Abstract
Integrative and conjugative elements (ICEs) are mobile genetic elements that contribute to horizontal gene transfer. The aim of this work was to study different types of ICEs in clinical isolates of the emergent pathogen Shewanella spp., to compare their transfer efficiency and their ability to integrate a new host. Here we show that 3 out of 10 clinical isolates contained an ICE. Two of these elements were similar to ICEs from the SXT/R391 family and the other one was similar to ICESh95, a hybrid platform. Mating assays showed that these elements co-exist for several generations in the same host. Furthermore, transfer rates and competition assays between ICESh95 and ICESh392, an SXT-like element, suggest that the latter has evolved into a well-oiled machine that efficiently spread to different bacteria. Our results provide strong evidence of the role that ICEs play in the dissemination of genetic traits in nature and the implications that they have in the global threat of antimicrobial resistance.
Similar content being viewed by others
Introduction
Integrative and conjugative elements (ICEs) are large genomic platforms capable of self-transferring. These elements play a key role in the genetic exchange between bacteria via horizontal transfer events1,2,3. ICEs encode in their platform the mechanisms of excision, integration, self-transfer, and regulation. Furthermore, they contain accessory modules identified as variable regions or hot spots (HS), which are commonly unique for each element1,2,3. The genetic information found in these regions can confer adaptive advantages to their host, such as antibiotic and heavy metal resistance, restriction modification systems, DNA repair systems or toxin, and antitoxin systems1,2,3,4,5. ICEs can be classified in different families2,3. Among them, the SXT/R391 family has been extensively studied and their members are widely disseminated among gamma-proteobacteria2,3. Recently, the criteria for classifying ICEs of the SXT/R391 family was redefined in four types based on their insertion site, structure, prevalence, and distribution in bacterial species6. While type I includes all ICEs integrated at the 5′ end of prfC gene found in Enterobacteriaceae and Vibrionaceae, types 2, 3 and 4 ICEs are integrated at the 3′ end of the tRNA-Ser gene and are exclusively found in Vibrio species6.
Different ICEs from the SXT/R391 family have been found in environmental and clinical strains of Shewanella7,8,9,10,11, a gram-negative rod that thrives in aquatic niches12. In recent years there has been an increase in the reports on Shewanella spp. isolated from clinical samples establishing it as an emerging opportunistic pathogen13,14,15,16,17. This bacterium is also known for its potential application in bioremediation, its versatile metabolism, and its genetic plasticity12.
We have previously described a hybrid and fully active ICE in the clinical isolate Shewanella sp. Sh95, named ICESh9510. This element contained a multidrug resistant (MDR) integron platform harboring two gene cassettes that confer resistance to trimethoprim and quaternary ammonium compounds. Moreover, we showed that ICESh95 has the core genes conserved among members of the SXT/R391 family and it was integrated at the gene pabA, a para-aminobenzoate synthase involved in folate biosynthesis.
This work aimed to characterize different types of ICEs found in clinical isolates of Shewanella spp. by assessing their transfer efficiency, maintenance, and activity. Our study evidenced that Shewanella spp. can carry different ICEs platforms, which in turn can spread to other hosts playing an important role in horizontal gene transfer (HGT). Furthermore, we suggest that the higher occurrence of ICEs from the type I SXT/R391 family in nature instead of hybrid elements is the result of an efficient transfer machinery. This study provides additional information on the impact of ICEs in bacterial genome evolution and on their contribution in the dissemination of antibiotic resistance determinants from environmental niches to clinical settings.
Results and Discussion
Identification of ICEs from Shewanella clinical isolates
We looked for ICEs from the SXT/R391 family and ICESh95-like in 10 clinical strains, which were obtained from a public hospital of Buenos Aires, Argentina10,18,19. The screening was done by PCR using specific primers to detect two conserved regions6,20, the origin of transfer, oriT, and the setR gene. Our results showed that 3 out of 10 isolates contained an ICE corresponding to strains Shewanella sp. Sh31, Shewanella sp. Sh82, and Shewanella sp. Sh39210. Identification of each ICE was done by PCR amplification using specific primers for the xis/int module of ICEs SXT/R391 and ICESh95. As a result, we observed that ICESh31 from Shewanella sp. Sh31 contained the xis/int module found in ICESh95, whereas Shewanella sp. Sh82 and Sh392 (ICESh82 and ICESh392, respectively) were positive for an SXT/R391-like ICE6,10,21. We then searched for the integron described in ICESh9510, located in the variable region HS3, in the strains bearing an ICE using specific primers. Similar to ICESh95, ICESh31 contained an MDR integron carrying the dfrA15 gene cassette, which confers resistance to trimethoprim.
