Abstract
The objective of this study was to determine the mechanism by which RU 486 (mifepristone) protects sperm to undergo premature capacitation during cryopreservation. For this, semen ejaculate (n = 20) was divided into four equal fractions and diluted using egg yolk-based extender supplemented with different concentrations of RU 486 (0, 5, 10 and 20 µM) and cryopreserved. We found that RU 486 did not impair the post-thaw sperm kinetics and motility but prevented cholesterol efflux, calcium influx, and protected CatSper channels during cryopreservation. The RU 486 protected sperm from premature capacitation which was confirmed by intracellular calcium level, expression of tyrosine phosphorylated proteins (75 and 80 kDa) and CTC (chlortetracycline) assay. Furthermore, antioxidant ability of RU 486 was reflected by the ferric reducing ability, lower production of sperm malondialdehyde and intracellular reactive oxygen species. Also, we demonstrated that RU 486 treated sperm underwent normal capacitation, zona pellucida binding and zygote cleavage indicating normal fertilizing ability of sperm. In conclusion, we report a new role of RU 486 in protecting buffalo sperm from premature capacitation during cryopreservation.
Similar content being viewed by others

Introduction
In spite of advancements in sperm cryopreservation, achieving optimum conception rate with frozen buffalo semen remains a challenge. Cryopreservation, being a damaging phenomenon causes the loss of viability to around 50% during this process1,2 and remaining motile sperm undergo premature capacitation (cry-capacitation)3. A variety of protocols, cryoprotectants and additives have been tried to protect sperm during cryopreservation with varied degree of success, but unable to prevent premature capacitation4,5,6,7. The mechanism of cryo-capacitation is not fully understood and is complicated as the onset of normal capacitation has yet to be elucidated. Therefore, capacitation-like changes in spermatozoa during cryopreservation process has fascinated and frustrated the task for reproductive biologists. Furthermore, the loss of cholesterol during cryopreservation increases the permeability of sperm membrane by stimulating several factors that allow frozen-thawed spermatozoa to undergo precocious capacitation8. In 2011, Strünker et al.9 and Lishko et al.10 reported that progesterone (7.7 nM) could activate a sperm-specific calcium ion channel (CatSper) resulting calcium influx which stimulate a cascade reaction responsible for capacitation. Therefore, higher progesterone concentration in egg yolk-based extender might play role in triggering signaling pathway leading to capacitation-like changes in already destabilized sperm membrane during cryopreservation. To our knowledge, no study has been attempted the use of antagonist of progesterone receptor to prevent cryo-capacitation in sperm. We report a new role of RU 486 to prevent premature capacitation of sperm during cryopreservation.
Results
Progesterone concentration in seminal plasma, egg yolk and semen extender
Progesterone activates CatSper channel resulting massive calcium influx that triggers cascade reaction responsible for capacitation9,10. Therefore, we estimated progesterone concentration in seminal plasma, hen egg yolk and semen extender. In this study, the average progesterone concentration in buffalo bull seminal plasma and hen egg yolk was 0.73 ± 0.09 ng/mL (n = 6), and 1753.77 ± 202.87 ng/mL (n = 8), respectively. The egg yolk contributed 20% of total volume of the semen extender; hence available concentration of progesterone in the extender was 438.44 ± 50.72 ng/mL. Thus, the contribution of progesterone from seminal plasma to the extender is negligible in comparison to egg yolk (Supplementary Fig. 1).
Sperm kinetics and motility parameters
In an attempt to make the assessment of semen quality more objective, computer-assisted sperm analysis (CASA) was used and found that RU 486 did not impair (P > 0.05) the sperm kinetics and sperm motility parameters (Table 1).
Thermal resistance test
The capability of sperm to survive at 38 °C was evaluated at 30 min interval for 2 h to observe any negative impact of RU 486 on post-thaw sperm motility parameters and found that there was no significance difference between the groups (Fig. 1A).
(A) Incubation test of RU 486 treated groups. (B) Plasma membrane integrity of RU 486 treated groups. (C) Concentration of cholesterol in post-thaw sperm. (D1) Western blot of CatSper 2 proteins. (D2) The optical intensity of CatSper proteins normalized to β-tubulin. Values with different letters (a–d) differ significantly (P < 0.05), n = 20.
Functional integrity of plasma membrane
Plasma membrane integrity is of key importance during freeze–thaw process and semen extender has to protect it against cryo-damage11. The functional intact plasma membrane was estimated by hypo-osmotic swelling test (HOST). The HOST predicts membrane integrity by determining the ability of the sperm membrane to maintain equilibrium between the sperm and its environment. The percent sperm with functional intact plasma membrane were higher (P < 0.05) in 10 µM group as compared to control and other treated groups (Fig. 1B).
Cholesterol concentration in sperm membrane
Cholesterol, an essential component of sperm membrane, stabilizes the membrane fluidity. It is well established that during cryopreservation cholesterol efflux occurs from the sperm plasma membrane. This result into calcium influx which triggers intracellular signaling cascade associated with capacitation8. Therefore, we estimated sperm cholesterol and found that cholesterol efflux decreases in dose dependent manner i.e. highest cholesterol was estimated in 20 µM treated sperm and lowest in untreated sperm (Fig. 1C).
Expression of CatSper proteins
Progesterone has been shown to trigger calcium influx into the sperm via activation of the sperm-specific calcium channel CatSper9,10. The cryopreservation decreased the quantity of the sperm membrane CatSper channel proteins12. To reveal the effect of RU 486 on CatSper channel expression during cryopreservation, we estimated expression of CatSper by immunoblotting. We observed three major bands of 55, 58 and 82 kDa (Fig. 1D1) and the bands’ optical densities were measured (Fig. 1D2). We found that the quantities of the CatSper proteins decreased in untreated sperm as compared to treated sperm (P < 0.05).
