Abstract
Arterial hypertension is a leading risk factor for developing atrial fibrillation. CHA2DS2-VASc score can help to decide if patients with atrial fibrillation need anticoagulation. Whether CHA2DS2-VASc may predicts incident atrial fibrillation and how it interacts with left atrial dilatation is unknown. We tested this hypothesis in a large registry of treated hypertensive patients. From 12154 hypertensive patients we excluded those with prevalent atrial fibrillation (n 51), without follow-up (n 3496), or carotid ultrasound (n 1891), and low ejection fraction (i.e. <50%, n 119). A CHA2DS2-VASc score ≥3 was compared with CHA2DS2-VASc score ≤2. Incident symptomatic or occasionally detected atrial fibrillation was the end-point of the present analysis. At baseline, 956 (15%) patients exhibited high CHA2DS2-VASc; they were older, most likely to be women, obese and diabetic, with lower glomerular filtration rate, and higher prevalence of left ventricular hypertrophy, left-atrial dilatation and carotid plaque (all p < 0.005). Prevalent Stroke/TIA was found only in the subgroup with high CHA2DS2-VASc. During follow-up (median = 54 months) atrial fibrillation was identified in 121 patients, 2.57-fold more often in patients with high CHA2DS2-VASc (95% Cl 1.71–4.86 p < 0.0001). In multivariable Cox analysis, CHA2DS2-VASc increased incidence of atrial fibrillation by 3-fold, independently of significant effect of left-atrial dilatation (both p < 0.0001) and other markers of organ damage. Incident AF is more than doubled in hypertensive patients with CHA2DS2-VASc ≥3. Coexisting CHA2DS2-VASc score >3 and LA dilatation identify high risk subjects potentially needing more aggressive management to prevent AF and associated cerebrovascular ischemic events.
Similar content being viewed by others
Introduction
Arterial hypertension (Hpt) is a major cardiovascular disorder and atrial fibrillation (AF) the most common arrhythmia. These conditions frequently overlaps and their prevalence increases with age. Different risk factors and clinical conditions predispose to the development of AF, but because of its high prevalence, Hpt is still the most common population-attributable risk for the development of this arrhythmia1.
Since the prevalence of both medical conditions is increasing paralleling human life expectation, AF in hypertensive patients will become a leading risk factor for cardiovascular morbidity and mortality in the next future1.
More than 30% of patient with AF presented with no obvious symptoms or impaired quality of life. Therefore, the first clinical evidence of this ‘silent’ AF could be a thromboembolic event2. At least 30% of patients presenting with strokes present previously unrecognized AF3. MRI studies revealed that up to 40% of patients with AF had one or more silent cerebral infarcts4. The attempt to accurately predict risk of thromboembolic events, and especially of cardioembolic stroke, has been a very important field of scientific interest in the recent years. CHA2DS2-VASc score has been demonstrated to be useful in early risk stratification of patients with non-valvular AF in terms of intracardiac thrombogenesis and cardioembolism5, and allows to start antithrombotic treatments with a net favourable balance between stroke prevention and bleeding complications6,7,8.
Furthermore, in patients with nonvalvular AF and stroke, CHA2DS2-VASc score was found to be associated with intracerebral atherosclerosis9, suggesting that these scores might reflect the global burden of atherosclerosis10. This association also suggests that the CHA2DS2-VASc might be used to stratify CV risk beyond the limits of current guidelines.
In the contest of the Campania Salute Network (CSN) registry, the development of AF has been recently considered as an incident CV event and used as soft end-point. Therefore, given the above rationale, in the present study we assess whether a high CHA2DS2-VASc score can predict incident AF in a registry of treated hypertensive patients.
Methods
Participants
The CSN is an open registry collecting information from general practitioners and community hospitals in the 5 districts of the Campania Region, in Southern Italy. General practitioners and community hospitals are networked with the Hypertension Research Center of the Federico II University Hospital in Naples4. The data-base generation of CSN was approved by “Federico II” institutional Ethic Committee and signed informed consent was obtained from all participants. All research was performed in accordance with relevant guidelines/regulations. All hypertensive patients of the network were referred for baseline echocardiograms and carotid ultrasound to our Hypertension Center. Detailed characteristics of CSN population have been previously repeatedly reported2,11,12.
