Abstract
As the broad spectrum pharmacological action, aspirin has been one of the most widely used medicines since its initial synthesis; however, the association between aspirin and erectile function is still controversial. We aim to explore whether long-term aspirin administration deteriorates or preserves erectile function from adult rats and ageing rat model. Twenty adult rats (10 weeks of age) and twenty ageing rats (80 weeks of age) were randomly divided into four groups as follows: Adult-Control (normal saline [NS]), Adult-Aspirin (aspirin, 10 mg/kg/d), Ageing-Control (NS), and Ageing-Aspirin (aspirin, 10 mg/kg/d) groups (n = 10 per group). For all rats, erectile function was assessed by maximum intracavernous pressure (ICP), total area under ICP curve (AUC), ICP/mean arterial pressure (MAP) ratio, and MAP. The total treatment duration was one month. Protein expression levels of cyclooxygenase-1 (COX-1), COX-2, endothelial nitric oxide synthase (eNOS), and nNOS of the corpus cavernosum were detected by Western blot. ELISA kits were used to determine 6-keto PGF1a, PGE2, TXB2, cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP) levels. Total nitric oxide (NO) concentration was measured using a fluorometric assay kit. As a result, Ageing-Control rats revealed significantly decreased ICP, AUC, and ICP/MAP ratios compared to Adult-Control rats, and these effects were accompanied by reduced eNOS protein expression and lower total NO and cGMP levels; however, no difference was found in nNOS protein expression. For adult rat groups, aspirin significantly inhibited the production of 6-keto PGF1a, PGE2, and TXB2; however, it neither changed the ICP, AUC, or ICP/ MAP ratios nor altered the protein expression of eNOS, nNOS, COX-1, and COX-2. Meanwhile, aspirin did not influence the concentrations of total NO, cAMP, or cGMP. The same tendency was also found in the ageing rat model, which confirmed that aspirin did not alter erectile function. Our data suggested that long-term aspirin administration did not strengthen or weaken erectile function in adult rats or ageing rat model. Thus, it had no impact on erectile function.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is defined as the inability to attain or maintain sufficient penile erection for satisfactory sexual performance1,2. It is a common condition that affects 31–52% of men aged >50 years3 and impairs people’s quality of life4. As medicine-induced ED leads to noncompliance with medical prescriptions, it is important to examine the association between medications and ED4.
Since its initial synthesis 100 years ago, aspirin has been one of the most widely used medicines3 for its broad spectrum indications. Aspirin is now available without prescription for arthritis, joint pain, muscle aches and chronic musculoskeletal pain; it is well known as an important prophylaxis for CVD and atherosclerotic disease3,5; other ‘magical’ effects are being explored, including a protective role against stroke, thrombosis, and cancer progression5,6,7. Thus, aspirin usage is common in the older population. Meanwhile, it is also popularly used for any type of febrile condition, acute or chronic pain, and dysmenorrhea3,5. As a consequence, it has been the single most important self-prescribed medicine for any aged patient, while the annual drug usage is tremendous. In addition, the usage of aspirin is also accompanied by many adverse effects, like gastrointestinal injury, cerebral bleeding, and anaphylaxis8,9,10; however, its potential impact on erectile function is still controversial8.
First, ED shares similar risk factors to cardiovascular diseases (CVD), such as ageing11,12, hypertension, diabetes, and hyperlipidaemia13. ED has been associated with endothelial dysfunction2,3,5,7 and is considered an early predictor of CVD13,14. With an excellent protective role against CVD, aspirin should be beneficial for ED. Meanwhile, low-grade inflammation processes or highly circulating pro-inflammatory markers are also related to the ED process3,15; it is rational to use aspirin as a preventive treatment against ED, considering its anti-inflammatory abilities3.