Intracellular calcium status
Physiologically, Ca2+ triggers multiple physiological events in spermatozoa, such as hyperactivation, capacitation, and acrosomal reaction which are essential for successful fertilization13 but if these phenomena occur during cryopreservation before reaching the female reproductive tract resulting poor fertilizing ability of sperm. Therefore, we estimated intracellular calcium in post-thaw sperm using fluorescent dye Fluo-3 AM by flow cytometry and found that 10 µM RU 486 treated sperm had more percent of live sperm with low calcium (Fig. 2A1,A2).
(A1) Flow cytometry dot plots of Fluo-3 AM/PI loaded sperm. Window lower left (LL): live sperm with low intracellular calcium, window lower right (LR): live sperm with high intracellular calcium, window upper left (UL): dead sperm with low intracellular calcium and window upper right (UR): dead sperm with high intracellular calcium. (A2) Intracellular calcium of sperm measured by Fluo-3 AM/PI stains. (B1) Western blot of tyrosine phosphorylated sperm proteins. (B2) The optical intensity of tyrosine phosphorylated sperm proteins normalized to β-tubulin. Values with different letters (a–c) differ significantly (P < 0.05), n = 4. (C1).Micrograph of sperm showing three patterns of chlortetracycline fluorescent staining (CTC): NC, non-capacitated sperm; C, capacitated sperm; AR, Acrosome reacted sperm. (C2) Percent mean of non-capacitated (NC), capacitated (C) and acrosome reacted (AR) RU 486 treated sperm. Values with different letters (a,b) differ significantly (P < 0.05), n = 20.
Cryo-capacitation status of sperm
We evaluated different concentration of RU 486 for inhibition of capacitation-like changes during cryopreservation by immunoblotting of tyrosine phosphorylated proteins and chlortetracycline (CTC) assay. In the cryopreserved sperm, expression of tyrosine phosphorylated proteins, a hallmark event of capacitation, were estimated by immunoblotting (Fig. 2B1). The quantitative digital image analysis of 85 and 75 kDa bands showed greater (P < 0.05) expression of the proteins in untreated group as compared to treated groups (Fig. 2B2). The results of CTC assay showed intracellular calcium related changes during the freeze-thaw process. The CTC assay not only allows discrimination between acrosome-intact cells and acrosome reacted ones, but also divides acrosome-intact cells into two categories, i.e. uncapacitated and capacitated (Fig. 2C1). The percentage of non-capacitated sperm in 10 µM group was higher (P < 0.05) than control and other treated groups (Fig. 2C2). Further, the percent of cryo-capacitated and acrosome reacted sperm were lower (P < 0.05) in 10 µM group than other groups.
Antioxidant activity of RU 486
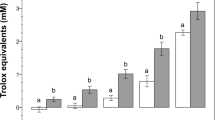
To confirm the antioxidant activity of RU 486, ferric reducing antioxidant power (FRAP), TBARS (thiobarbituric acid reactive substances) and intracellular ROS (reactive oxygen species) assays were performed. In FRAP assay, the antioxidants act as reducing agents by donating electrons to free radicals to stabilize them. The reducing ability of RU 486 increased in a dose-dependent manner (Fig. 3A). Further, malondialdehyde (MDA), a marker of lipid peroxidation and oxidative stress, was found to be higher in untreated sperm as compared to treated sperm (Fig. 3B). Among treated group, lower (P < 0.05) concentration of MDA was found in 10 µM group. To know whether RU 486 prevents ROS production during cryopreservation we estimated intracellular ROS level and found that 10 µM group had lower level of intracellular ROS (Fig. 3C).
(A) The ferric reducing antioxidant power of RU486 fortified egg- yolk extender. Values with different letters (a–c) differ significantly (P < 0.05), n = 4. (B) MDA (malondialdehyde) concentration in sperm membrane. Values with different letters (a–c) differ significantly (P < 0.05), n = 20. (C) Intracellular reactive oxygen species (ROS) level in RU 486 treated sperm. (D1,D2.) CTC assay shows that most of the sperm irrespective of concentration of RU 486 underwent capacitation in vitro capacitation media.
In vitro capacitation and zona binding
To confirm that RU 486 treated sperm undergo normal capacitation, acrosome reaction and ability to attach with zona pellucida of oocyte; in vitro capacitation and zona binding assays were performed. In vitro capacitation test showed that all treated and untreated sperm underwent capacitation indicating that treatment does not inhibit normal sperm capacitation (Fig. 3D1,D2). The zona binding assay is based on ability of sperm to bind zona pellucida to predict the fertilizing potential of sperm14. The untreated sperm were found to be loosely attached to zona as compared to treated sperm (Fig. 4A1). The RU 486 treated sperm had higher (P < 0.05) zona binding ability than that of control. Among the treated groups, 10 µM groups showed higher zona binding ability than others (Fig. 4A2).
(A1) Micrographs showing ability of RU 486 treated sperm to attach zona pellucida in phase contrast (Magnification 10X) and fluorescence (blue) microscopes. (A2) Bar graph of number of sperm tightly attach to zona under different treatment. Values with different letters (a–c) differ significantly (P < 0.05), n = 5. (B1) In vitro fertilized buffalo oocytes (Magnification 10X) fertilized with RU 486 treated sperm. UC- uncleaved; C- cleaved. (B2) Percent cleaved zygote produced using RU 486 treated sperm.
