Abstract
Taste buds are comprised of taste cells, which are classified into types I to IV. Transient receptor potential (TRP) channels play a significant role in taste perception. TRP vanilloid 4 (TRPV4) is a non-selective cation channel that responds to mechanical, thermal, and chemical stimuli. The present study aimed to define the function and expression of TRPV4 in taste buds using Trpv4-deficient mice. In circumvallate papillae, TRPV4 colocalized with a type IV cell and epithelial cell marker but not type I, II, or III markers. Behavioural studies showed that Trpv4 deficiency reduced sensitivity to sourness but not to sweet, umami, salty, and bitter tastes. Trpv4 deficiency significantly reduced the expression of type III cells compared with that in wild type (WT) mice in vivo and in taste bud organoid experiments. Trpv4 deficiency also significantly reduced Ki67-positive cells and β-catenin expression compared with those in WT circumvallate papillae. Together, the present results suggest that TRPV4 contributes to sour taste sensing by regulating type III taste cell differentiation in mice.
Similar content being viewed by others
Introduction
We sense five basic tastes (bitter, sweet, umami, salty, and sour) across all regions of the tongue where taste buds exist. Taste buds are comprised of taste cells, which are classified into types I to IV1. Type I cells are generally thought to have a support function in the taste bud2. Type II cells detect sweet, bitter, and umami tastants via a common intracellular transduction cascade including PLCβ23. Type III taste cells are sour detectors and respond to acid taste stimulation by releasing serotonin4,5. The cell type mediating salty taste remains ambiguous6. Type IV basal cells differentiate into type I, II, and III taste cells during rapid cell turnover in taste buds2. Taste afferent nerve fibres transmit information from taste buds to the brain7.
Transient receptor potential (TRP) channels are non-selective cation channels activated by a variety of chemical and physical stimuli, such as temperature, oxidative stress, and osmotic pressure, and by food-derived products, such as capsaicin, menthol, and various lipids8. Proteins related to TRP channels, including TRP-melastatin 5 (TRPM5), polycystic kidney disease-1-like 3 (PKD1L3), and polycystic kidney disease-2-like 1 (PKD2L1), are also expressed in taste cells9. It is thus possible that these TRP channels play some roles in taste sensing10. In fact, TRPM5 has a well-defined role in the detection of bitter, sweet, and umami stimuli and is exclusively expressed in type II taste cells11.
TRPV4 is a non-selective cation channel that responds to mechanical, thermal, and chemical stimuli in addition to various endogenous ligands, such as arachidonic acid metabolites12. We recently reported that TRPV4 was expressed in the mouse gastrointestinal tract, where it regulated pathophysiological functions13. Furthermore, several studies have demonstrated possible mechano- and osmosensing roles of TRPV4 in taste buds14,15. TPRV4 is reportedly localized in sensory neurons16,17 and epithelial cells but not in taste buds of the tongue15. In contrast, TRPV4 immunostaining was observed in zebrafish taste buds18. At this stage, the localization and implied functions of TRPV4 in the taste buds and in taste sensation remain undefined. The present study aimed to define the expression of TRPV4 in the taste buds and investigate the roles of TRPV4 in taste perception using Trpv4-deficient mice.
Results
TRPV4 immunoreactivity is detected in type IV taste cells and epithelial cells of mouse circumvallate papillae
First, we investigated the expression of TRPV4 in the mouse tongue, oesophagus, stomach, ileum, and colon by immunohistochemistry (Fig. 1a). TRPV4 immunoreactivity was detected in epithelial-like cells of the mouse tongue, oesophagus, stomach, ileum, and colon. Within the mouse tongue, oesophagus, stomach, ileum, and colon, TRPV4 expression was highest in the tongue according to fluorescence intensities (Fig. 1b). We also found TRPV4 expression in the mouse circumvallate, fungiform, and foliate papillae (Fig. 1c).
Expression of TRPV4 in mouse tissues. (a) Immunohistochemistry of TRPV4 in the tongue, oesophagus, stomach, ileum, and colon. (b) Quantitative analysis of TRPV4 expression in the tongue, oesophagus, stomach, ileum, and colon. Data presented as the means ± SEM for 8 mice. (c) TRPV4 expression in the circumvallate, fungiform, and foliate papillae. Scale bars = 50 μm.