In contrast, aspirin inhibits the cyclooxygenase (COX) pathway to decrease vasodilative agents of prostaglandin I2 (PGI2) and prostaglandin E2 (PGE2)6. Considering their vasodilation effects, intracavernous or intraurethral injection (PGE1)1,8,16 and COX-2-linker-PGIS gene therapies17 have been used for penile rehabilitation. Therefore, aspirin should deteriorate normal erectile function, as it reduces these vasodilative prostaglandin agents4,5.
In addition, some recent studies revealed that aspirin had no impact on erectile function2,3,18. These studies claimed that, similar to arthritis, joint pain, muscle aches, chronic musculoskeletal pain, or atherosclerotic disease, most medical indications of aspirin were also risk factors for ED3,4. Therefore, it was necessary to clarify whether these associations were attributed to aspirin or the disease condition itself 3. However, these claims were not supported by any basic evidence which might help to elucidate this relationship19.
Considering the broad spectrum indications and tremendous annual drug prescriptions for aspirin, we investigated the relationship between aspirin and erectile function. As more recent clinical studies report that aspirin does not affect erectile function, the positive or negative associations reported may potentially be the result of confounding by indication bias; thus, we also hypothesized that aspirin administration has no impact on erectile function. Moreover, we tested this hypothesis on both adult rats and ageing rat model, considering that aspirin is commonly taken by both adult and aged populations.
Materials and Methods
Animals
The experimental male Sprague-Dawley rats were purchased from Dashuo Experimental Animal Co. Ltd., Chengdu, Sichuan Province, China. We confirmed that all experiments were performed in accordance with relevant guidelines and regulations; all rats were housed and cared for under strict guidelines, and this study was approved by the Animal Ethics Committee of West China Hospital, Sichuan University (NO. 20160461A).
Specifically, twenty healthy adult rats (10 weeks of age) and twenty ageing rats (80 weeks of age) were randomly divided into four groups (n = 10 per group) as follows: Adult-Control (normal saline [NS]), Adult-Aspirin (aspirin, 10 mg/kg/d), Ageing-Control (NS), and Ageing-Aspirin (aspirin, 10 mg/kg/d). The total treatment duration was one month.
Erectile function measurement
With a one-week washout period after the treatment duration, erectile function was assessed in all rats (n = 10 per group) by recording the maximum intracavernous pressure (ICP) and ICP/mean arterial pressure (MAP) ratio according to a previous method20,21. Briefly, anesthesia induction was first given at a concentration of 5% (volume/volume) isoflurane (RWD Life Science, Guangdong Province, China) mixed with air20,22. The isoflurane concentration was then downgraded and maintained at 2%, while the left carotid was carefully exposed and cannulated with heparinized (200 IU/ml) detaining venipuncture (26 G; Closed IV Catheter System, Becton Dickinson Medical Devices Co. Ltd., Franklin Lakes, NJ, USA) connected to a pressure transducer to measure arterial pressure. The left cavernous nerve was carefully exposed and isolated after a low midline abdominal incision was made. After the penis was denuded of skin, a heparinized (200 IU/ml) 24-gauge needle (SGJS Medical Equipment Group Co. Ltd., Luohe, Henan Province, China) connected with a BL420 bio-function experiment system (Chengdu TME Technology Co. Ltd. Chengdu, Sichuan Province, China) was inserted into the penile crus to record the ICP. The concentration of isoflurane was then decreased to 1%, and the cavernous nerve was electrically stimulated as follows: using 2.5 V, 5 V, 7.5 V at a frequency of 20 Hz, pulse width of 5 millisecond and duration of 60 seconds20,22,23. Maximum ICP and arterial pressure were recorded simultaneously, and total ICP was monitored by calculating the area under erectile curve (AUC) from the beginning of cavernous nerve stimulation to return of the ICP to the baseline, while MAP and the ratio of ICP/MAP were also calculated for data analysis.
Tissue harvesting
At the completion of erectile function assessment, the rats were then sacrificed by cervical dislocation. Then the penile corpus cavernosum (below the cartilage of the glans to penile crus) was harvested and washed with phosphate buffered saline (PBS). The cavernous tissue was cut into distal (near to penile glans), medial, and proximal (near penile crus) sections and stored with liquid nitrogen. The rats were then killed by cervical dislocation.