In vitro fertility
The fertilizing ability of RU 486 treated sperm was evaluated by oocytes cleavage percentage after in vitro fertilization and we found that 10 µM group had higher (P < 0.05) cleavage rate than that of control group (Fig. 4B1,B2).
Discussion
We estimated very low progesterone concentration (0.73 ng/mL) in buffalo seminal plasma indicating the low progesterone requirement for normal physiology of a spermatozoon after ejaculation. Surprisingly, we observed very high progesterone concentration (1753 ng/mL) in hen egg yolk used for semen extender. As 20% egg yolk is used in semen extender, the final progesterone concentration (438.44 ng/mL) in extender was as high as the physiological concentration of progesterone in oviduct15. In addition to progesterone, testosterone and estradiol are present in egg yolk16. The non-genomic progesterone receptors on sperm membrane are also activated by testosterone and estradiol but lesser intensity and induce calcium influx into spermatozoa17. Therefore, we hypothesized that the higher concentration of progesterone in egg yolk extender might be responsible for premature capacitation during the cryopreservation. Since higher incidence (51.3 to 69%) of sperm cryo-capacitation of using egg yolk base extender have been reported of buffalo sperm in different studies18,19,20. Hence, we anticipated anti-progesterone (RU 486) supplementation to egg yolk based extender would reduce the incidence of premature capacitation during cryopreservation process.
Our results showed that RU 486 fortification in extender did not affect the sperm kinetics and motility parameters as assessed by CASA. Our results were in line with previous observations that RU 486 has either small or negligible effect on sperm motility21,22,23. This substantiated the use of RU 486 in semen extender without hampering the post-thaw sperm motility. It is established that the cryopreservation process restructures the membranes due to phospholipids redistribution and cholesterol removal24,25. The loss of cholesterol from the sperm membrane results in decrease in cholesterol/phospholipids ratio which is responsible for premature capacitation during cryopreservation20. For the first time, the present study demonstrated that RU 486 prevents cholesterol efflux in dose dependent manner, but exact mechanism is not known. We found higher cholesterol concentration after cryopreservation in RU 486 treated sperm which indicates the cryoprotection tendered by RU 486. Since cholesterol maintains the fluidity of sperm membrane, it protects the sperm membrane proteins from denaturation11.
The progesterone actually activates CatSper channel resulting Ca2+ influx was recently reported9,10. During cryopreservation, there is reduction in the quantity of CatSper proteins due to protein denaturation11. In this study, we found expression of three variants of CatSper-2 proteins were higher in treated sperm than control. These results indicate that CatSper channels are more intact and functional in treated sperm as compared to untreated sperm confirmed by the intracellular calcium level. The intracellular calcium is key regulator of cascade reaction of capacitation, but in frozen-thaw sperm, it is negatively correlated with bull fertility26. In the untreated sperm, relatively higher intracellular calcium was found as compared to RU 486 treated sperm. We hypothesised that more intact and functional CatSper channels in RU 486 treated sperm after cryopreservation might be due to its antioxidant activity with lesser cholesterol efflux and sperm membrane lipid peroxidation. On the other hand, in untreated sperm more number of partially damaged CatSper channels and presence of progesterone in extender activate the channels resulting uncontrolled calcium influx and premature capacitation after cryopreservation of sperm. The ultimate biomarker of capacitation is phosphorylation of tyrosine protein in sperm27. This study demonstrated a decrease in phosphorylation of tyrosine proteins indicating the signal transduction pathways that lead to protein tyrosine phosphorylation is not triggered in sperm in the presence of RU 486 during cryopreservation. Earlier study has shown that sperm cryo-capacitation has been correlated with an increase in protein tyrosine phosphorylation in buffalo18. Finally, we tested capacitation status in treated and untreated sperm to know percent of sperm underwent premature capacitation and acrosome reaction by CTC assay and found that 10 µM RU 486 effectively prevented cryo-capacitation consistent with earlier findings21,23.
During sperm cryopreservation, excess free radicals are generated and progesterone promotes the generation of superoxide anion in sperm28. Higher production of free radicals is involved in lipid peroxidation of plasma membrane29. The endogenous antioxidant capacity of semen is insufficient in preventing lipid peroxidation during the cryopreservation process30. But, in the present study, RU 486 effectively protects cholesterol efflux, decreased intracellular free radicals and MDA from sperm membrane. This hinted us to test the reducing ability of RU 486 by FRAP assay. We, for the first time, found that ferric reducing ability of RU 486 increases in a dose-dependent manner that proving antioxidant activity of RU 486, in addition to anti-hormonal action. RU 486 consists of a progesterone-like steroid ring with a non-steroid moiety named di-methylaminophenyl (Supplementary Fig. 2) and the antioxidant activity of RU 486 resides in the non-steroid moiety31. Earlier, an antioxidant containing di-methylaminophenyl group named as B16 [5-(4-dimethylamino-phenyl)-2-phenyl-penta-2,4-dienoic acid] has been used in canine semen extender for storage semen at 4 °C for 72 h and found to inhibit superoxide anion by 92% and reduce total ROS production during storage32. From this study, it is evident that RU 486 protects sperm from premature capacitation by its anti-progesterone and anti-oxidant properties. Therefore, it is interesting to find whether RU 486 treated sperm in the oviduct would undergo normal capacitation, acrosome reaction and fertilize to the oocytes. To confirm this, we conducted three experiments viz. in vitro capacitation, zona binding assays and in vitro fertilization. We found that treated sperm capacitated normally and better fertilize the oocytes compared to untreated sperm. The reduced binding ability of the untreated sperm might be due to higher proportion of premature capacitated and acrosome reacted sperm losing the viability before attachment. Similar results were reported in bull14 and human33. In addition, our results are supported by the earlier finding34 wherein it was shown that RU 486 did not prevent fertilization or implantation in female reproductive tract. Thus, it can be anticipated that when the treated sperm reach to oviduct after artificial insemination, they are exposed to higher level of progesterone secreted by the cumulus cells and the corpus luteum35. RU 486 acts as a competitive progesterone receptor antagonist and presence of high concentrations of progesterone would replace RU486 from the receptor site36 resulting into the sperm would undergo normal capacitation and fertilization.