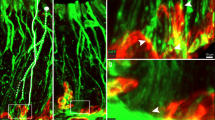
TRPV4 immunoreactivity in the circumvallate papillae was abolished in Trpv4 knockout (KO) mice (Fig. 2a). There were no structural differences between wild type (WT) and Trpv4 KO circumvallate papillae (Fig. 2b). Double-labelling experiments of TRPV4 with the type I taste cell marker NTPdase2, type II taste cell marker PLCβ2, type III taste cell markers CAR4 and 5-HT, type IV taste cell marker Sonic hedgehog, and basal keratinocyte marker keratin 14 were performed in circumvallate papillae (Fig. 2c). TRPV4 colocalized with Sonic hedgehog and keratin 14 but did not colocalize with any of the other markers.
TRPV4 expression in WT and Trpv4 KO mice. (a) TRPV4 expression in the circumvallate papillae. (b) Representative images of haematoxylin and eosin staining of WT and Trpv4 KO. (c) Double labelling experiments of TRPV4 (green) with NTPdase2, PLCβ2, CAR4, 5-HT, Sonic hedgehog, and keratin 14 (red) in the circumvallate papillae. Scale bars = 50 μm (a,b) and 20 μm (c). Arrows indicate colocalization of signals of TRPV4 and keratin 14.
Trpv4-deficient mice exhibit decreased behavioural response to sour tastants
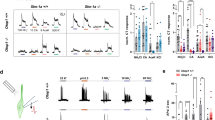
To determine the taste sensitivities of Trpv4 KO mice, we performed a two-bottle test (Fig. 3a). WT and Trpv4 KO mice were subjected to a preference test between water and an appetitive concentration of sucrose and sodium glutamate, followed by an aversion test between water and an aversive concentration of sodium chloride and denatonium benzoate. Trpv4 KO mice showed diminished avoidance of the citric acid solution compared with that of WT mice.
Behavioural responses of WT and Trpv4 KO mice to sour tastants. (a) Two-bottle tests were used to evaluate behavioural responses to sweet (sucrose, 30 mM), umami (monosodium glutamate, 100 mM), bitter (denatonium benzoate, 1 mM), salty (sodium chloride, 300 mM), and sour taste (citric acid, 10 mM) stimuli. Data presented as the means ± SEM for 8 mice. For statistical analysis, Student’s t-test was used. *P < 0.05 vs. WT. (b) Two-bottle (left) and brief-access (right) tests were used to measure behavioural responses to sour taste stimuli. Data presented as the means ± SEM for 8 mice. For statistical analysis, two-way ANOVA followed by Bonferroni’s multiple comparison test was used. *P < 0.05 vs. WT.
To assess the change in sour taste sensitivity in more detail, we performed two-bottle and brief-access tests using different concentrations of citric acid solutions (Fig. 3b). A statistical analysis by two-way analysis of variance (ANOVA) showed a significant difference in sour taste sensitivity between WT and Trpv4 KO mice for both tests (two-bottle test: p = 0.0009, F(1, 12) = 19.39; brief-access test: p = 0.014, F(1, 14) = 7.868). A post-hoc comparison test showed that the preference ratios in the Trpv4 KO mice for the 1, 3, 10, and 30 mM citric acid solutions were significantly higher than those in WT mice in the two-bottle test. Similarly, for the brief-access test, a post-hoc comparison test showed that the lick ratios in the Trpv4 KO mice for the 10 and 30 mM citric acid solutions were significantly lower than those in WT mice.
Trpv4 deficiency reduces type III taste marker expression in circumvallate papillae
In mice, taste buds are composed of collections of type I, II, and III taste cells. We next assessed changes in type I, II, and III taste cells and taste nerve cells in Trpv4 KO mice using NTPdase2, PLCβ2, CAR4, and P2X2 as markers. There was no significant difference in the fluorescence intensity of NTPdase2, PLCβ2, or P2X2 between WT and Trpv4 KO mice (Fig. 4a). In contrast, the fluorescence intensity and cell numbers of CAR4 in Trpv4 KO mouse taste buds were significantly reduced compared to those in WT mice.