Western blot (WB)
Distal corpus cavernosum samples (n = 10 per group) were snipped and homogenized in RIPA lysis buffer supplemented with 1* protease inhibitor. The supernatants were collected, and protein concentrations were calculated by Coomassie brilliant blue. Protein (30 µg/lane) was loaded and separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane according to standard procedures20,21. After blocking with 5% non-fat dry milk in TBS-T, the membrane was incubated with primary antibodies against COX-1 (1:1000, Abcam, Cambridge, MA, USA), COX-2 (1:1000, Abcam), endothelial nitric oxide synthase (eNOS) (1:1000, Abcam), and neuronal NOS (nNOS) (1:1000, Abcam) for 24 hours at 4 °C. The membranes were washed and then incubated with secondary antibody (GAPDH, 1:200, Zen BioScience Co., Ltd. Chengdu, Sichuan Province, China). Protein band densitometry was collected by a Bio-Rad ChemiDoc MP (Bio-Rad, Berkeley, CA, USA), while the band intensities were quantified by Image J software (National Institute of Health, Bethesda, MD, USA).
Prostaglandins
6-keto PGF1a, thromboxane B2 (TXB2) (stable metabolites of PGI2 and TXA2, respectively), and PGE2 were measured by enzyme linked immunosorbent assay (ELISA) (Cayman Chemical, Ann Arbor, MI, USA) kits. The medial section of the corpus cavernosum (n = 10 for each group) was snipped and homogenized in homogenization buffer (1 mM ethylenediaminetetraacetic acid, 0.1 M phosphate buffer [pH 7.4], and 20 μg/ml indomethacin) at 4 °C to inhibit the metabolism of arachidonic acid (AA) to prostaglandins24. The homogenate was centrifuged at 12,000 g for 20 min at 4 °C, and the supernatant was used for measurements, according to the manufacturer’s instructions. The final prostaglandin concentrations were normalized to total protein and expressed as pg/mg protein.
Cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), and total nitric oxide (NO)
Proximal corpus cavernosum (n = 10 per group) was cut into pieces, homogenized in PBS and centrifuged (12,000 g for 20 min, at 4 °C) for supernatant collection. cAMP and cGMP were measured by ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China) according to the manufacturer’s instructions. For total NO level, a commercially available Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical) was used to determine the total nitrate + nitrite concentration (metabolites of NO).
Statistical analysis
The erectile function index (ICP, AUC, ICP/MAP ratio, and MAP) and all biomarker results were analysed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The data are shown as the mean ± SEM (standard error mean). Differences between Adult-Control and Ageing-Control groups were performed to explore the changes between adult rats and ageing rat models, while differences between Adult-Control and Adult-Aspirin groups, as well as between Ageing-Control and Ageing-Aspirin groups were compared to investigate the effects of aspirin administration on adult rats and ageing rat model, respectively. We chose the statistical method of Student’s t-test after proving Gaussian distribution using Kolmogorov-Smirnov-test, and a p-value < 0.05 was considered significant.
Results
Erectile function
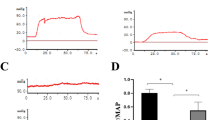
Typical ICP tracings (5 V for 60 s) are presented in Fig. 1A. At the voltage of 5 V, our results showed that the Ageing-Control group revealed a significantly decreased ICP (p < 0.0001), AUC (p < 0.0001), and ICP/MAP ratio (p < 0.001) compared with the Adult-Control rats. However, no significant difference was observed in the ICP (p = 0.7026), AUC (p = 0.3161), and ICP/MAP ratio (p = 0.2370) between the Adult-Control and Adult-Aspirin groups. Meanwhile, aspirin also did not significantly change the ICP (p = 0.7499), AUC (p = 0.1679), or ICP/MAP ratio (p = 0.7499) between the Ageing-Control and Ageing-Aspirin groups (Fig. 1B–D).