Taken together, the present results showed that RU 486 (mifepristone) in semen extender acts as a un-capacitation factor and protect sperm from premature capacitation during cryopreservation. Based on current and previous studies, it can be postulated that RU 486 in semen extender binds to the receptor site of progesterone, prevents activation of CatSper channel and calcium influx, minimizes tyrosine phosphorylation and prevents the initiation of capacitation during the cryopreservation process. In addition, RU 486 decreases cholesterol efflux, ROS production and lipid peroxidation in sperm due to its antioxidant activity during cryopreservation. In female reproductive tract, RU 486 is displaced from the receptor sites in presence of high concentration of progesterone and sequence of events is reverted.
Conclusion
To the best of our knowledge, this is the first report describing a new role of RU 486 in sperm cryopreservation. Supplementation of RU 486 protects sperm from capacitation-like changes during cryopreservation without hampering the sperm post-thaw motility. Its ability to reduce ferric ions confirms its antioxidant activity in addition to anti-progesterone activity. The RU 486 treated sperm underwent normal capacitation, and attach to zona pellucida and fertilized the oocytes. These findings potentially open a new avenue of research against premature capacitation of sperm during cryopreservation.
Methods
Chemicals and reagents
All reagents were purchased from Sigma–Aldrich, Inc., St. Louis, MO, USA unless otherwise stated.
Animal ethics
Animal experiments were conducted following the guidelines laid down by Institute Animal Ethics Committee, ICAR-CIRB, Hisar, and protocol approved by SERB, DST, Govt. of India.
Estimation of progesterone in egg yolk and buffalo seminal plasma
To determine progesterone concentration, egg yolk was extracted to eliminate potential interfering substances and concentrate as described by Kozlowski et al.37. Briefly, 1 mL of PBS was added to 1 g of egg yolk and vortexed for 5 min. Thereafter, in the 100 µL of homogenised egg yolk, 400 µL of PBS was added and incubated at 37 °C for 1 h. After incubation, 500 µL of 100% ethanol was added and immediately vortexed for 1 min and allowed to incubate at room temperate for 10 min. After incubation, the sample was centrifuged at 13,000 rpm for 10 min. The supernatant was stored at −70 °C and used for progesterone estimation using progesterone enzyme immuno assay as per instruction of kit’s manufacturer (Xema-Medica, Moscow, Russia). Similarly, progesterone was estimated in seminal plasma. The standard curve was plotted using the standards and concentration for each sample is calculated from standard curve and expressed as ng/mL.
Semen collection and cryopreservation
Semen of four Murrah buffalo bulls was collected twice a week using artificial vagina technique. Five ejaculates from each bull were used for the study. Each ejaculate was divided into four equal aliquots and extended using egg yolk- based extender fortified with different concentration of RU 486 (0, 5, 10 and 20 μM) and cryopreserved as described by Kumar et al.38.
Sperm kinetics and motility
After cryopreservation, sperm kinetics and motility were assessed using CASA system as described earlier39. Before analysis under CASA, the semen sample was diluted with pre-warmed Tris buffer to give a sperm concentration of about 40 × 106 spermatozoa/mL. One μL prepared semen sample was loaded in a pre-warmed (38 °C) 8 chamber Leja slide (depth 20 μm) and analyzed for sperm motility characteristics. For each sample, 5 optical fields around the central reticulum of the chamber were used to count spermatozoa. The following motion characteristics were recorded: total motility (TM, %), progressive motility (PM, %), rapid motility (RM %), straight linear velocity (VSL, µm/s), average path velocity (VAP, µm/s), curvilinear velocity (VCL, µm/s), average lateral head displacement (ALH, µm/s), beat cross frequency (BCF, Hz), straightness (STR, %) and linearity (LIN,%) of the spermatozoa. The CASA software settings were as follows: temperature = 38 °C, frame rate = 60 Hz, frames acquired = 30, minimum contrast = 35, minimum cell size = 5 pixels, cell size = 9 pixels, cell intensity = 110 pixels, progressive cells (VAP cut-off = 50μm/s, STR cut-off = 70%), slow cells (VAP cut-off = 30 μ/s and VSL cut-off = 15μ/s).
Incubation test
Each semen sample was thawed in water bath at 37 °C for 30 s. The content of straw was transferred to 2 mL tubes maintained in a water bath at 37 °C. Sperm motility was assessed at 0, 30, 60, 90 and 120 min by using phase contrast microscope to rule out any detrimental effect of RU 486 on sperm motility during incubation period.
Functional sperm membrane integrity
Functional integrity of sperm was evaluated by HOST as described by Swami et al.40 to know the ability of sperm to withstand stress during cryopreservation. The assay was performed by mixing 100 μL of frozen-thawed semen with 1 mL hypo-osmotic solution (0.735 g sodium citrate 2H2O and 1.351 g fructose in 100 mL distilled water). After incubation for 60 min at 37 °C, sperm tail bending/coiling was assessed by placing 15 μL of well-mixed sample on a warm slide (37 °C) under light microscopy at 400× magnification. At least 200 spermatozoa were observed per slide. Sperm with coiled tail after incubation was considered having intact plasma membrane.