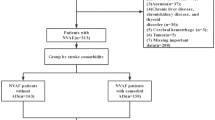
From a population of 12154 hypertensive patients we excluded patients with AF at baseline (n 51), no follow-up (n 3496), unavailable carotid ultrasound (n 1891), low left ventricular (LV) ejection fraction, (<50%, n 119). Thus, the study population comprised 6597 hypertensive patients.
Outcome
All prevalent and incident CV or cerebrovascular events (including AF), were adjudicated by the Committee for Event Adjudication in the Hypertension Research Center. Adjudication was based on patients’ history, contact with the reference general practitioner or community hospital and clinical records documenting the occurrence of the event/arrhythmia2,13.
Incident AF was the end-point of the present analysis. Incident AF was demonstrated in symptomatic patients going to the emergency department or in asymptomatic patients during a routine visit. AF was confirmed by an ECG performed at our outpatient clinics or in other hospitals at the time of hospitalization or by the GP at the time of the control visit2.
Measurements and definitions
Diabetes was defined according to 2007 ADA criteria (fasting plasma glucose >125 mg/dl or anti-diabetic treatment)14. Obesity was defined as a BMI ≥30 kg/m2. Systolic and diastolic BP were measured by standard aneroid sphygmomanometer after 5 minutes resting in the sitting position, according to current guidelines15. Glomerular filtration rate (GFR) was measured by the CKD-EPI (Chronic Kidney Disease Epidemiology collaboration) equation16,17.
Echocardiography
Echocardiograms were recorded in our Hypertension Research Center on videotapes, using commercial machines and a standardized protocol, were digitally mastered and read off line by one expert reader under the supervision of a senior faculty member, using dedicated work-stations (MediMatic, Genova, Italy1).
Measurements were made according to the EACVI/ASE recommendations18. LA antero-posterior diameter was measured from the parasternal long axis view19. LA volume (LAv) was estimate from the LA diameter (LAd) in centimeters, based on an elliptic model, by a best-fitting validated nonlinear equation20:
LAv was normalized by height in meters to the second power, as recently indicated and prognostically validated in a large cohort study16. LAv dilatation was adjudicated for LAV ≥17.5 ml/m2 in men or ≥14.8 ml/m2 in women, based on the 95th sex-specific percentile of our healthy population participating in the multicenter EchoNoRMAL Study18,21. LV mass was estimated from a necropsy-validated formula and normalized for height in meters to the power of 2.7 (LVMi)22,23,24. LV hypertrophy (LVH) was defined as LVMi ≥50 g/m2.7 in men and ≥47 g/m2.7 in women22,23. LV volumes were estimated by the z-derived method25, and used to compute ejection fraction and stroke volume26.
Carotid ultrasound
Carotid ultrasound was performed with the patients in the supine position and the neck extended in mild rotation. Examinations were recorded on S-VHS videotapes and analyzed as previously described1,27. The maximal arterial intima-media thickness (IMT) was estimated offline in up to 12 arterial walls, including the right and the left, near and far distal common carotid (1 cm), bifurcation, and proximal internal carotid artery, and using an image-processing dedicated workstation (MediMatic, Genova, Italy). Evidence of IMT value higher than 1.5 mm was considered as ‘plaque’28,29,30,31,32,33.
Statistical analysis
Data were analyzed using SPSS (version 23.0; SPSS, Chicago, IL) and expressed as mean ± 1 SD. Due to the presence of one factor in all participants (hypertension), we considered a CHA2DS2-VASc ≥3 as representative of high risk sub-population (corresponding to an annual risk of stroke of 3.2%), whereas a score <3 was considered at low-moderate risk. ANOVA was used to compare baseline characteristics of patients with high and low-moderate CHA2DS2-VASc. The chi-square distribution was used to compare categorical variables, with the MonteCarlo simulation to obtain precise p values. LV mass index, LAVI and IMT were also dichotomized according to the presence of LV hypertrophy, LA dilatation and carotid plaque. Cumulative incidence of AF during follow-up was estimated by product limit Kaplan –Meier survival function, to compare AF-free survival in groups of patients with low-moderate CHA2DS2-VASc and normal LAVI, high CHA2DS2-VASc and normal LAVI, low-moderate CHA2DS2-VASc and dilated LAVI and with coexistence of both high CHA2DS2-VASc and dilated LAVI.