(A) Representative intracavernous pressure (ICP) tracings in response to cavernous nerve stimulation (5 V for 60 seconds). (B–D) Statistical analysis for voltage (2.5 V, 5 V, and 7.5 V) dependent ICP, AUC, and ICP/MAP Ratio to cavernous nerve stimulation (CNS), respectively. (E) Statistical analysis for MAP. The differences were analyzed with Student’s t-test between Adult-Control and Aging-Control, between Adult-Control and Adult-Aspirin, as well as between Aging-Control and Aging-Aspirin (n = 10 per group). ***< 0.0001. ICP, intracavernous pressure; AUC: total area under the ICP curves; ICP/MAP, intracavernous pressure/mean arterial pressure; MAP, mean arterial pressure.
Compared with 5 V, both lower (2.5 V) and higher (7.5 V) voltages revealed the same tendency, that was, Ageing-Control rats showed a significantly lower ICP (p = 0.0002 at 2.5 V, p < 0.0001 at 7.5 V), AUC (p < 0.0001 at 2.5 V, p = 0.0002 at 7.5 V), and ICP/MAP ratio (p = 0.0004 at 2.5 V, p < 0.0001 at 7.5 V) than Adult-Control rats. While aspirin did not significantly alter the ICP (p = 0.5782 at 2.5 V, p = 0.8241 at 7.5 V), AUC (p = 0.6381 at 2.5 V, p = 0.3528 at 7.5 V), and ICP/MAP ratio (p = 0.4464 at 2.5 V, p = 0.9738 at 7.5 V) between the Adult-Control and Adult-Aspirin groups, it also did not significantly change the ICP (p = 0.7197 at 2.5 V, p = 0.4371 at 7.5 V), AUC (p = 0.7736 at 2.5 V, p = 0.3000 at 7.5 V), and ICP/MAP ratio (p = 0.5885 at 2.5 V, p = 0.5691 at 7.5 V) between the Ageing-Control and Ageing-Aspirin groups (Fig. 1B–D).
In addition, Ageing-Control rats presented a slightly higher MAP (p = 0.2139) than Adult-Control rats, while no significant difference was found between Adult-Control and Adult-Aspirin (p = 0.5484), as well as between Ageing-Control and Ageing-Aspirin (p = 0.9669) groups (Fig. 1E).
COX-1/2 protein expression
In WB analysis, Ageing-Control rats exhibited significantly increased COX-1 expression compared to Adult-Control rats (p = 0.0027); however, this difference was not observed between the Adult-Control and Adult-Aspirin groups (p = 0.2944), or between the Ageing-Control and Ageing-Aspirin rats (p = 0.5967) (Fig. 2A,B). In addition, no significant difference was found in terms of COX-2 levels (p = 0.8297) (Fig. 2C,D).
(A,C) Representative cyclooxygenase-1 (COX-1) and COX-2 bands in WB analysis (selected from gel 1 and 5 in the Supplementary File, respectively). (B,D) Statistical analysis for COX-1 and COX-2, respectively; the differences were analyzed with Student’s t-test between Adult-Control and Aging-Control, between Adult-Control and Adult-Aspirin, as well as between Aging-Control and Aging-Aspirin (n = 10 per group). *<0.05.
Prostaglandins-cAMP pathway
As the results of ELISA analysis show, Ageing-Control rats revealed significantly increased PGE2 levels (p = 0.0057) compared to Adult-Control rats; however, no significant difference was found for 6-keto PGF1a (p = 0.3713) and TXB2 (p = 0.6481) between the two groups. For the adult groups, aspirin significantly decreased the 6-keto PGF1a (p = 0.0019), PGE2 (p = 0.0080), and TXB2 (p = 0.0113) levels compared with the control group. The aspirin inhibitory effects on 6-keto PGF1a (p = 0.0152), PGE2 (p = 0.0163), and TXB2 (p = 0.0405) were also observed in the ageing groups (Fig. 3A–C). Moreover, there was no significant difference in cAMP concentrations among groups (Fig. 3D).