Determination of sperm intracellular calcium
The frozen-thawed sperm was washed to separate from semen extender and other somatic cells contamination using Bovi Pure™ (Nidacon Sweden). Intracellular calcium of sperm was analysed as per the method given by Harrison et al.41 with slight modification. Briefly, the washed sperm (∼10 million/ml) was loaded with fluorescent probe Fluo-3 AM (1 µM final concentration) and incubated for 15 min. Then, PI was added to a final concentration of 5 µM and incubated for 5 min and analyzed by flowcytometer (BD FACSVerseTM San, Jose, USA). The total of 10,000 events/sample acquired at the rate of 500 events/sec. The Fluo-3 AM fluorescence was detected using a 527/32 nm band pass filter and the fluorescence of PI was detected using 586/42 nm band pass filter. The sperm population were separated into four groups: live sperm with low calcium, live sperm with high calcium, dead sperm with low calcium and dead sperm with high calcium.
Determination of capacitation status
To confirm status of premature capacitation of sperm treated with RU 486 during cryopreservation of sperm, chlortetracycline (CTC) assay was performed42,43. For this, cysteine buffer (20 mM Tris, 130 mM NaCl, 5 mM cysteine; pH 7.8) was prepared and stored at 4 °C. The 750 µM chlortetracycline (Sigma-Aldrich # C4881) was prepared freshly in cysteine buffer on the day of staining and kept in light protected tube at 4 °C till use. The frozen-thawed semen was centrifuged at 140 g at 28 °C for 10 minutes to remove extender and seminal plasma. The obtained sperm pellet was re-suspended in 1 mL PBS and re-centrifuged. Consequently, the sperm pellet was re-suspended with 100 µL of PBS. A 50 µL of the sperm suspension was mixed with 100 µL CTC working solution kept for 20 minutes at 37 °C. After this, 5 µL of 12.5% glutaraldehyde (in 20 mM Tris) was added as fixative to the sperm suspension and vortexed. On clean grease free slide, 12 µL of the above sperm suspension and 6 µL glycerol was taken and cover slip was placed. A total of 200 sperm per slide were observed under FITC filter within 24 hours using fluorescence microscope (Nikon Eclipse Ti Tokyo, Japan). Sperm were evaluated according to 1 of 3 CTC staining patterns (Fraser et al., 1995): 1. Fluorescence over the entire head was considered non-capacitated sperm; 2. Fluorescence-free band in the post-acrosomal region was capacitated cells and 3. Low fluorescence over the entire head except for a thin bright fluorescent band along the equatorial segment was acrosome-reacted cells. A total of 200 sperm per slide were observed.
Determination of tyrosine phosphorylation of sperm proteins
Extraction of sperm protein
Sperm cells from each sample were separated from semen extender and other somatic cells contamination using Bovi Pure™ (Nidacon Sweden). Proteins from the entire spermatozoa were extracted as described by Li et al.44. Briefly, the sperm pellet obtained above was solubilised in 100 µL of lysis buffer containing 4% CHAPS, 40 mM Tris-base, 75 mM DDT, 1 mM PMSF, 1 mM EDTA, 7 mM urea, 2 mM thiourea, 1 mM sodium orthovanadate and protease inhibitor cocktail @10 µL/mL. The mixture was incubated at room temperature for 60 min followed by centrifugation at 21130 g for 30 min at 4°C. After centrifugation, the supernatant was separated and stored at −20 °C for further use. Protein concentration was determined by Quick Start™ Bradford Protein Assay Kit (BioRad, Hercules, USA).
SDS-PAGE and Western blotting
For one dimensional gel electrophoresis, 10 µg of solubilized proteins were loaded per lane and resolved by SDS–PAGE (Mini-PROTEAN tetra cell (Bio-Rad) using stacking and resolving gels with 4% and 10% of acrylamide, respectively. Transfer of proteins from gel to PVDF membrane was done using iBlot® Dry Blotting System (Invitrogen, USA). Immunoblotting was done by using WesternBreez® Chromogenic immunodetection system kit (Invitrogen USA), using anti-Phosphotyrosine antibody, clone 4G10® (Merck Life Science, Mumbai, India) as primary antibody. As an internal control, β-tubulin was detected using the anti-β-tubulin mouse antibody and the expression levels of the proteins were normalized to those of β-actin using myImageAnalysis software from Thermo Fisher Scientific.
Determination of CatSper proteins
Sperm protein extraction, SDS-PAGE and western blotting were performed as described above. Immunoblotting was done by using WesternBreez® Chromogenic immunodetection system kit, anti-rabbit (Thermo Fisher Scientific, Rockford USA,), and CatSper-2 Polyclonal Antibody (Thermo Fisher Scientific, Rockford, USA) as primary antibody.
TBARS assay
The extent of lipid peroxidation (Malondialdehyde, MDA) in samples was determined using TABARS assay kit (Cayman Chemical Company, Ann Arbor, USA) as described by Kumar et al.30. Briefly, frozen-thawed semen was centrifuged to remove seminal plasma and extender. The sperm pellet was re-suspended in PBS and again centrifuged. After centrifugation, the pellet was suspended in PBS and concentration of sperm was estimated. To each tube 100 µL of samples/standards, 100 µL of SDS solution and 4 mL color reagent was added. The mixture was kept in boiling water bath for 1 h. After 1 h, the samples and the standards were removed immediately and placed in ice bath for 10 min to stop the reaction. After cooling, the suspension was centrifuged for 10 min at 1600 × g at 4 °C. A 150 µL of the suspension was loaded into the colorimetric plate and absorbance was measured at 535 nm. The standard curve was prepared using the MDA standards and expressed as MDA µM/million of sperm.