Type III taste cell marker expression in circumvallate papillae and taste organoids of WT and Trpv4 KO mice. (a) Representative images and quantitative analysis of NTPdase2, PLCβ2, CAR4, and P2X2 expression in circumvallate papillae. (b) Quantitative analysis of NTPdase2, T1r2, T1r3, Pkd1l3, and CAR4 mRNA expression in circumvallate papillae. (c) Representative images and quantitative analysis of PLCβ2 and CAR4 expression in taste organoids derived from WT and Trpv4 KO. A taste organoid was derived from each WT or Trpv4 KO mouse. Data are presented as the mean ± SEM for 6–8 mice. For statistical analysis, Student’s t-test was used. *P < 0.05 vs. WT.
Next, we investigated the mRNA expression levels of NTPdase2, T1r2, T1r3, Pkd1l3, and CAR4 in WT and Trpv4 KO mice (Fig. 4b). Consistent with the immunofluorescence results, the mRNA expression levels of the type III markers Pkd1l3 and CAR4 in the circumvallate papillae of Trpv4 KO mice were significantly reduced compared to WT levels. There was no significant difference in the expression levels of the type I taste cell marker NTPdase2 or type II taste cell markers T1r2 and T1r3 between WT and Trpv4 KO mice.
Next, the effect of Trpv4 ablation on type III taste cell differentiation was tested in taste organoids derived from WT and Trpv4 KO circumvallate papillae (Fig. 4c). Immunostaining of Trpv4 KO organoids showed that the expression level of CAR4-immunopositive cells was significantly reduced compared with that in WT organoids, while that of PLCβ2-immunopositive cells was unchanged.
Trpv4 deficiency affects the expression of β-catenin in circumvallate papillae
To investigate the role of TRPV4 in the differentiation of taste cells, we compared the proportions of Ki67 and β-catenin expression in WT and Trpv4 KO mice (Fig. 5). Quantitative analysis showed that proliferating cell marker Ki67 was significantly decreased in the circumvallate papillae of Trpv4 KO mice compared with that in WT mice (Fig. 5a). β-catenin is a representative key regulator of taste cell renewal in mice. TRPV4 immunoreactivity in basal cells colocalized with that of β-catenin in circumvallate papillae (Fig. 5b). Western blotting analysis showed that expression of β-catenin was significantly reduced in Trpv4 KO mice compared with that WT mice (Fig. 5c). Finally, we performed subcellular fractionation of protein lysates and analysed cytosolic, membrane, and nuclear fractions by immunoblotting for β-catenin proteins in WT and Trpv4 KO mouse circumvallate papillae (Fig. 5d). The expression of β-catenin in all fractions was significantly lower in Trpv4 KO mice than in WT mice.
Effect of Trpv4 deficiency on Ki67 and β-catenin expression in circumvallate papillae and analysis of TRPV4 expression in circumvallate papillae subfractions. (a) Number of Ki67-immunopositive basal cells per 400 μm2 of epithelium in the circumvallate papillae of WT and Trpv4 KO mice. (b) Double-labelling of TRPV4 (green) and β-catenin (red) in circumvallate papillae. (c) β-catenin expression in circumvallate papillae of WT and Trpv4 KO mice. (d) β-catenin expression in cytoplasmic, membrane, and nuclear fractions of circumvallate papillae. Data are presented as the mean ± SEM for 8 mice. For statistical analysis, Student’s t-test was used. *P < 0.05 vs. WT.
Discussion
The sense of taste is responsible for the detection of bitter, sweet, umami, salty, and sour tastants by taste buds. TRP channels play an important role in taste, with channels responding to basic tastants. The current study demonstrated for the first time that TRPV4 is expressed in type IV cells of the taste buds. Trpv4 deficiency significantly reduced the sensitivity of sourness and the expression of type III cells compared with those in WT mice. These findings suggest that TRPV4 contributes to sour taste sensing by regulating type III taste cell differentiation in mice.