(A–D) Statistical analysis for 6-keto PGF1a, PGE2, thromboxane B2 and cAMP levels; the differences were analyzed with Student’s t-test between Adult-Control and Aging-Control, between Adult-Control and Adult-Aspirin, as well as between Aging-Control and Aging-Aspirin (n = 10 per group). *<0.05, **<0.01.
NOS-NO-cGMP pathway
Ageing-Control group presented significantly decreased eNOS protein expression (p = 0.0056) compared with the Adult-Control group based on WB analysis. However, no significant difference was found between the Adult-Control and Adult-Aspirin (p = 0.6073) groups, or between the Ageing-Control and Ageing-Aspirin (p = 0.6458) groups. Moreover, there was no significant difference in nNOS expression (p = 0.7880) between the groups (Fig. 4A,B).
(A,B) Representative eNOS and nNOS bands in WB analysis (selected from gel 1 and 5 in Supplementary File, respectively). (C,D) Statistical analysis for eNOS and nNOS for protein expression from WB. (E,F) Statistical analysis for total NO and cGMP, respectively; the differences were analyzed with Student’s t-test between Adult-Control and Aging-Control, between Adult-Control and Adult-Aspirin, as well as between Aging-Control and Aging-Aspirin (n = 10 per group). *<0.05, **<0.01, ***<0.0001.
Ageing-Control rats had lower total NO (p = 0.0394) and cGMP (p = 0.0026) levels than Adult-Control rats. However, aspirin did not significantly reduce the concentrations of total NO (p = 0.9427) and cGMP (p = 0.8765) between the Adult-Control and Adult-Aspirin groups. For the ageing groups, aspirin also did not change the total NO (p = 0.2291) and cGMP (p = 0.7297) levels (Fig. 4C,D).
Discussion
ED is a natural consequence of ageing, while a survey from the US National Health and Nutrition Examination revealed that the incidence increased from 8.2% in men aged 40–49 years to 77.5% in those aged over 75 years11,25. This can be mainly attributed to endothelial dysfunction2,3,5,7,12 and reduced NOS protein26. In Fig. 1B–D, Ageing-Control rats exhibited significantly decreased ICP, AUC, and ICP/MAP ratios (at all voltages of 2.5 V, 5 V, and 7.5 V) compared to Adult-Control rats, which confirmed decreased erectile function with age. Meanwhile, although with constant nNOS protein expression, Ageing-Control rats showed lower eNOS protein expression, and total NO and cGMP levels than Adult-Control rats (Fig. 4C,E,F), which might explain the age-dependent decreased erectile function. In addition, COX-1 protein expression was significantly higher in Ageing-Control rats than in Adult-Control rats; however, this difference was not found in COX-2 protein expression.
It has been reported that basal release of prostaglandins exhibited protective roles in many pathophysiological conditions, including normal penile erection6. PGI2 binds to the endothelial PGI receptor (IP) to activate the Gs protein-coupled receptor, which stimulates adenylyl cyclase to produce cAMP. The elevated cAMP then causes smooth muscle relaxation and induces penile erection17. PGE2 exerts a similar relaxation effect by combining PGE receptors (EP2/4)8,16. As vasoactive prostaglandins were formed via active COX-1/2 pathways, the release of vasodilator prostaglandins was impaired by insulin resistance, leading to enhanced vasoconstriction and blunted endothelium-dependent vasodilation27. Thus, the study revealed that indomethacin and diclofenac, the other COX inhibitors that reduced relaxant agents of PGI2 and PGE24,5,8, adversely affected erectile responses in rats8. As a consequence, the authors believed that the most popular aspirin should impair normal penile erection as well.