Estimation of cholesterol
To know whether RU 486 prevent cholesterol efflux from sperm plasma membrane, cholesterol was estimated by using cholesterol quantification Kit (Sigma Aldrich, St. Louis, MO, USA). Briefly, sperm was washed as described by Vijayalakshmy et al.45. The sperm pellet was mixed with 200 µL of chloroform: isoporpanol: IGEPAL CA-630 (7:11:0.1), vortexed and centrifuged at 13000 g for 10 min to remove insoluble material. The pellet was discarded and organic phase was air dried at 50 °C to remove any residue organic solvent. The dried lipid was dissolved with 200 mL of the cholesterol assay buffer and vortexed for two min. To 50 µL samples and standards, 50 µL of reaction mix was added to each corresponding wells to initiate reaction and kept for shaking for 1 hr at 37 °C. The absorbance was measured at 570 nm and cholesterol concentration was calculated from standard curve and expressed as µg/100 million sperm.
Determination of ferric reducing ability
Ferric reducing antioxidant power (FRAP) of RU 486 was determined as per procedure described by Benzie et al.46 with slight modifications. Briefly, FRAP reagent is prepared by mixing 300 mM sodium acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM hydrochloric acid, and 20 mM ferric chloride (III) solution at the ratio 10:1:1 (v/v/v), respectively. The methanol extract of extenders was used as sample for the assay. A 10 µL sample was added to 300 µL of FRAP reagent and incubated at room temperature for 3 min. The absorbance at 593 nm was measured immediately using the microplate reader. Aqueous solution of known FeSO4.7H2O concentration (0, 0.2, 0.4, 0.6, 0.8 and 1.0 mM) was used as standard curve. The values obtained were expressed as μM of ferrous equivalent Fe (II).
Detection of intracellular reactive oxygen species (ROS)
The intracellular ROS level was measured using fluorometric intracellular ROS kit (Sigma Aldrich, St. Louis, MO, USA). The 100 µL sample and 100 µL master reaction mix was added in each well and incubated the cells in a 5% CO2, 37 °C incubator for one hour. The fluorescence signal was monitored at ex/em = 490/525 nm.
Assessment of in vitro capacitation
Induction of in vitro capacitation of sperm was done as per the procedure described by Harrison et al.39. Single semen straw from each sample was thawed and centrifuged at 140 g at 28 °C for 10 min to remove semen plasma and extender. The obtained sperm pellet was re-suspended in 1 mL PBS (pH 7.4) and re-centrifuged. Supernatant was discarded and sperm pellet was re-suspended in 200 µL sperm TALP-H media15 and incubated for 5 h. After incubation, CTC assay was performed.
Zona binding assay
To know ability of RU 486 treated sperm to bind zona pellucida was assessed as described previously47 with some modifications. The washed sperm was re-suspended in BO media without heparin to make final concentration of 2 million sperm per mL. Fresh ovaries were obtained from slaughter house and washed two to three times with PBS. Oocytes along with follicular fluid were aspirated from antrum follicles (diameter of 4 to 8 mm) of different ovaries and pooled together. Oocytes with cumulus were recovered under zoom stereo microscope and vortexed for 2 min to remove cumulus cells. The good quality cumulus free oocytes were washed with PBS and kept at 4 °C until they were incubated with spermatozoa. A small sperm droplet of 10 µL was placed in 35 mm multi-well Petri dish and an oocyte was added to each droplet. The sperm droplet with oocyte was covered with mineral oil and incubated at 38.5 °C (5% CO2 and 98% humidity) for 4 h. After incubation, the oocyte-sperm complex was rinsed five times in PBS to remove loosely attached spermatozoa and fixed with 2.5% glutaraldehyde in PBS for 10 min. The fixed oocyte-sperm complex was washed in PBS and stained with 1 µg/mL of Hoechst 33342 dye for 10 min. The sperm-oocyte complex was washed again in PBS to remove excess stain and placed on a glass slide under coverslip. The number of spermatozoa bound to zona pellucida was counted under epifluorescent microscope.
In vitro fertilization
For assessment of fertilizing ability of RU 486 treated spermatozoa, in vitro maturation, fertilization and culture was performed in three replicates as described by Saugandhika et al.48. For oocyte maturation, compact cumulus oocyte complexes (COCs) with an unexpanded cumulus mass having ≥3 layers of cumulus cells and with homogenous evenly granular ooplasm were taken. The oocytes were washed 4–5 times with the washing medium (TCM-199 + 10% FBS + 0.81 mM sodium pyruvate + 100 U/mL penicillin, and 100 μg/mL streptomycin) and twice with the IVM medium (TCM-199 + 10% FBS + 5 µg/mL porcine FSH + 1 µg/mL estradiol-17β + 0.81 mM sodium pyruvate + 100 U/mL penicillin, and 100 μg/mL streptomycin). The washed COCs were then placed in 80 μL droplets (15–20 oocytes/droplet) of the IVM medium, covered with sterile paraffin oil, in a 35 mm Petri dish and cultured for 24 h in a CO2 incubator (5% CO2 in air, 90–95% relative humidity) at 38.5 °C. In vitro matured oocytes with expanded cumulus cells were subjected to in vitro fertilization. The frozen-thaw semen washed twice with washing BO medium containing 10 µg/mL heparin, 137.0 µg/mL sodium pyruvate and 1.942 mg/mL caffeine sodium benzoate. The pellet was re-suspended in 0.5 mL of the washing BO medium. The matured oocytes were rinsed in washing BO medium and transferred to 50 μL droplets (15–20 oocytes/droplet) of the capacitation and fertilization BO medium (washing BO medium + 10 mg/mL fatty acid-free BSA). The spermatozoa in 50 μL of the capacitation and fertilization BO medium were then added to the droplets containing the oocytes, covered with sterile mineral oil and placed in a CO2 incubator (5% CO2 in air) for 18 h at 38.5 °C. After the end of sperm-oocyte incubation, the cumulus cells were washed off the oocytes by gentle pipetting. The oocytes were then washed several times with the modified Charles Rosenkrans medium with amino acids (mCR2aa) as IVC medium. After 40 to 42 h of insemination, presumptive zygotes were evaluated under stereo zoom microscope for evidence of cleavage. The cleavage rate was determined by dividing the number of oocytes cleaved out of the total number of oocytes inseminated.
