Mol. Cell Cardiol. 92, 158–162 (2016).
Sakuma, T. & Yamamoto, T. Engineering Customized TALENs Using the Platinum Gate TALEN Kit. Methods Mol. Biol. 1338, 61–70 (2016).
Matsunari, H. et al. Hollow fiber vitrification: a novel method for vitrifying multiple embryos in a single device. J. Reprod. Dev. 58, 599–608 (2012).
Nakano, K. et al. Generating porcine chimeras using inner cell mass cells and parthenogenetic preimplantation embryos. PLos One 8, e61900 (2013).
Yoshioka, K., Suzuki, C. & Onishi, A. Defined system for in vitro production of porcine embryos using a single basic medium. J. Reprod. Dev. 54, 208–213 (2008).
Fine, E. J. et al. An online bioinformatics tool predicts zinc finger and TALE nuclease off-target cleavage. Nucleic Acids Res. 42, e42 (2014).
Acknowledgements
The authors thank H. Kadoi for help with maintenance of pigs. This work was supported by grants from the Japan Science and Technology Agency [Exploratory Research for Advanced Technology (ERATO), Nakauchi Stem Cell and Organ Regeneration Project] (to H. Nakauchi), the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18gm0010002 (to H. Nagashima), and Meiji University International Institute for Bio-Resource Research (to H. Nagashima).
Author information
Authors and Affiliations
Contributions
H. Nagashima, R.N. and H. Nakauchi conceived and designed experiments and research; M.W., S.Y., H. Nagashima, R.N. and H. Nakauchi wrote the paper; M.W., K.N., A.U., H.M., S.Y., K.U., S.T., T.S., T.Y, S.M., T.H., H.I. and H. Nagashima performed experiments; M.W., R.N., H. Nakauchi and H. Nagashima analyzed data; M.W., H. Nagashima, R.N. and H. Nakauchi discussed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
One author (H. Nagashima) is a cofounder and shareholder of ChimaERA Corporation and PorMedTec Inc. and is an unpaid scientific adviser for PorMedTec Inc. These do not alter the authors’ adherence to all the journal’s policies on sharing data and materials. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, M., Nakano, K., Uchikura, A. et al. Anephrogenic phenotype induced by SALL1 gene knockout in pigs. Sci Rep 9, 8016 (2019). https://doi.org/10.1038/s41598-019-44387-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44387-w
This article is cited by
-
Pigs with δ-sarcoglycan deficiency exhibit traits of genetic cardiomyopathy
Laboratory Investigation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.