However, our study showed that although aspirin reduced prostaglandin production in both adult rats and the ageing rat model (Fig. 3A–C), it did not change erectile function, as demonstrated by the ICP, AUC, and ICP/MAP ratios (at all voltages of 2.5 V, 5 V, and 7.5 V) (Fig. 1B–D), while aspirin had no impact on the downstream cascade of cAMP (Fig. 3D). This was in accordance with previous basic28 and clinical2,3,18 studies. They insisted and explained that penile hypercoagulability induced by TXA2 plays a key initiating role in penile vascular changes to reduce penile erection8,29; however, contractive and harmful of TXA2 was also inhibited by aspirin when relaxant and protective agents of PGI2 and PGE2 were reduced16,17,29. This might explain why aspirin did not impair erectile function.
In addition, there was a broad range of cellular responses in thromboxane receptor (TP) signalling. TPα stimulated adenylyl cyclase to produce cAMP, whereas TPβ reduced it30,31. Such counteracting effects occur in other receptors, such as PGE receptor (EP)2/4, which increased cAMP, but EP3 decreased it32. This might contribute to the unchanged cAMP level33 in corpus cavernosum even when aspirin blocked prostaglandin production, and it also provided further evidence that aspirin does not influence erectile function.
In fact, NO synthesized by NOS isoforms is the principal neurotransmitter in maintaining erections7,34,35. However, the interaction between prostaglandins and NO signalling pathways occurs on multiple levels, which requires further investigation6,17.
Studies revealed that PGIs increased eNOS levels17 while repeated PGE1 injection enhanced constitutive NOS isoforms (eNOS and nNOS) by stimulating prostaglandin production8,36, which could promote NO accumulation and strengthen erectile response to nerve stimulation8,36. By inhibiting prostaglandin production, aspirin or indomethacin markedly decreased NOS activity and suppressed NO concentration6,7,8 to decrease penile erectile function7,8,16. Therefore, aspirin could reduce erectile function by impairing the eNOS/nNOS pathway.
In contrast, some researchers believed that aspirin had the ability to restore the impaired NOS isoforms7,37 to increase NO bioavailability7. Thus, they claimed that aspirin or indomethacin could promote endothelial-dependent relaxation of the corpus cavernosum7,35 and improve impaired erectile function7,34,38.
However, both our adult rats and ageing rat model demonstrated that aspirin neither inhibited eNOS/nNOS protein expression nor reduced total NO levels. It also did not change the downstream cGMP concentrations. The constant NOS-NO-cGMP pathways were in accordance with ICP, AUC, and ICP/MAP indices, which verified that long-term aspirin administration had beneficial effects on erectile function. This was also consistent with previous clinical studies2,3,18,39,40.
In addition, although they share the similar pharmacological action of COX inhibition, aspirin, indomethacin, and diclofenac should be considered for a separate study on their effects on erectile processes, considering their diverse actions affecting smooth muscle tone, NO production, and blood coagulation8.
Our study has several limitations. First, we showed decreased prostaglandins and unchanged NO levels; however, this relationship has been controversial, and we were not able to clarify it6,17. Second, we chose an ageing rat model to investigate whether aspirin would change erectile function; however, the ageing model may not accurately represent all processes of ED progression, such as diabetes mellitus, dyslipidaemia, or other disease conditions. Finally, although all rats underwent ICP measurements, it was worth noting that ICP measurement activates eNOS/nNOS protein expression and cAMP/cGMP concentrations; thus, the subsequent biomarker detection might have been influenced.
Conclusion
From the results of adult rats and the ageing rat model, although aspirin administration decreased prostaglandin levels, it did not strengthen or weaken erectile function. This is also in accordance with recent clinical studies that claim that aspirin usage has no influence on erectile function.
References
Fazio, L. & Brock, G. Erectile dysfunction: management update. CMAJ 170, 1429–37 (2004).
Bohm, M. et al. Sexual function, satisfaction, and association of erectile dysfunction with cardiovascular disease and risk factors in cardiovascular high-risk patients: substudy of the ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNT Study in ACE-INtolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND). Am Heart J 154, 94–101 (2007).
Patel, D. P. et al. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: results from the Prostate Cancer Prevention Trial. BJU Int 117, 500–6 (2016).