Statistical analysis
All statistical analyses were carried out using IBM SPSS Statistics software (IBM Corporation, USA) for windows. Statistical significance was set at 0.05 probability level. Percent data were arcsine-square root transformed before statistical analysis. Data were analysed using one-way ANOVA and comparison of means were done by Duncan multiple range test. Results are expressed as mean ± standard error of mean. Progesterone concentration measured in seminal plasma and egg yolk was analysed by independent samples t-test. The bands intensity in western blot was calculated by using my Image Analysis software from Thermo Fisher Scientific and their means was compared by using Turkey post-hoc test.
References
Watson, P. F. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev. 7, 871–91 (1995).
Watson, P. F. The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci. 60, 481–492 (2000).
Bailey, J. L., Bilodeau, J. F. & Cormier, N. Semen cryopreservation in domestic animals, a damaging and capacitating phenomenon. J Androl. 21, 1–7 (2000).
Cormier, N., Sirard, M. A. & Bailey, J. L. Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J. Androl. 18, 461–468 (1997).
Singh, V. K., Kumar, R. & Atreja, S. K. Cryo-survival, cryo-capacitation and oxidative stress assessment of buffalo spermatozoa cryopreserved in new soya milk extender. Livest. Sci. 160, 214–218 (2014).
Setyawan, E. M. N. et al. Spermine reduces reactive oxygen species levels and decreases cryocapacitation in canine sperm cryopreservation. Biochem. Biophys. Res. Commun. 479, 927–932 (2016).
Kumar, P. et al. Incubation of spermatozoa with Anandamide prior to cryopreservation reduces cryocapacitation and improves post-thaw sperm quality in the water buffalo (Bubalus bubalis). Anim. Reprod. Sci. 189, 77–83 (2018).
Leahy, T. & Gadella, B. M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J Androl. 17, 561 (2015).
Strünker, T. et al. The CatSper channel mediates progesterone-induced Ca 2+ influx in human sperm. Nature. 471, 382 (2011).
Lishko, P. V., Botchkina, I. L. & Kirichok, Y. Progesterone activates the principal Ca 2+ channel of human sperm. Nature. 471, 387 (2011).
Kumar P. et al. Liposome-based semen extender is suitable alternative to egg yolk-based extender for cryopreservation of buffalo (Bubalus bubalis) semen. Anim Reprod Sci. 159, 38–45 (2015).
Chen, B. et al. Cryopreservation of cynomolgus macaque (Macaca fascicularis) sperm with glycerol and ethylene glycol, and its effect on sperm-specific ion channels—CatSper and Hv1. Theriogenology. 104, 37–42 (2017).
Rahman, M. S., Kwon, W. S., & Pang, M. G. Calcium influx and male fertility in the context of the sperm proteome: an update. Bio Med Res Int, 1–13 (2014)
Fazeli, A. R., Steenweg, W., Bevers, M. M. & Colenbrander, B. Development of a sperm zona pellucida binding assay for bull semen. Vet Rec. 132, 14–16 (1993).
Panda, M. et al. Effect of exogenous progesterone on cumulus characteristics of buffalo oocytes by allowing passage of more number of sperm through cumulus but not essentially fertilization. Asian Pac J Reprod. 7, 79–86 (2018).
Aslam, M. A. et al. Yolk concentrations of hormones and glucose and egg weight and egg dimensions in unincubated chicken eggs, in relation to egg sex and hen body weight. Gen Comp Endocrinol. 187, 15–22 (2013).
Alexander, N. J., Kim, H. K., Blye, R. R. & Blackmore, P. F. Steroid specificity of the human sperm membrane progesterone receptor. Steroids. 61, 116–125 (1996).
Kadirvel, G., Kathiravan, P. & Kumar, S. Protein tyrosine phosphorylation and zona binding ability of in vitro capacitated and cryopreserved buffalo spermatozoa. Theriogenology. 75, 1630–1639 (2011).
Elkhawagah, A. R. et al. Evaluation of in vitro capacitation of buffalo frozen/thawed sperm by different techniques. J Buffalo Sci. 3, 3–11 (2014).
Longobardi, V. et al. Cholesterol-loaded cyclodextrins prevent cryocapacitation damages in buffalo (Bubalus bubalis) cryopreserved sperm. Theriogenology. 89, 359–364 (2017).
Uhler, M. L., Leungt, A., Chan, S. Y. & Wang, C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil Steril. 58, 1191–1198 (1992).
Yang, J. et al. Progesterone and RU486, opposing effects on human sperm. Proc. Natl. Acad. Sci. USA 91, 529–533 (1994).
Wang, C., Sinha-Hikim, A. & Leung, A. The anti-progestin CDB 2914 has no antifertility effect in male rats. Contraception. 51, 215–218 (1995).
Ko, J. K. Y. et al. An in vitro study of the effect of mifepristone and ulipristal acetate on human sperm functions. Andrology. 2, 868–874 (2014).