We calculated hazard ratios (HR) and 95% confidence intervals (CI), by multivariable Cox proportional hazard regression models, using an enter model building procedure with hierarchical steps. The null hypothesis was rejected at a two-tailed α-value of ≤0.05.
Results
Patient characteristics
Table 1 shows the CHA2DS2-VASc score used to classify patients.
At baseline, 956 (15%) patients exhibited high baseline CHA2DS2-VASc score; they were older, most likely to be women, obese and diabetic, exhibited lower GFR, and higher prevalence of LVH, LAVI dilatation and carotid plaque (p < 0.005) (Table 1). Prevalent Stroke/TIA was found only in the subgroup with high baseline CHA2DS2-VASc score and not in the group with low-moderate CHA2DS2-VASc score. During a median follow-up of 54 months, a first episode of AF was identified in 121 patients, significantly more often in those with high CHA2DS2-VASc (p < 0.0001, Table 2).
Incident AF at follow-up
Absolute risk for incident AF was 8.8/1000 patients/year in subjects with high CHA2DS2-VASc score vs 4.4/1000 patients/year in those with low-moderate CHA2DS2-VASc score, with an Odds Ratio of 2.57 (95% Cl 1,71–4,86, p < 0.0001).
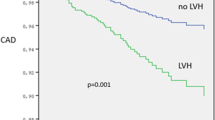
In the Kaplan-Meier plot, AF-free survival was significantly reduced in patients with high CHA2DS2-VASc score (p < 0.0001, Fig. 1).
The multivariable Cox analysis confirmed that patients with high CHA2DS2-VASc score had increased hazard of incident AF (HR = 2.99; 95%CI = 1.64–5.43; p < 0.0001), independently of significant effect of LA dilatation (HR = 2.41; 95%CI = 1.35–4.29; p < 0.003). When other markers of target organ damage, including GFR, LVH and carotid plaque, the relative contribution of high CHA2DS2-VASc score and LA dilatation remained similar and very significant (Table 3).
Table 3 also shows that there was a progressive increase in the hazard ratio from the subgroup with normal CHA2DS2-VASc score but dilated left atrium to the subgroup with high CHA2DS2-VASc score and normal LAD, up to the subgroup carrying both abnormalities, exhibiting more than eight-fold higher hazard than patients with low-moderate CHA2DS2-VASc score and normal LAD (Table 3 and Fig. 2). This result remained similar also including GFR, LVH and carotid plaque as covariables.
Discussion
Our study demonstrates that in a large population of hypertensive treated patients:
-
(1)
CHA2DS2-VASc score predicts development of AF, independently of markers of target organ damage, including chronic kidney disease, LVH, carotid plaque and LA dilatation.
-
(2)
The coexistence of CHA2DS2-VASc score ≥3 and of LA dilatation has a synergistic effect, amplifying the risk of incident AF.
In a previous large population-based study, CHADS2 and CHA2DS2-VASc predicted new-onset AF34. However, that cohort included a heterogeneous population of individuals 50 years of age or more. Moreover, no information was available on target organ damage. In contrast, our study focused on a specific population of patients with arterial hypertension including any patient above 18 years of age. We also conducted an extensive evaluation of hypertensive target organ damage (i.e. chronic kidney disease, LVH, carotid plaque and LA dilatation).
Thus, we identify a sub-population of hypertensive patients with high risk of AF, potentially needing more aggressive diagnostic and therapeutic strategies. In addition, we combined the CHA2DS2-VASc score with a marker of target organ damage, LA dilation13,19, that is specific for incident AF, and found that the coexistence of high CHA2DS2-VASc score and of LA dilatation substantially increases the probability of incident AF. This finding could be of great clinical interest. In fact, this is the first paper demonstrating an interaction between CHA2DS2-VASc and LA dilation with a hazard ratio that almost triplicates when both conditions coexist.