The common features found between ICESh95 and ICESh31 were consistent with phylogenetic studies previously reported10, in which it was shown that strains Sh31 and Sh82 are closely related to Sh95, whereas strain Sh392 belonged to the S. algae/S. haliotis lineage10. Taken together, these results confirmed that Shewanella spp. can acquire either an ICE of the SXT/R391 family (Sh82 and Sh392 strains) or a hybrid element such as ICESh95 (Sh95 and Sh31 strains) regardless its source. Thus, we observed that ICEs could contribute to the evolution of bacterial pathogens to extensive drug resistance by acting as vectors spreading key traits to different hosts.
Transfer ability of ICEs in Shewanella spp
In order to evaluate the ability of each ICE to self-transfer to other bacteria we did a conjugative assay and looked for the excised forms by PCR. Our results showed that ICESh31 and ICESh392 were able to form circular intermediaries and self-transfer to other hosts, whereas ICESh82 did not exhibit any transfer activity nor its circular form was detected, which suggest that it may have lost its ability.
ICESh31 was able to transfer to Shewanella sp. Sh10 with a frequency of 2.5 × 10−5 (±0.90 × 10−5) and to E. coli HB101 with a frequency of 2.62 × 10−4 (±0.37 × 10−5). On the other hand, ICESh392 showed transfer rates of 2.33 × 10−4 (±1.53 × 10−5) for Shewanella sp. Sh10 and 2.86 × 10−3 (±0.34 × 10−4) for E. coli HB101 (Fig. 1).
Previously, we have reported that ICESh95 was able to transfer to other Shewanella spp. with a frequency of 2.85 × 10−3 (±2.15 × 10−3) and to E. coli with a frequency of 1.04 × 10−5 (±0.60 × 10−5)10. Taken together, these results confirm that both ICESh392 and ICESh31 efficiently participate in the genetic exchange and genome diversity of different bacteria. Since ICESh392 is an active mobile element and its recombination module is similar to those from the SXT/R391 family we decided to sequence the host genome and further study this ICE.
ICESh392 is similar to members of the type I SXT/R391 family
De novo assembly of the genome of strain Sh392 resulted in 4,803,735 bp with an N50 contig size of 96,236 bp (max. length 367,520 bp) and a G + C content of 52.9%. Sequence annotation using RAST led to the identification of 4,200 coding genes and 123 tRNA genes22. We calculated the average nucleotide identity (ANI) to estimate the proximity between species, which resulted in 98.38% (±1.42%) identity to the closest relative S. haliotis JCM 14758 (cut-off: 95%)23, now proposed S. algae24. It is worth to mention that S. algae is considered the causative agent of skin and soft tissue infections, bacteremia and osteomyelitis14,15,17.
We then focused in the region comprising ICESh392, which revealed that it contained the complete backbone of the ICE of the family SXT/R391 (Fig. 2). Its G + C content was 47.31%, which is lower than the average G + C content of S. algae Sh392 genome (52.9%). This is in line with Cury et al. (2017), who reported that ICEs are elements rich in As and Ts. Furthermore, we did a phylogenetic analysis using the setCD operon and setR to identify the closest relative of ICESh392. The concatenated tree showed that these elements, setCD and setR, clustered with those from ICE SXTMO10 and R391, which indicates that ICESh392 belonged to the type I SXT/R391 lineage (Fig. S1).
Comparison of ICESh95 and ICESh392 genetic architecture. Structural core genes shared between both ICEs (light gray and white) are shown in the middle of the figure. Hot spots for each ICE are depicted in grey boxes. The attachment sites are depicted in black (attL and attR), the xis/int module of ICESh95 and the eexS gene are in black.