Shiri, R. et al. Effect of nonsteroidal anti-inflammatory drug use on the incidence of erectile dysfunction. J Urol. 175(5), 1812–5 discussion 1815–6 (2006).
Gleason, J. M. et al. Regular nonsteroidal anti-inflammatory drug use and erectile dysfunction. J Urol 185, 1388–93 (2011).
Mollace, V., Muscoli, C., Masini, E., Cuzzocrea, S. & Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 57, 217–52 (2005).
Hafez, G. et al. Acetylsalicylic acid protects erectile function in diabetic rats. Andrologia 46, 997–1003 (2014).
Senbel, A. M. Functional inhibition of erectile process in rats by indomethacin. World J Urol 29, 523–7 (2011).
Christen, W. G. & Chew, E. Y. Does long-term aspirin use increase the risk of neovascular age-related macular degeneration? Expert Opin Drug Saf 13, 421–9 (2014).
Small, K. W., Garabetian, C. A. & Shaya, F. S. Macular Degeneration And Aspirin Use. Retina 37, 1630–5 (2017).
Mulhall, J. P., Luo, X., Zou, K. H., Stecher, V. & Galaznik, A. Relationship between age and erectile dysfunction diagnosis or treatment using real-world observational data in the USA. Int J Clin Pract 70, 1012–8 (2016).
Bayraktar, Z. & Albayrak, S. Antiplatelet (aspirin) therapy as a new option in the treatment of vasculogenic erectile dysfunction: a prospective randomized double-blind placebo-controlled study. Int Urol Nephrol 50, 411–8 (2018).
Sullivan, M. E. et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res 43, 658–65 (1999).
Goldstein, I., Chambers, R., Tang, W., Stecher, V. & Hassan, T. Real-world observational results from a database of 48 million men in the United States: Relationship of cardiovascular disease, diabetes mellitus and depression with age and erectile dysfunction. Int J Clin Pract 72, e13078 (2018).
Vlachopoulos, C., Rokkas, K., Ioakeimidis, N. & Stefanadis, C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. Eur Urol 52, 1590–600 (2007).
Javier Angulo, P. C. Regulation of human penile smooth muscle tone by prostanoid receptors. Br J Pharmacol (2002).
Lin, H. et al. COX-2-10aa-PGIS gene therapy improves erectile function in rats after cavernous nerve injury. J Sex Med 10, 1476–87 (2013).
Kupelian, V., Hall, S. A. & McKinlay, J. B. Common prescription medication use and erectile dysfunction: results from the Boston Area Community Health (BACH) survey. BJU Int 112, 1178–87 (2013).
Cole, A. P., Leow, J. J. & Trinh, Q. D. New evidence from the Prostate Cancer Prevention Trial may exculpate cyclooxygenase (COX) blockers in erectile dysfunction. BJU Int 117, 385–6 (2016).
Li, J. et al. Reduction in Peyronie’s-like plaque size using a vacuum erection device in a rat model of Peyronie’s disease via the TGF-beta/SMAD signalling pathway. Andrologia 50, e13051 (2018).
Mulhall, J. P. et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med 5, 1126–36 (2008).
Li, J. et al. Isoflurane inhalation anesthesia should be a new requirement in intracavernosal pressure detection-the gold standard of erectile function assessment. Sci Rep 7, 14949 (2017).
Yuan, J. et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol 58, 773–80 (2010).
Cong, P., Xiao, Z. L., Biancani, P. & Behar, J. Prostaglandins mediate tonic contraction of the guinea pig and human gallbladder. Am J Physiol Gastrointest Liver Physiol 292, G409–18 (2007).
Saigal, C. S., Wessells, H., Pace, J., Schonlau, M. & Wilt, T. J. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 166, 207–12 (2006).
Ding, Z., Shen, X. & Li, Y. Association of nNOS and Rho-kinase with age-associated erectile dysfunction in Sprague-Dawley rats. Exp Ther Med 13, 1133–6 (2017).