Previous studies have detected TRPV4 immunoreactivity in the epithelial cells of the mammalian alimentary tract13,19,20. We also detected TRPV4 immunoreactivity in epithelial cell-like structures in the gastrointestinal tract. Within the mouse tongue, oesophagus, stomach, ileum, and colon, TRPV4 expression was highest in the tongue. We further observed the expression of TRPV4 in circumvallate, fungiform, and foliate papillae. Circumvallate papillae, located on the surfaces of the back part of the tongue, contain higher numbers of taste buds than other papillae. It is thus likely that TRPV4 expressed on the oral side, in the circumvallate papillae, plays a role in the taste function.
TRPV4-immunopositive cells were previously identified in zebrafish taste buds18. In contrast, TRPV4 was found to be expressed in the epithelial cells but not the taste buds of the mouse tongue15. In the present study, we observed TRPV4 immunoreactivity in Sonic hedgehog-positive type IV cells in mouse circumvallate papillae. However, TRPV4 immunoreactivity did not colocalize with type I, II, and III taste cell markers. Sonic hedgehog-positive cells are precursors for all taste cell types within taste buds21. It has been previously reported that TRPV4 is expressed in keratin 14-positive epidermal keratinocytes and in the olfactory and airway epithelia22,23. In this study, we also observed TRPV4 immunoreactivity in keratin 14-positive basal epithelial cells. Keratin 14-positive epithelial cells located outside of the taste bud are bipotential progenitor cells that can generate both taste bud cells and keratinocytes24. These results suggest that TRPV4 is expressed in type IV cells and keratin 14-positive epithelial cells in the circumvallate papillae.
Some TRP channels are expressed in taste buds and have functional roles as detectors of taste. TRPM5 is known to be abundantly expressed in type II taste cells, where it participates in sweet, bitter, and umami taste perception25,26. In the present study, we found that Trpv4 deficiency reduced the sensitivity to sour taste but not to sweet, umami, salty, or bitter tastes in two-bottle tests. As previously reported, TRPV4 agonist 4α-phorbol 12,13-didecanoate administration did not affect the intake of sucrose solution27. Consequently, we compared the behaviour of Trpv4 KO and WT mice using different concentrations of citric acid solution presented in brief-access and two-bottle tests. Trpv4 deficiency tended to reduce taste aversion to sourness in both tests. These results imply that TRPV4 has an important role in sour taste sensing in mice. Type III cells respond directly to a variety of acids, such as citric acid, acetic acid, and HCl, suggesting that type III cells are involved in sour taste sensing4,5,28. Basal cells differentiate into new type I, II, and/or III taste cells during cell turnover2. Presynaptic type III taste cells survive for approximately one month, while type II taste cells have a life span of about one week and type I taste cells survive for 2–3 days29,30. Sour compounds are detected by type III taste cells, which represent a small population of cells within taste buds (~15%)4,31. The activation of type III taste cells in mammalian taste buds is implicated in the transduction of sour taste. To investigate the relationship between TRPV4 and sour taste detection, we compared the expression of type III taste cell markers between WT and Trpv4 KO mice. PKD2L1 and PKD1L3 may serve as sour taste sensors28,32. Trpv4 deficiency significantly reduced the mRNA levels of PKD1L3 and CAR4 and protein expression levels of the type III cell marker but did not affect type I or II cell marker expression levels. Taste bud organoids derived from circumvallate papillae can stably express differentiated cell types specific to the native organ33. Because taste organoids are not influenced by signals from other tissues, the results of genetic or other manipulations can be interpreted in a more straightforward manner33,34. The effect of Trpv4 deficiency in taste organoids reproduced the observations of in vivo experiments involving PLCβ2 and CAR4 expression in taste buds. These results suggest that TRPV4 regulates type III taste cell differentiation without affecting type I or II taste cells in mouse taste buds.
In brief-access and two-bottle tests, Trpv4 deficiency did not completely diminish taste aversion to sourness. Purinergic signalling, including P2X2 and/or P2X3 expressed on taste nerves, also contributes to taste sensing. Indeed, several studies have demonstrated that mice lacking P2X2 and P2X3 receptors exhibit essentially no response to all classes of tastants, including acids35,36. In the present study, Trpv4 deficiency did not affect the expression levels of taste nerve markers (P2X2 receptor) compared with those in WT. In contrast, Trpv4 deficiency significantly reduced the expression levels of type III cell markers (CAR4) compared with those in WT but did not completely abolish expression. Thus, it is likely that the remaining sour taste aversion in Trpv4 KO mice may be based on sour sensing via taste nerves and remaining type III taste cells.