The analysis of all ICESh392 modules showed that its integrase shared 98% amino acid identity with the integrase of SXTMO10 while the other core regions also showed strong similarities (>95% nucleotide identity). In addition, we identified all HS and variable regions from this element (Fig. 2). In HS5 we found a putative type III restriction and modification system with poor nucleotide identity to the one found in ICESh95; whereas, the variable region IV contained a mercury resistance operon, a common feature of most ICE reported in Shewanella spp.8,9,10,11. Noteworthy, ICESh392 did not encode antimicrobial resistance determinants.
Insertion site of ICESh392
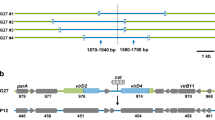
Analysis of the insertion site of ICESh392 showed that this element was located at the prfC gene similarly to type I SXT/R391 family members (Fig. 2)4. We examined the extremities of ICESh392 and found the putative sequences for the attL and attR attachment sites (5′-ATCATCTCTCACCCGGA-3′ and 5′-ATCATTTCTCACCCTGA-3′, respectively). PCR amplification of the exconjugant product and sequence analysis revealed that the attachment site attP was 5′-ATCATCTCTCACCCTGA-3′, and based on attP, attL and attR sequences we were able to infer that the attB sequence was 5′-ATCATCTCTCACCCGGA-3′. Alike other SXTs, our ICE also integrated at the gene prfC without altering the open reading frame, which shows that these elements have a bias towards that gene.
Co-existence and competition of ICEs
We wondered whether an ICE of the SXT/R391 family and an ICESh95-like could co-exist. Based on the entry exclusion group eex gene25, we were able to determine that ICESh95 belonged to type S group (gene eexS)10. On the other hand, we could not identify a recognizable eex gene in ICESh392. This gene is commonly located downstream of the gene traG, instead we found a mercury resistance operon (mer) at that locus. Since no exclusion gene was detected, we proceeded to test the ability of the host to acquire and maintain both ICEs using strains Sh95 and Sh392 as donor and recipient cells. We performed mating assays using Shewanella sp. Sh95 as recipient strain and S. algae Sh392 as donor strain and vice-versa. We selected the different transconjugants using trimethoprim (150 μg/ml) and meropenem (6 μg/ml) and checked for the presence of either ICE by PCR using specific primers for the respective xis/int modules (Fig. S2A). Our results showed that these ICEs co-exist in the same strain regardless of their presence in the recipient cell (Fig. S2A). This suggests that none of these elements exclude themselves. Moreover, we assessed the stability of these elements after serial growth in the same host and we observed that both ICEs were present after 384 generations (Fig. S2B). Since entry exclusion is a key feature for ICEs survival in a new host25, the absence of a known eex gene in ICESh392 could reflect a major advantage for its dissemination to organisms already harboring an ICE.
As various ICEs co-exist in a niche, we wanted to expose different ICEs to a single recipient and evaluate their efficiency to invade a new cell. We did a competition assay using Shewanella sp. Sh95 and S. algae Sh392 as donor strains, and E. coli HB101 as the recipient strain. We determined the quantity of each ICE in the recipient cell (E. coli) after mating by qPCR. We used primers IntSXTqPCR-F and IntSXTqPCR-R to quantify the integrase gene from ICESh392, primers Int95qPCR-F and Int95-R-qPCR for the integrase gene from ICESh95 and primers traV-F and traV-R for the gene traV from both ICEs. We obtained the Ct values from three independent assays for a specific gene from ICESh95, intSh95 (33 ± 0.85), a specific gene from ICESh392, intSh392 (27.7 ± 1.05), and a gene conserved in both ICEs, traV (22.3 ± 1.07). We calculated the normalized ratio for each ICE taking into account the respective primer efficiencies. As a result, we observed that ICESh392 was 58 times more efficient than ICESh95 as a self-transfer element.