Although the European Society of Cardiology guidelines recommends screening for AF in patients 65 years or older, by pulse palpation, followed by an electrocardiogram (ECG) in those with irregular pulse frequency, unrecognized AF remains an important cause of morbidity and mortality in high-risk patients, strongly encouraging the research of dedicated strategies for early detection36,35. In fact, asymptomatic AF detected by implanted devices, is associated with increased risk of ischemic stroke and systemic embolism37. Furthermore. hypertensive patients with pacemaker exhibit silent brain infarcts associated with silent AF38. More recently, the REVEAL-AF study demonstrated a substantial proportion of undiagnosed AF (nearly 30%) in patients demographically at high risk of both AF and stroke39. In cardiac implantable electronic device population, a very recent paper also demonstrated that “atrial high-rate” episodes increase as CHA2DS2-VASc score increases40. However, in this study only a modest ability to identify AF was demonstrated.
This disappointing result is due to several reasons:
-
1.
Limited time span of observation. In our study a much longer follow-up is available.
-
2.
Only CHA2DS2-VASc score was tested. It is reasonable to speculate that a multi parametric approach would be more accurate. In our analysis CHA2DS2-VASc score and LAV dilatation synergistically predict incident AF.
-
3.
Patients included so far were heterogeneous. Our population comprised only patients with arterial hypertension which is the most important risk factor for AF development.
New electronic tools might be of great help in the identification of silent AF, however waiting their implementation and spread, asymptomatic AF remains a critical problem in the prevention of stroke.
Our study offers a new tool, helping identification of a specific risk phenotype for AF, using the same score already validated for identification of high risk of stroke in patients with non-valvular AF. We demonstrate that the risk of incident AF is near doubled in hypertensive patients with CHA2DS2-VASc ≥3 and, especially important for risk stratification, that this risk is independent of coexisting target organ damage, as LVH, carotid plaque and renal function. There are aspects in our analysis that need to be highlighted.
Firstly, as usual, not all patients with ascertained AF episodes were symptomatic and went to hospital settings. The others were detected incidentally at the time of a doctor visit. Thus, it is reasonable that a number of episodes of AF could not be censored. At least one-third of patients with incident AF do not experience any obvious symptoms or noticeable degradation of quality of life41,42. Therefore, the initial manifestation of this ‘silent’ AF could be a thromboembolic event2, which can be in turn predicted by CHA2DS2-VASc score. It is relevant that the same score could also predict cardiac event most associated with ischemic stroke or microstroke, indirectly adding evidence to the close link between AF and stroke. Therefore, one could speculate that a more aggressive diagnostic strategy might be the use of an implantable cardiac monitor, in order to obtain an early diagnosis of AF in those at high risk.
Secondly, our work can be used as a hypothesis generating study. In fact, hypertensive patients with high CHA2DS2-VASc and LA dilatation might need more aggressive strategy to prevent development of AF or at least its consequence (cerebrovascular ischemic events). The possibility to prescribe oral anticoagulation therapy to prevent both silent brain infarcts and clinically evident strokes might be therefore open. Ad hoc studies should be performed at least in the population with coexisting CHA2DS2-VASc score >3 and LA dilatation, to establish the actual incidence of AF (both symptomatic and sub-clinical) and the subsequent need of anticoagulant therapy.
Conclusions
We demonstrated, in a population of treated hypertensive patients, that combining information from CHA2DS2-VASc score and LA dimensions predict the incidence of AF. Co-existence of high CHA2DS2-VASc score with LA dilation substantially increases the probability of incident AF and allows to identify a subpopulation of patients potentially needing more aggressive diagnostic and therapeutic strategies.
References
Manolis, A. J. et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the Working Group ‘Hypertension Arrhythmias and Thrombosis’ of the European Society of Hypertension. J Hypertens 30, 239–252, https://doi.org/10.1097/HJH.0b013e32834f03bf (2012).
Savelieva, I. & Camm, A. J. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol 4, 369–382 (2000).
Petersen, P. et al. Silent cerebral infarction in chronic atrial fibrillation. Stroke 18, 1098–1100 (1987).