Type III cells may also respond to high concentrations of NaCl37. A previous study also showed amiloride-insensitive salt taste-like responses in a subset of bitter-sensitive type II cells38. Based on these findings, the relationship between salty taste detection and TRPV4 is unclear at present, although it is possible that TRPV4 affects the type III taste cell function and/or expression.
Wnt/β-catenin signalling is involved in taste cell fate determination38,39. β-catenin activates the transcription of target genes in type IV basal cells to induce their differentiation into type I, II, and/or III cells. A previous study reported that TRPV4 interacts with ß-catenin at adherens junctions in keratinocytes40 and in the membranes of endothelial cells41. We found that TRPV4 colocalized with β-catenin in the basal cells of taste buds. Furthermore, Trpv4 deficiency significantly reduced β-catenin expression in cytosolic, membrane, and nuclear fractions. In endothelial cells, shear stress stimulation disrupted the interaction of TRPV4 with β-catenin in the basal membrane42. From these results, we speculate that Trpv4 deletion interrupts the interaction with β-catenin in the basal cell membrane and affects β-catenin expression in the taste buds. Conditional β-catenin deletion in mouse taste progenitor cells leads to taste bud loss and alterations in behavioural taste sensitivity43. This mutant also exhibited reductions in type I, II, and III taste cells. Conditional β-catenin deletion significantly reduced the perception of artificial sweetener SC45647 (sweet), denatonium (bitter), and a low concentration (1 mM) of citric acid (sour) but did not affect perception of high concentrations (30 and 100 mM) of citric acid. In the present study, we found that Trpv4 deficiency significantly reduced selective type III taste cells and citric acid (1 to 30 mM) perception compared with those of WT mice. However, the mechanism for the selective regulation of type III taste cells remains unclear. Further studies are needed to clarify the involvement of TRPV4 in regulating β-catenin expression and type III taste cell differentiation in circumvallate papillae. Additional molecular factors likely contribute to the regulation of the fate of type IV basal cells. The Sonic hedgehog and Notch pathways are also thought to be involved in taste cell fate determination2,34. Thus, further investigations of the relationship between TRPV4 and these other molecular factors are needed.
In conclusion, TRPV4 is involved in sour taste perception through the differentiation of type III taste cells via the regulation of β-catenin expression. Our results imply that TRPV4 is a key molecule in the differentiation of sour taste cells in mammalian taste bud stem cells.
Methods
Animals
Male C57BL/6 mice (8–10 weeks) weighing 22–27 g were purchased from Japan SLC Inc. (Shizuoka, Japan). Trpv4 KO mice were generated in a C57BL/6J background as described previously15. As previously reported, female hormones affect behavioural taste sensitivity in mice41. Therefore, we used only male mice in this study. All mice were maintained in plastic cages with free access to food and water and housed at 22 ± 1 °C under a 12-h light/dark cycle. This study was carried out in strict accordance with ARRIVE guidelines. The protocols were approved by the Committee on the Ethics of Animal Research of Kyoto Pharmaceutical University (permit numbers: 16-12-034, 17-12-034, and 18-12-034). The number of animals was kept to the minimum necessary for a meaningful interpretation of the data, and animal discomfort was minimized. WT and/or Trpv4 KO mice were divided into experimental groups according to the random comparison group method.
Tissue processing and histology
Animals were sacrificed by CO2 gas inhalation. Tissues were removed, washed with cold phosphate-buffered saline (PBS), and immersed in 4% paraformaldehyde for 2 h at 4 °C. Tissues were cryoprotected overnight in 20% sucrose solution prior to embedding in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan) mounting medium. They were then sectioned using a cryostat (Leica Instruments, Nussloch, Germany) at a thickness of 20 µm and thaw-mounted onto Superfrost Plus slides (Matsunami, Osaka, Japan). Immunohistochemical procedures were performed as previously described13. Sources of all primary and secondary antibodies, as well as the optimised dilutions, are listed in Supplementary Table 1. TRPV4 immunoreactivity was detected using the fluorescein-conjugated tyramide amplification method (Perkin Elmer Life Sciences, Boston, MA, USA). Cytokeratin 14 and β-catenin were detected using the MOM Immunodetection Kit (FMK-2201; Vector Laboratories, Burlingame, CA, USA). NTPDase2, CAR4, CGRP, 5-HT, PLCβ2, and Ki67 were detected by indirect staining with specific antibodies. P2X2 receptor-ATTO-594 and PLCβ2-Cy5 were detected by direct staining with specific antibodies. The specificity of the TRPV4 antibody was demonstrated by the loss of immunostaining in Trpv4 KO mice (Fig. 2a).