In order to evaluate that these ICEs maintained their ability to re-transfer once inserted in a new genome we did a mating assay using Shewanella sp. Sh95 carrying both ICEs as a donor strain and E. coli HB101::pCR2.1 as recipient strain. Our results showed that ICESh392 was able to transfer successfully to E. coli but no positive results were detected for ICESh95 (Fig. S2C). This could be due to the higher conjugation efficiency showed by ICESh392. On the other hand, we observed that ICESh95 was able to self-transfer from strain Sh392 carrying ICESh392 to E. coli (data not shown). Furthermore, to confirm that there was no cross-complementation between ICEs, we assessed the individual re-transfer abilities of each element. We incubated E. coli HB101 carrying either ICESh95 or ICESh392 with Shewanella sp. Sh10 and observed that both ICEs retained their ability to conjugate to a new host (Fig. S3A–C).
Taken together, our data suggest that both ICE are active elements capable to re-transfer regardless of the host or the presence of other genomic islands. Furthermore, we propose that type I SXT/R391 ICEs are more efficient mobile elements, which is consistent with their prevalence reported in nature2,6,10,26.
Since the ICE integrases play a major role during the transfer process, we did a phylogenetic analysis including representatives of all known platforms. The integrase of ICESh392 clustered with those from ICE SXTMO10 and R391. This data together with ICESh392 sequence analysis (QFDC00000000) and the concatenated setCD/setR tree (Fig. S1) confirm that this element is a type 1 SXT/R391 member. On the other hand, the integrase from ICESh95 was closely related to Int from type 2 and 3 SXT/R391 ICEs, which have a different integration module and are integrated at the 3′ end of the tRNA-Ser gene6 (Fig. 3). The differences observed between other SXT/R391 ICEs and ICESh95 indicate that the latter was subjected to a distinct recombination event that originated a unique hybrid platform10.
Despite ICESh95 is less efficiently transferred to a new host, it has evolved into a multidrug resistant platform. Taking together our results and those reported for other SXT/R391 ICEs2,6,11, we can infer that ICESh392 from S. algae might adapt and acquire multidrug resistance genes upon antibiotic pressure.
SXT/R391 ICEs have evolved into well-oiled and highly efficient elements that either have or can recruit antimicrobial resistance genes in their platforms. Elements such as ICESh392 represent a troublesome scenario in the antimicrobial resistance field, where we can foresee the evolution of opportunistic bacteria acquiring mobile platforms that can carry resistance genes or gain novel determinants, and subsequently disseminate these traits into a new niche.
Concluding remarks
The comparative analysis of ICEs found in Shewanella spp. evidenced the plasticity of these mobile genetic elements, making them a potential threat facing the problem of multidrug resistance and exposing their adaptability. This report demonstrates that different ICEs can co-exist in a single strain and evaluates their ability to compete for a new host providing compelling information on the significant spread of ICEs SXT/R391 in nature.
Our results suggest that the ICEs studied here contribute to the evolution and the exchange of the genetic information between aquatic niches and nosocomial environments, where the antimicrobial pressure play a key role in the selection and survival of these bacteria.
Methods
Strains, plasmids and general molecular techniques