Kempster, P. A., Gerraty, R. P. & Gates, P. C. Asymptomatic cerebral infarction in patients with chronic atrial fibrillation. Stroke 19, 955–957 (1988).
Puwanant, S. et al. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol 54, 2032–2039, https://doi.org/10.1016/j.jacc.2009.07.037 (2009).
Lip, G. Y., Tse, H. F. & Lane, D. A. Atrial fibrillation. Lancet 379, 648–661, https://doi.org/10.1016/S0140-6736(11)61514-6 (2012).
Lip, G. Y. & Tse, H. F. Management of atrial fibrillation. Lancet 370, 604–618, https://doi.org/10.1016/S0140-6736(07)61300-2 (2007).
Lip, G. Y. H. et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 19, 891–911, https://doi.org/10.1093/europace/eux091 (2017).
Kim, Y. D. et al. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke 42, 930–934, https://doi.org/10.1161/STROKEAHA.110.602987 (2011).
Dzau, V. J. et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 114, 2850–2870, https://doi.org/10.1161/CIRCULATIONAHA.106.655688 (2006).
Mancusi, C. et al. Effect of diabetes and metabolic syndrome on myocardial mechano-energetic efficiency in hypertensive patients. The Campania Salute Network. J Hum Hypertens 31, 395–399, https://doi.org/10.1038/jhh.2016.88 (2017).
de Simone, G. et al. Depressed myocardial energetic efficiency is associated with increased cardiovascular risk in hypertensive left ventricular hypertrophy. J Hypertens 34, 1846–1853, https://doi.org/10.1097/HJH.0000000000001007 (2016).
Losi, M. A. et al. Cardiovascular ultrasound exploration contributes to predict incident atrial fibrillation in arterial hypertension: the Campania Salute Network. Int J Cardiol 199, 290–295, https://doi.org/10.1016/j.ijcard.2015.07.019 (2015).
Association, A. D. Diagnosis and classification of diabetes mellitus. Diabetes Care 30(Suppl 1), S42–47, https://doi.org/10.2337/dc07-S042 (2007).
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520, https://doi.org/10.1001/jama.2013.284427 (2014).
Kuznetsova, T. et al. Impact and pitfalls of scaling of left ventricular and atrial structure in population-based studies. J Hypertens 34, 1186–1194, https://doi.org/10.1097/HJH.0000000000000922 (2016).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150, 604–612 (2009).
Marwick, T. H. et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE)†. Eur Heart J Cardiovasc Imaging 16, 577–605, https://doi.org/10.1093/ehjci/jev076 (2015).
Losi, M. A. et al. Atrial Dilatation Development in Hypertensive Treated Patients: The Campania-Salute Network. Am J Hypertens 29, 1077–1084, https://doi.org/10.1093/ajh/hpw043 (2016).
Canciello, G. et al. Validation of Left Atrial Volume Estimation by Left Atrial Diameter from the Parasternal Long-Axis View. J Am Soc Echocardiogr 30, 262–269, https://doi.org/10.1016/j.echo.2016.11.017 (2017).
Collaboration, E. N. R. M.-AotL. H. Ethnic-Specific Normative Reference Values for Echocardiographic LA and LV Size, LV Mass, and Systolic Function: The EchoNoRMAL Study. JACC Cardiovasc Imaging 8, 656–665, https://doi.org/10.1016/j.jcmg.2015.02.014 (2015).
Lønnebakken, M. T. et al. Left Ventricular Hypertrophy Regression During Antihypertensive Treatment in an Outpatient Clinic (the Campania Salute Network). J Am Heart Assoc 6, https://doi.org/10.1161/JAHA.116.004152 (2017).
Mancusi, C. et al. Left atrial dilatation: A target organ damage in young to middle-age hypertensive patients. The Campania Salute Network. Int J Cardiol. 15, 229–233, https://doi.org/10.1016/j.ijcard.2018.03.69 (2018).
de Simone, G. et al. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens 18, 191–196, https://doi.org/10.1016/j.amjhyper.2004.08.032 (2005).
de Simone, G. et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol 78, 801–807 (1996).