Microscopy and image analysis
Sections were viewed using a confocal microscope (A-1R+; Nikon, Tokyo, Japan), and images were captured using Nikon NIS-Elements AR 4.20.00 software. Multiple images in Z-stacks were projected onto a single plane and reconstructed using NIS-Elements AR 4.20.00 software. For quantitative analyses, circumvallate papillae were viewed at 200× magnification using a confocal microscope, and quantitative determinations were made from three random fields for each mouse. For analysis of the TRPV4-, NTPdase2-, PLCβ2-, CAR4-, and P2X2-immunopositive intensities, active areas were measured after interactive thresholding using the NIS-Elements AR 4.20.00 software image analysis system. The relative intensity of the TRPV4 KO was calculated by comparison with the intensity of WT. For analysis, the number of CAR4-immunopositive cells was counted per trench.
Western blotting
Gastrointestinal tissue preparation was performed as described previously13. Lingual epithelial tissue was exfoliated from the tongue by injection of an enzyme cocktail comprising 2.5 mg/mL dispase II (Wako Chemicals, Tokyo, Japan), 1.0 mg/mL collagenase D, and 1.0 mg/mL trypsin inhibitor (Roche Diagnostics, Basel, Switzerland) for 20 min at 25 °C, and then the epithelial tissue was peeled off. Proteins were separated by 7.5% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) by electroblotting. Three subcellular fractions (cytoplasmic, membrane, and nuclear) of circumvallate papillae were prepared utilizing the subcellular protein fractionation kit (Thermo Fisher Scientific, Rockford, IL, USA) and characterized by western blotting using fraction-specific protein antibodies. The membranes were stained with rabbit anti-β-catenin (1:500; Cell Signaling Technology, Danvers, MA USA) and rabbit anti-β-actin (1:2000; Gene Tex, San Antonio, TX, USA) antibodies (see full-length blot strips in the Supplementary Information). Immunoreactivity was detected by enhanced chemiluminescence (Perkin Elmer Life Sciences, Inc.), and band density was determined using the FUSION solo5 (Vilber Lourmat, Marne-la-Vallée, France).
Two-bottle tests
Taste preferences were assessed using an ascending concentration series of two-bottle choice tests for 48 h with the following taste compounds: sucrose, sodium glutamate, sodium chloride, denatonium benzoate, and citric acid. In each test series, the mice were trained on two drinking tubes containing deionized water for 1 week, and then a choice between deionized water and the taste compound was given, with each test lasting 96 h. The positions of the two drinking tubes were switched every 24 h. Intake from each tube was obtained by recording the weight of fluid at the beginning and end of each 48-h test. Total fluid intakes were obtained by adding together the intakes from both drinking tubes. Preference ratios were calculated as the intake of each solution divided by total intake and expressed as a percentage.
Brief-access test
A brief-access test was performed as described previously44. In brief, the lick number for distilled water over 10 s was determined, followed by a 15-s inter-presentation interval, and then the taste solution was presented for 10 s, followed by a 5-s water presentation. Because the lick number is affected by differences in motivation to drink solutions among mice, data are expressed as lick ratios as a quantitative index of taste sensitivity and were calculated by dividing the lick number over 10 s for the taste solution by that for distilled water. The number of licks of water used for the lick ratio calculation was recorded preceding the trial with the first sour taste solution (3, 10, 30, and 100 mM citric acid). Data were excluded when mice were unable to complete a series of brief-access tests in each session.