Clinical strains of Shewanella spp., including Shewanella sp. Sh9, Shewanella sp. Sh10, Shewanella sp. Sh31, Shewanella sp. Sh47, Shewanella sp. Sh74, Shewanella sp. Sh78, Shewanella sp. Sh82, Shewanella sp. Sh117, Shewanella sp. Sh256 and Shewanella sp. Sh392, the strain harbouring the ICESh95 as control, Shewanella sp. Sh9510,18,19 and Escherichia coli HB101 were grown at 37 °C in Luria-Bertani medium (10 g/l tryptone, 5 g/l yeast extract and 10 g/l NaCl in dH2O) with shaking at 200 rpm. Total DNA extraction was done using the Wizard Genomic DNA Purification kit (Promega). All PCR reactions were done using 1 U of Taq DNA polymerase (InBio Highway) in 1× Taq buffer (InBio Highway) supplemented with 2 mM MgCl2, 0.14 mM dNTP mix and 0.4 mM of each primer in a final volume of 50 μl. The PCR conditions were 3 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at the appropriate annealing temperature and 1 min at 72 °C, followed by a final extension of 5 min at 72 °C. Amplification of the 16s rRNA gene of clinical isolates of Shewanella spp. was done using specific primers FD2 and RP227. All positive PCR amplification products were confirmed by sequencing. Occurrence of SXT-ICE family in Shewanella genus was determined by PCR amplification of the origin of transfer (oriT) with primers oriT-SXT-F (5′-TGTATCCCTTGTCAGGTATG-3′) and oriT-SXT-R (5′-ACCCCAAAAGCCAAAACCAC-3′) and the setR gene with primers setR-SXT-F (5′-TACTGGTTACTGGCTTTCA-3′) and setR-SXT-R (5′-CATCAACTCAAAACAGCAA-3′). Detection of the excised form of ICESh392 was done by using ICE-3′ end-F (5′-GTGAACAGAAGTACCTAAA-3′) and primer Sh392-5-end-F (5′-ATAACTCTAGGTCCAGTAC-3′). Characterization of the insertion site was done by PCR using primers ICE-3′ end-F (5′-GTGAACAGAAGTACCTAAA-3′), pabA-R (5′-CTCTTGCCCTAACTGCT-3′), primer pabAEc-R (5′GCACGCTGGTAATGAGTCAG-3′), primer prfCSh95-R (5′-GCGTTTCCGAATAACAGCA-3′) and prfCEc-R (5′-GAGAAATAATGGCAAAAGTG-3′). The xis/int module was detected using primers Xis-ICE-F (5′-GTTGAGTATATGATGCTCTGG-3′) and int-ICE-R for ICESh95-like elements (5′-CAAGTGGTCCGAAAACTACA-3′) and IntSXT-F (5′-AGACTCTGCCGGTAAGCAAG-3′) and IntSXT-R (5′-GCAACTTCTTGCGTCGTGAT-3′) for SXT-like elements. For HS3 characterization we used the following primers: inti9-Sh95R (5′-TTGAGTAGACGCCGTAACT-3), Sh95-GII-IntF (5′-TTACAGCAGGCATGGAAACA-3), Sh95-GII-IntR (5′-CGCCATTGCCGCCAATAG-3′), dfrA15-F (5′-CTATCACTAATGGCAGCA-3′), dfrA15-R (5′-GAACGAGTTACAACGGCA-3′), TraF-ICESh95-R (5′-GAACCAGGCAGCACTGA-3) and IS4-ICE-F (5′-ACCGACTTTCCGCACCTGAT-3′). Lastly, for qPCR assays we used the following primers: IntSXTqPCR-F (5′-GAGTTCGTTTGCCTTTCTTAC-3′), IntSXTqPCR-R (5′-ACTGTCTTCGTGAGGTTTCG-3′), Int95qPCR-F (5′-CGTACCATTATCCAAACAAGC-3′), Int95-R-qPCR (5′-TGCCGTACCCCT GAATCCA-3′), traV-F (5′-CAGTCGGCTCAACCTTATTG-3′) and traV-R (5′-ATCAGATTGTGAATACCCAG-3′).
Mating assay
Mating assays were carried out on LB agar plates. Strains Sh95 (TmpR), Sh31 (TmpR), Sh82 (TmpR) and Sh392 (TmpR) were used as donor strains, while E. coli HB101::pCR2.1 (KanR) and Shewanella sp. Sh10::pCR2.1 (KanR) were used as recipient strains. Donor and recipient strains were diluted from saturated overnight cultures into 10 ml and grown for 7 h at 37 °C. The cells were harvested by centrifugation, poured onto LB agar plates and incubated at 37 °C for 18 h. The cells were scraped off the mating plates and serial dilutions were plated onto LB agar with the specific antibiotic to select for donor, recipient or transconjugant cells. Frequency of transfer was expressed as the number of transconjugant cells per donor cell in the mating mixture at the time of plating. Transconjugants obtained with both recipient strains were checked for the presence of ICESh95 and SXT by PCR with the original recipient strains used as negative controls.
Co-existence assays were done using strains Sh95 and Sh392 as donor and recipient strains and transconjugant cells were tested by PCR with specific primers for each xis/int module. Re-transfer assay was done using Shewanella sp. Sh95::ICESh392 as donor and E. coli HB101 as recipient strains. Transconjugant cells were tested by PCR for each xis/int module as described before. To evaluate the transfer efficiency of both ICEs, we did a conjugative assay using strains Sh95 and Sh392 as donors strains and E. coli HB101::pCR2.1 as recipient strain. Conjugative assays were done as described above using both donor strains in each assay so that both ICEs compete for the recipient (ratio 1:1:1). The cells were scraped off the mating plates and serial dilutions were plated onto LB agar with kanamycin (50 μg/ml) to select for the transconjugant cells. Lastly, we did a total DNA extraction of the transconjugant cells and the controls using the Wizard Genomic DNA Purification kit (Promega) and analyzed the amount of each ICE by qPCR.