Sanchez, A. et al. Altered arachidonic acid metabolism via COX-1 and COX-2 contributes to the endothelial dysfunction of penile arteries from obese Zucker rats. Br J Pharmacol 159, 604–16 (2010).
Kim, Y. C., Kim, J. H., Davies, M. G., Hagen, P. O. & Carson, C. C. 3rd. Modulation of vasoactive intestinal polypeptide (VIP)-mediated relaxation by nitric oxide and prostanoids in the rabbit corpus cavernosum. J Urol 153, 807–10 (1995).
Bornman, M. S., Franz, R. C., Jacobs, D. J. & Du Plessis, D. J. Effect of single dose aspirin on the development of penile hypercoagulability during erection. Br J Urol 59, 267–71 (1987).
Huang, J. Cell signalling through thromboxane A2 receptors. Cellular Signalling 16, 521–33 (2004).
Hirata, T., Ushikubi, F., Kakizuka, A., Okuma, M. & Narumiya, S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J Clin Invest 97, 949–56 (1996).
Iwasaki, R. et al. Molecular and pharmacological characterization of zebrafish ‘contractile’ and ‘inhibitory’ prostanoid receptors. Biochem Biophys Res Commun 438, 353–8 (2013).
Levine, R. A., Nandi, J. & King, R. L. Aspirin potentiates prestimulated acid secretion and mobilizes intracellular calcium in rabbit parietal cells. J Clin Invest 86, 400–8 (1990).
Sadeghipour, H., Ghasemi, M., Nobakht, M., Ebrahimi, F. & Dehpour, A. R. Effect of chronic lithium administration on endothelium-dependent relaxation of rat corpus cavernosum: the role of nitric oxide and cyclooxygenase pathways. BJU Int 99, 177–82 (2007).
Minhas, S., Cartledge, J. J., Eardley, I., Joyce, A. D. & Morrison, J. F. The interaction of nitric oxide and prostaglandins in the control of corporal smooth muscle tone: evidence for production of a cyclooxygenase-derived endothelium-contracting factor. BJU Int 87, 882–8 (2001).
Escrig, A., Marin, R. & Mas, M. Repeated PGE1 treatment enhances nitric oxide and erection responses to nerve stimulation in the rat penis by upregulating constitutive NOS isoforms. J Urol 162, 2205–10 (1999).
Gao, H. et al. Aspirin attenuates monocrotaline-induced pulmonary arterial hypertension in rats by suppressing the ERK/MAPK pathway. Clin Exp Hypertens 39, 34–41 (2017).
Saroukhani, S. et al. Aspirin for treatment of lithium-associated sexual dysfunction in men: randomized double-blind placebo-controlled study. Bipolar Disord 15, 650–6 (2013).
Bener, A., Al-Hamaq, A. O., Kamran, S. & Al-Ansari, A. Prevalence of erectile dysfunction in male stroke patients, and associated co-morbidities and risk factors. Int Urol Nephrol 40, 701–8 (2008).
Li, T. et al. Association between use of aspirin or non-aspirin non-steroidal anti-inflammatory drugs and erectile dysfunction: A systematic review. Medicine (Baltimore) 97, e11367 (2018).
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (No. 81671453 & 81270691) and Sichuan Science and Technology Program (No. 2018SZ0019 & 2018TJPT0018).
Author information
Authors and Affiliations
Contributions
T.L., C.J.W., F.D.F., W.F.X., F.Q. and J.H.Y. conceived of the study, and participated in its design and coordination and helped to draft the manuscript; T.L., F.D.F. and W.F.X. carried out the ICP test, participated in the manuscript drafting; T.L., C.J.W. and F.Q. performed the experimental test, like ELISA and WB analysis. J.H.Y. approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, T., Wu, C., Fu, F. et al. Long-Term Aspirin Administration Has No Effect on Erectile Function: Evidence from Adult Rats and Ageing Rat Model. Sci Rep 9, 7941 (2019). https://doi.org/10.1038/s41598-019-44386-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44386-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.