Reverse transcription and real-time quantitative polymerase chain reaction (PCR) analyses
Total RNA was extracted using a NucleoSpin RNA® XS kit (Macherey-Nagel, Düren, Germany) and a PrimeScriptTM RT reagent kit with gDNA Eraser (Takara, Shiga, Japan) and was analysed by quantitative PCR on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA) using SYBR Premix ExTaq II (Takara). Specific primer sets for β-actin, NTPdase2, T1r2, T1r3, Pkd1l3, and CAR4 are listed in Supplementary Table 2. The expression of each target gene was calculated by the comparative Ct method and was normalized to that in WT mice.
Organoid culture
Organoid culture was performed as described previously35. The tongue was isolated, and dispase II (Roche, 1 mg/mL) was injected under the epithelium. After 30 min of incubation at room temperature, the epithelium was peeled away under a dissecting scope, and the circumvallate papilla tissue was isolated. Tissues were incubated with 0.25% trypsin/EDTA for 30 min at 37 °C and centrifuged at 800 × g for 5 min. The tissue was suspended in Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and the suspended tissue was seeded into 12-well culture plates (50 μL Matrigel). After Matrigel polymerization at 37 °C, advanced DMEM/F12 supplemented with 2 mM GlutaMax, 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 × N2 and 1 × B27 supplements (Thermo Fisher Scientific, Rockford, IL, USA) plus the following growth factors was added to the wells and replaced every 4 days: taste bud organoid medium: Wnt-conditioned medium (50%), R-spondin-conditioned medium (10%), EGF (50 ng/mL, Pepro Tech, Rocky Hill, NJ, USA), and Noggin (100 ng/mL, Pepro Tech).
Organoid passage
Organoids in Matrigel were collected with cold DPBS (without Ca2+/Mg2+) and centrifuged at 150 × g for 5 min at 4 °C, followed by removal of supernatants including Matrigel. Taste bud organoids were incubated with 0.25% trypsin/EDTA for 30 min at 37 °C and then dissociated into single cells through a 31 G insulin needle. After centrifugation at 800 × g for 5 min, cells were re-suspended in Matrigel. Taste bud organoid medium was first changed after 5 days of culturing and then every 3 days. A taste organoid was derived from each WT or Trpv4 KO mouse. Immunohistochemical staining of taste bud organoids was conducted at 10 days after first passage.
Statistical analysis
Data are reported as mean ± standard error of the mean and were analysed with GraphPad Prism 6.07 (GraphPad Software, La Jolla, CA, USA). Multiple groups were compared by two-way (genotype and citric acid concentration) ANOVA followed by Bonferroni’s multiple comparison test. Parametric data were tested by Student’s t-test. Results were considered statistically significant at P-values < 0.05.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Murray, R. G. The ultrastructure of taste buds in The ultrastructure of sensory organs (ed. Friedman, I.) (Elsevier, 1974).
Barlow, L. A. & Klein, O. D. Developing and regenerating a sense of taste. Curr. Top. Dev. Biol. 111, 401–419 (2015).
Chaudhari, N. & Roper, S. D. The cell biology of taste. J. Cell Biol. 190, 285–296 (2010).
Huang, Y. A. et al. Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 586, 2903–2912 (2008).
Yoshida, R. et al. Discrimination of taste qualities among mouse fungiform taste bud cells. J. Physiol. 587, 4425–4439 (2009).
Barlow, L. A. Progress and renewal in gustation: new insights into taste bud development. Development 142, 3620–3629 (2015).
Yarmolinsky, D. A., Zuker, C. S. & Ryba, N. J. Common sense about taste: from mammals to insects. Cell 139, 234–244 (2009).
Nilius, B. & Szallasi, A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 66, 676–814 (2014).
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Roper, S. D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 22, 827–871 (2014).
Talavera, K. et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025 (2005).
Watanabe, H. et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 (2003).
Matsumoto, K. et al. Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 175, 84–99 (2018).
Boulais, N. et al. Rat Merkel cells are mechanoreceptors and osmoreceptors. PLoS One 4, e7759 (2009).
Suzuki, M. et al. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–2268 (2003).
Brierley, S. M. et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134, 2059–2069 (2008).
Fichna, J. et al. Transient receptor potential vanilloid 4 inhibits mouse colonic motility by activating NO-dependent enteric neurotransmission. J. Mol. Med. 93, 1297–1309 (2015).