Real Time PCR assays
Real-time quantitative PCR assays were designed to measure the transfer rate of the elements SXT or ICESh95-like to E. coli HB101 and evaluate their transfer efficiency. To measure the rates of each element we calculated the amount of each integrase of ICESh95 and ICESh392, which were normalized with the amount of chromosomal DNA in each sample using the conserved gene traV, encoded in both ICEs (adapted from ref.21). Briefly, each primer set was tested at different concentrations (5–30 pmol/μl) to optimize their performance. The StepOnePlus Real-Time PCR System was used to quantify the increase in fluorescence emission during PCR that resulted from the binding of the dye EVA Green to double-stranded DNA. Each 20 μl reaction mixture contained 4 μl of 5x HOT FIREPol® EvaGreen® qPCR Mix Plus (ROX) (Solis BioDyne), 5 pmol/μl of each primer and 8 ng of the DNA template. All qPCR products were electrophoresed on 1.2% agarose gels to detect the presence of a single band. We performed three independent assays. A standard curve was generated for each gene by using the donors DNA (Shewanella sp. Sh95 and Shewanella sp. Sh392) as the template and by plotting the Ct values as a function of the concentration of DNA in order to calculate the PCR efficiencies (E) as described elsewhere21. The efficiency of intSh95 was 2.00, intSXT was 1.90, and traV were 1.98 and 2.02 (with both donors, Sh95 and Sh392 respectively). We calculated the relative ratio of each int based on the E and the Ct deviation (ΔCt) of the transconjugant sample compared with each donor DNA (control samples). Each value was normalized by the calibrator sequence (traV gene) by using the following equation:
where Eint is the PCR efficiency of the int site tested (intSh95 or intSXT), EtraV is the PCR efficiency of the calibrator sequence (traV gene), ΔCt(int) is the difference between the Ct value of the donor strain and the Ct value of the transconjugant strain [Ct(Sh95) − Ct(sample)] for the intSh95 gene tested or [Ct(Sh392) − Ct(sample)] for the intSXT, and ΔCt(traV) is the difference between the Ct value of the donors strains and the Ct value of the recipient strain [Ct(Sh95 or Sh392) − Ct(sample)] for the calibrator sequence.
Genome sequencing, assembly and annotation
Total DNA of Shewanella sp. Sh392 was obtained using the Wizard Genomic DNA Purification kit (Promega). A draft sequence of this genome was developed using Illumina MiSeq at the Argentinian Consortium of Genomic Technology (ACGT). A total of 9938334 high-quality paired-end reads were produced, with an average insertion size of 436 bp. De novo assembly was performed with the SPAdes assembler version 3.6.228. 4900160 pairs of reads plus 15837 unpaired reads, 98.8% of the generated reads, were assembled resulting in mean nucleotide coverage of 332 (and a k-mer coverage of 117). Corrected reads showed an average length of 164 bp. This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession QFDC00000000.
Phylogenetic analysis
ICEs integrases protein sequences were downloaded from GenBank and aligned using Clustal Omega v1.2.129. The datasets comprise 46 amino acid sequences of various integrases of representative ICEs and other integrases as outgroups, such as XerD, XerC, IntI1 and P4 Int (Table S1). SetC, SetD and SetR protein sequences were downloaded from GenBank and independently aligned using Clustal Omega v1.2.129 (Table S2). The alignments were concatenated and the final dataset comprises 34 sequences including 492 amino acid positions. A maximum likelihood phylogenetic analysis was done for each dataset by means of the PHYML v3.1 software30. The LG + G model was used31, with 8 substitution rate categories and 5 random starting trees. The default SH-like test32 was used for branch support assessment.
Accession codes
The genome of Shewanella sp. Sh392 was deposited in GenBank as accession number QFDC00000000.