De Marco, M. et al. Influence of Left Ventricular Stroke Volume on Incident Heart Failure in a Population With Preserved Ejection Fraction (from the Strong Heart Study). Am J Cardiol 7, 1047–1052 (2017).
Mancusi, C. et al. Determinants of decline of renal function in treated hypertensive patients: the Campania Salute Network. Nephrol Dial Transplant. 33, 435–440, https://doi.org/10.1093/ndt/gfx062. (2018).
Mancusi, C. et al. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (the Campania Salute Network). Int J Cardiol 244, 260–264, https://doi.org/10.1016/j.ijcard.2017.06.045 (2017).
Mancia, G. et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34, 2159–2219, https://doi.org/10.1093/eurheartj/eht151 (2013).
Li, Y. et al. Relationship of CHA2DS2-VASc and CHADS2 score to left atrial remodeling detected by velocity vector imaging in patients with atrial fibrillation. PLoS One 8, e77653, https://doi.org/10.1371/journal.pone.0077653 (2013).
Casaclang-Verzosa, G., Gersh, B. J. & Tsang, T. S. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol 51, 1–11, https://doi.org/10.1016/j.jacc.2007.09.026 (2008).
Vaziri, S. M., Larson, M. G., Benjamin, E. J. & Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 89, 724–730 (1994).
Casaclang-Verzosa, G. et al. C-reactive protein, left atrial volume, and atrial fibrillation: a prospective study in high-risk elderly. Echocardiography 27, 394–399, https://doi.org/10.1111/j.1540-8175.2009.01039.x (2010).
Saliba, W., Gronich, N., Barnett-Griness, O. & Rennert, G. Usefulness of CHADS2 and CHA2DS2-VASc Scores in the Prediction of New-Onset Atrial Fibrillation: A Population-Based Study. Am J Med 129, 843–849, https://doi.org/10.1016/j.amjmed.2016.02.029 (2016).
Camm, A. J. et al. 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33, 2719–2747, https://doi.org/10.1093/eurheartj/ehs253 (2012).
January, C. T. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64, e1–76, https://doi.org/10.1016/j.jacc.2014.03.022 (2014).
Healey, J. S. et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366, 120–129, https://doi.org/10.1056/NEJMoa1105575 (2012).
Benezet-Mazuecos, J. et al. Silent brain infarcts in high blood pressure patients with cardiac implantable electronic devices: unmasking silent atrial fibrillation. J Hypertens 34, 338–344, https://doi.org/10.1097/HJH.0000000000000787 (2016).
Reiffel, J. A. et al. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol 2, 1120–1127, https://doi.org/10.1001/jamacardio.2017.3180 (2017).
Rovaris, G. et al. Does CHA2DS2-VASc Score reliably predict atrial arrhythmias? Analysis of a nationwide database of Remote Monitoring data daily transmitted from Cardiac Implantable Electronic Devices. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2018.02.023 (2018).
Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 18, 1609–1678, https://doi.org/10.1093/europace/euw295 (2016).
Barbarossa, A., Guerra, F. & Capucci, A. Silent Atrial Fibrillation: A Critical Review. J Atr Fibrillation 7, 1138, https://doi.org/10.4022/jafib.1138 (2014).
Acknowledgements
This study was supported by the Telematic Network Center project Fund; by grant “P.O.R. Campania FESR 2007–2013 - O.O. 2.1/CUP B25C13000280007”; and by grant PRIN 2015EASE8Z_004/MIUR.
Author information
Authors and Affiliations
Contributions
A.R., M.A.L. wrote the main manuscript text. C.M. prepared figures and tables. C.M., G.C., R.I., T.S., N.D.L., G.A., B.T. critically revised the manuscript. Gd.S., M.A.L. and C.M. performed statistical analysis and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rapacciuolo, A., Mancusi, C., Canciello, G. et al. CHA2DS2-VASc score and left atrial volume dilatation synergistically predict incident atrial fibrillation in hypertension: an observational study from the Campania Salute Network registry. Sci Rep 9, 7888 (2019). https://doi.org/10.1038/s41598-019-44214-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44214-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.