Amato, V. et al. TRPV4 in the sensory organs of adult zebrafish. Microsc. Res. Techniq. 75, 89–96 (2012).
Yamawaki, H. et al. Role of transient receptor potential vanilloid 4 activation in indomethacin-induced intestinal damage. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G33–G40 (2014).
D’Aldebert, E. et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140, 275–285 (2011).
Miura, H. et al. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev. Dyn. 243, 1286–1297 (2014).
Chen, Y. et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 155, 2662–2672 (2014).
Ueda, T. et al. Basal cells express functional TRPV4 channels in the mouse nasal epithelium. Biochem. Biophys. Rep. 4, 169–174 (2015).
Okubo, T. et al. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 27, 442–450 (2009).
Perez, C. A. et al. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 5, 1169–1176 (2002).
Clapp, T. R. et al. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 4, 7–10 (2006).
Tsushima, H. & Mori, M. Antidipsogenic effects of a TRPV4 agonist, 4alpha-phorbol 12,13-didecanoate, injected into the cerebroventricle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1736–R1741 (2006).
Chang, R. B. et al. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl. Acad. Sci. USA 107, 22320–22325 (2010).
Hamamichi, R., Asano-Miyoshi, M. & Emori, Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience 141, 2129–2138 (2006).
Perea-Martinez, I., Nagai, T. & Chaudhari, N. Functional cell types in taste buds have distinct longevities. PLoS One 8, e53399 (2013).
Kataoka, S. et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses 33, 243–254 (2008).
Ishii, S. et al. Acetic acid activates PKD1L3-PKD2L1 channel-a candidate sour taste receptor. Biochem. Biophys. Res. Commun. 385, 346–350 (2009).
Aihara, E. et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci. Rep. 5, 17185 (2015).
Ren, W. et al. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci. Rep. 7, 4004 (2017).
Finger, T. E. et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495–1499 (2005).
Ohkuri, T. et al. Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (P2X2/P2X3 double-KO) mice. Chem. Senses 37, 523–532 (2012).
Oka, Y. et al. High salt recruits aversive taste pathways. Nature 494, 472–475 (2013).
Gaillard, D. et al. Beta-catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLoS Genet. 11, e1005208 (2015).
Thirumangalathu, S. & Barlow, L. A. Beta-catenin signaling regulates temporally discrete phases of anterior taste bud development. Development 142, 4309–4317 (2015).
Sokabe, T. et al. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J. Biol. Chem. 285, 18749–18758 (2010).
Amico, J. A. et al. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1798–R1806 (2005).
Baratchi, S. et al. Shear stress regulates TRPV4 channel clustering and translocation from adherens junctions to the basal membrane. Sci. Rep. 7, 15942 (2017).
Gaillard, D. et al. β-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice. PLoS Genet. 13, e1006990 (2017).
Ohishi, A. et al. Bortezomib alters sour taste sensitivity in mice. Toxicol. Rep. 4, 172–180 (2017).
Acknowledgements
We thank Drs. Makoto Suzuki and Atsuko Mizuno (Jichi Medical School) for providing the Trpv4-deficient mice. This work was supported by Kyoto Pharmaceutical University Fund for the Promotion of Collaborative Research (to Kenjiro Matsumoto and Akihiro Ohishi), Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (#25860395 and 16K08287 to Kenjiro Matsumoto) and Takeda Science Foundation (to Kenjiro Matsumoto).
Author information
Authors and Affiliations
Contributions
K.M. and A.O. planned and designed the experiments. K.M., A.O., K.I., K.Y., M.T. (Kyoto Pharmaceutical University), S.T., D.U. and T.T. performed the experiments. K.M., A.O. and S.K. analysed the data. K.M., A.O., K.N. and S.K. wrote the manuscript. K.M., A.O., K.I., E.A., T.T., M.T. (Okazaki Institute for Integrative Bioscience), K.N. and S.K. reviewed and discussed the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsumoto, K., Ohishi, A., Iwatsuki, K. et al. Transient receptor potential vanilloid 4 mediates sour taste sensing via type III taste cell differentiation. Sci Rep 9, 6686 (2019). https://doi.org/10.1038/s41598-019-43254-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43254-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.