References
Wozniak, R. A. et al. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet 5(12), e1000786, https://doi.org/10.1371/journal.pgen.1000786 (2009).
Cury, J., Touchon, M. & Rocha, E. P. C. Integrative and conjugative elements and their hosts: composition, distribution and organization. Nucleic Acids Res. 45(15), 8943–8956, https://doi.org/10.1093/nar/gkx607 (2017).
Delavat, F., Miyazaki, R., Carraro, N., Pradervand, N. & van der Meer, J. R. The hidden life of integrative and conjugative elements. FEMS Microbiol Rev. 41(4), 512–537, https://doi.org/10.1093/femsre/fux008 (2017).
Hochhut, B. & Waldor, M. K. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol Microbiol 32, 99–110 (1999).
Ryan, M. P., Armshaw, P. & Pembroke, J. T. SXT/R391 Integrative and Conjugative Elements (ICEs) Encode a Novel ‘Trap-Door’ Strategy for Mobile Element Escape. Front Microbiol. 7, 829, https://doi.org/10.3389/fmicb.2016.00829 (2016).
Bioteau, A., Durand, R. & Burrus, V. Redefinition and unification of the SXT/R391 family of integrative and conjugative elements. Appl Environ Microbiol, 84(13), https://doi.org/10.1128/AEM.00485-18 (2018).
Peters, S. E., Hobman, J. L., Strike, P. & Ritchie, D. A. Novel mercury resistance determinants carried by IncJ plasmids pMERPH and R391. Mol Gen Genet. 228(1-2), 294–299 (1991).
Pembroke, J. T. & Piterina, A. V. A novel ICE in the genome of Shewanella putrefaciens W3-18-1: comparison with the SXT/R391 ICE-like elements. FEMS Microbiol Lett 264, 80–88, https://doi.org/10.1111/j.1574-6968.2006.00452.x (2006).
Rodríguez-Blanco, A., Lemos, M. L. & Osorio, C. R. Integrating conjugative elements as vectors of antibiotic, mercury, and quaternary ammonium compound resistance in marine aquaculture environments. Antimicrob Agents Chemother. 56(5), 2619–2626 (2012).
Parmeciano Di Noto, G., Jara, E., Iriarte, A., Centrón, D. & Quiroga, C. Genome analysis of a clinical isolate of Shewanella sp. uncovered an active hybrid integrative and conjugative element carrying an integron platform inserted in a novel genomic locus. Microbiology. 162(8), 1335–1345, https://doi.org/10.1099/mic.0.000310 (2016).
Fang, Y. et al. Distribution and Genetic Characteristics of SXT/R391 Integrative Conjugative Elements in Shewanella spp. From China. Front. Microbiol. 9, 920, https://doi.org/10.3389/fmicb.2018.00920 (2018).
Fredrickson, J. K. et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6, 592–603, https://doi.org/10.1038/nrmicro1947 (2008).
Janda, J. M. & Abbott, S. L. The genus Shewanella: from the briny depths below to human pathogen. Crit Rev Microbiol. 40(4), 293–312, https://doi.org/10.3109/1040841X.2012.726209 (2014).
Srinivas, J., Pillai, M., Vinod, V. & Dinesh, R. K. Skin and Soft Tissue Infections due to Shewanella algae - An Emerging Pathogen. J Clin Diagn Res. 9(2), DC16–20, https://doi.org/10.7860/JCDR/2015/12152.5585 (2015).
Guinetti-Ortiz, K., Bocanegra-Jesús, A. & Gómez de la Torre-Del Carpio, A. Osteomyelitis due to Shewanella putrefaciens: case report and literature review. Medwave. 16(10), e6642, https://doi.org/10.5867/medwave.2016.10.6642 (2016).
Yousfi, K., Bekal, S., Usongo, V. & Touati, A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur J Clin Microbiol Infect Dis. 36(8), 1353–1362, https://doi.org/10.1007/s10096-017-2962-3 (2017).
Takata, T. et al. Shewanella algae Bacteremia in an End-stage Renal Disease Patient: A Case Report and Review of the Literature. Intern Med. 56(6), 729–732, https://doi.org/10.2169/internalmedicine.56.7616 (2017).