Langlais, J. & Roberts, K. D. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res. 12, 183–224 (1985).
Lin, Y. & Kan, F. W. Regionalization and redistribution of membrane phospholipids and cholesterol in mouse spermatozoa during in vitro capacitation. Biol. Reprod. 55, 1133–1146 (1996).
Collin, S. et al. Sperm calcium levels and chlortetracycline fluorescence patterns are related to the in vivo fertility of cryopreserved bovine semen. J. Androl. 21, 938–943 (2000).
Ickowicz, D., Maya, F. & Haim, B. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl. 14, 816–821 (2012).
Lamirande, E., Harakat, A. & Gagnon, C. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J. Androl. 19, 215–225 (1998).
Bucak, M. N. et al. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen, microscopic and oxidative stress parameters after freeze–thawing process. Theriogenology. 67, 1060–1067 (2007).
Kumar, P., Saini, M., Kumar, D., Bharadwaj A. & Yadav, P. S. Estimation of endogenous levels of osteopontin, total antioxidant capacity and malondialdehyde in seminal plasma, Application for fertility assessment in buffalo (Bubalus bubalis) bulls. Reprod. Domes. Anim, 1–6 (2016).
Parthasarathy, S., Morales, A. J. & Murphy, A. A. Antioxidant, a new role for RU-486 and related compounds. J. Clin. Investig. 94, 1990–1995 (1994).
Michael, A. et al. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology. 68, 204–212 (2007).
Amann, R. P., Shabanowitz, R. B., Huszar, G. & Broder, S. J. In Vitro Sperm‐Binding Assay to Distinguish Differences in Populations of Human Sperm or Damage to Sperm Resulting From Cryopreservation. J. Androl. 20, 648–654 (1999).
Gemzell-Danielsson, K. & Marions, L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum. Reprod. Update. 10, 341–348 (2004).
Osman, R. A., Andria, M. L., Jones, A. D. & Meizel, S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem. Biophys. Res. Commun. 160, 828–833 (1989).
Moe, B. G., Vereide, A. B., Ørbo, A. & Sager, G. High concentrations of progesterone and mifepristone mutually reinforce cell cycle retardation and induction of apoptosis. Anticancer Res. 29, 1053–1058 (2009).
Kozlowski, C. P., Bauman, J. E. & Caldwell Hahn, D. A simplified method for extracting androgens from avian egg yolks. Zoo. Biol. 28, 137–143 (2009).
Kumar, D., Kumar, P., Singh, P., Yadav, S. P. & Yadav, P. S. Assessment of sperm acrosome, plasma membrane, mitochondrial potential and DNA integrity by fluorescent probes in fresh, equilibrated and frozen-thawed buffalo semen. Cytotechnology. 68, 451–458 (2016).
Swami, D. S. et al. Cysteamine supplementation revealed detrimental effect on cryosurvival of buffalo sperm based on computer-assisted semen analysis and oxidative parameters. Anim. Reprod. Sci. 177, 56–64 (2017a).
Swami, D. S. et al. The cryoprotective effect of iodixanol in buffalo semen cryopreservation. Anim Reprod Sci. 179, 20–26 (2017b).
Harrison, R. A. P., Mairet, B. & Miller, N. G. A. Flow cytometric studies of bicarbonate mediated Ca2+ influx in boar sperm populations. Mol. Reprod. Dev. 35, 197–208 (1993).
Ward, C. R. & Storey, B. T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 104, 2 (1984).
Fraser, L. R., Abeydeera, L. R. & Niwa, K. Ca2+ Regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol. Reprod. Dev. 40, 233–241 (1995).
Li, L. W. et al. Establishment of a high‐resolution 2‐D reference map of human spermatozoal proteins from 12 fertile sperm‐bank donors. Asian. J. Androl. 9, 321–329 (2007).
Vijayalakshmy K., et al. A novel combination of silane-coated silica colloid with hybrid RNA extraction protocol and RNA enrichment for downstream applications of spermatozoal RNA. Andrologia. e13030, https://doi.org/10.1111/and.13030 (2018).
Benzie, I. F. & Strain, J. J. Ferric reducing/antioxidant power assay, Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 299, 15–27 (1999).
Saugandhika, S. et al. Effect of nitric oxide on in vitro development of buffalo (Bubalus bubalis) embryos. Reprod. Dom. Anim. 45, 931–933 (2010).
Acknowledgements
This study was supported by Science and Engineering Research Board (SERB) through project No- YSS/2014/000272. The authors thank the Director, ICAR-CIRB for providing facility for conducting this study and technical staffs of semen freezing laboratory for assistance in semen collection and cryopreservation.
Author information
Authors and Affiliations
Contributions
Design and conduct of the study: P.K., J.D., R.K.C., S.P., S.S., R.R., D.K. Analysis and interpretation of data: J.D., P.K., R.K.C., D.K. and J.A. Writing of the article: P.K. and J.D. Critical revision of the article: R.K.C., J.A., S.S. and D.K. Statistical expertise: J.D. and J.A. Literature search: J.A., D.K. and J.D. Preparation of figures: J.D. and P.K. Final approval of the article: all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The 32 kDa blot of Figure 2 (B1) was inadvertently duplicated as β-Tubulin in Figure 1 (D1). The original, un-processed blots for Figure 1 (D1) and Figure 2 (B1) β-Tubulin were also omitted from the Supplementary Information file.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dalal, J., Kumar, P., Chandolia, R.K. et al. A new role for RU486 (mifepristone): it protects sperm from premature capacitation during cryopreservation in buffalo. Sci Rep 9, 6712 (2019). https://doi.org/10.1038/s41598-019-43038-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43038-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.