Ramírez, M. S., Merkier, A. K., Almuzara, M., Vay, C. & Centrón, D. Reservoir of antimicrobial resistance determinants associated with horizontal gene transfer in clinical isolates of the genus Shewanella. Antimicrob Agents Chemother 54, 4516–4517, https://doi.org/10.1128/AAC.00570-10 (2010).
Almuzara, M. et al. Genetic analysis of a PER-2-producing Shewanella sp. strain harbouring a variety of mobile genetic elements and antibiotic resistance determinants. J Glob Antimicrob Resist. 11, 81–86, https://doi.org/10.1016/j.jgar.2017.06.005 (2017).
Ceccarelli, D., Daccord, A., René, M. & Burrus, V. Identification of the origin of transfer (oriT) and a new gene required for mobilization of the SXT/R391 family of integrating conjugative elements. J Bacteriol. 190(15), 5328–5338, https://doi.org/10.1128/JB.00150-08 (2008).
Burrus, V. & Waldor, M. K. Control of SXT integration and excision. J Bacteriol. 185(17), 5045–5054 (2003).
Aziz, R. K. et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics. 9, 75, https://doi.org/10.1186/1471-2164-9-75 (2008).
Kim, D. et al. Shewanella haliotis sp. nov., isolated from the gut microflora of abalone, Haliotis discus hannai. Int J Syst Evol Microbiol. 57(Pt 12), 2926–2931, https://doi.org/10.1099/ijs.0.65257-0 (2007).
Szeinbaum, N., Kellum, C. E., Glass, J. B., Janda, J. M. & DiChristina, T. J. Whole-genome sequencing reveals that Shewanella haliotis Kim et al. 2007 can be considered a later heterotypic synonym of Shewanella algae Simidu et al. 1990. Int J Syst Evol Microbiol. 68(4), 1356–1360, https://doi.org/10.1099/ijsem.0.002678 (2018).
Marrero, J. & Waldor, M. K. Determinants of entry exclusion within Eex and TraG are cytoplasmic. J Bacteriol. 189, 6469–6473, https://doi.org/10.1128/JB.00522-07 (2007).
Taviani, E. et al. Genomic analysis of ICEVchBan8: An atypical genetic element in Vibrio cholerae. FEBS Lett. 586(11), 1617–21, https://doi.org/10.1016/j.febslet.2012.03.064 (2012).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173(2), 697–703 (1991).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19, 455–477, https://doi.org/10.1089/cmb.2012.0021 (2012).
Sievers, F. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7, 539, https://doi.org/10.1038/msb.2011.75 (2011).
Guindon, S. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3), 307–321, https://doi.org/10.1093/sysbio/syq010 (2010).
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol Biol Evol. 25(7), 1307–1320, https://doi.org/10.1093/molbev/msn067 (2008).
Anisimova, M. & Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 55(4), 539–552, https://doi.org/10.1080/10635150600755453 (2006).
Acknowledgements
G.P.D.N. is a recipient of a doctoral scholarship from Consejo Nacional de Investigaciones en Ciencia y Tecnología (CONICET). C.Q. and D.C. are career investigators from CONICET. This work was supported by grants BID/OC ANPCyT (2013–1978) to C.Q. and International Cooperation CONICET (Argentina)-ANII (Uruguay) Res. 4360/16 to C.Q. and A.I.
Author information
Authors and Affiliations
Contributions
C.Q. conceived of the presented work with support from G.P.D.N., C.Q. and G.P.D.N. designed the experiments, G.P.D.N. performed the experiments and bioinformatics studies, A.I. performed the bioinformatics studies, C.Q. and G.P.D.N. analyzed and interpreted the results. D.C. and M.S.R. provided essential bacterial strains, D.C. and C.Q. contributed with reagents/materials/analysis tools and C.Q. and G.P.D.N. wrote the manuscript with support from A.I., M.S.R. and D.C. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parmeciano DI Noto, G., Iriarte, A., Ramírez, M.S. et al. ICE SXT vs. ICESh95: Co-existence of Integrative and Conjugative Elements and Competition for a New Host. Sci Rep 9, 8045 (2019). https://doi.org/10.1038/s41598-019-44312-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44312-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.