Abstract
In this work, we introduce a water electrolysis and CO2 hydrogenation tandem system which focuses on methane generation. The concept consists of a water electrolyzer thermally coupled to a CO2 hydrogenation reactor, where the power required to generate hydrogen comes from renewable energy. A thermodynamic analysis of the tandem system was carried out. Our analysis exposes that it is possible to increase the exergy efficiency of the water electrolyzer and CO2 hydrogenation system by thermal coupling, where the thermal energy required to split water into H2 and O2 during the electrolysis process is compensated by the heat generated during the CO2 hydrogenation reaction. Here, the conditions at which high exergy efficiency can be achieved were identified.
Similar content being viewed by others
Introduction
According to measurements done by the greenhouse gases observing satellite (GOSAT), the concentration of CH4 and CO2 in earth’s atmosphere has been increasing continuously in the recent years1. This indicates that the exploitation of fossil fuels to generate the energy required to meet our modern needs is not only threating a shortage in our limited fuel sources but also damaging our environment. In order to decarbonize our energy supply, alternative energy technologies must be considered2,3. The use of hydrogen as energy carrier is a promising way to encourage the decarbonization of energy, since it can be produced from the splitting process of H2O (water electrolysis), which generates H2 and O2 without exhausting carbon to the environment as long as the energy required to achieve this process comes from renewable sources4,5. The hydrogen obtained from the electrolysis process can be used to hydrogenate a CO2 source where CH4 and H2O are produced, this process is known as Power-to-Gas (PtG)6,7,8,9,10. PtG is a technology that connects the electrical power grid with the gas lines by converting excess electricity into a gas that can be injected to the existing gas distribution pipelines.
The PtG process is similar to life support systems for closed environments like the one that is currently used at the International Space Station (ISS), where O2 and H2O are the desired products to support life11,12. O2 and H2 are obtained from water electrolysis. H2 is used to convert the metabolic CO2 generated by the ISS crew to produce H2O which generates CH4 as a byproduct. The obtained H2O can be used to generate O2 while the obtained CH4 is vented to outer space.
Power-to-Gas technology is a promising alternative to recycle a global CO2 and reduce carbon emissions to our atmosphere, especially when the energy required to generate H2 comes from renewable sources like photovoltaic systems and wind turbines5,7,10. Since the supply of energy coming from renewable sources is not constant, it fluctuates depending on demand and weather conditions, a H2 generation system able to operate intermittently has to be adopted. A Polymer electrolyte membrane (PEM) water electrolysis system is a good option to meet the required intermittent operation. These systems are more operational flexible comparing to alkaline and solid oxide electrolysis cell systems13,14,15.
PEM water electrolysis in combination with a CO2 hydrogenation reactor is an attractive way for converting electric power coming from renewable sources into CH4. Other alternative to generate CH4 from renewable energy is the co-electrolysis of H2O and CO2 in solid oxide cells (SOECs)16,17. However, these cells are operated at very high temperatures, which make their design and operation more complicated than PEM electrolysis cells.
The hydrogenation process to convert CO2 into CH4, also known as the Sabatier reaction, is a highly exothermic process which generates a lot of heat18,19,20. This heat has to be removed from the Sabatier reactor in order to maintain a relatively low temperature. Avoiding high operation temperatures of the Sabatier reactor helps to prevent catalyst sintering which decreases the catalyst lifetime12,18,19,20,21.
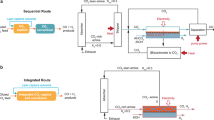
The efficiency of a water electrolyzer–Sabatier reactor system can be improved by thermally coupling the system. To split water into H2 and O2, the electrolyzer demands electrical and thermal energy. The removed heat from the Sabatier reactor can be supplied to the electrolyzer, which will reduce the thermal energy demand and then the overall efficiency of the system is improved. A schematic illustration of the system concept is shown in Fig. 1a. This PtG system consists of supplying renewable energy to power a water electrolyzer to generate H2, and then this H2 is used to hydrogenate CO2 and produce CH4. The efficiency of the system can be increased by exchanging the heat generated during the hydrogenation process of CO2 to the water electrolysis process. The thermodynamic concept of the system is shown in Fig. 1b. The theoretical energy required to split liquid H2O into gaseous H2 and O2 is equal to the enthalpy change (ΔHelec) of H2O, which is the sum of the Gibbs free energy change (ΔG, electrical demand) and entropy change times temperature (TΔS, thermal demand). By exchanging the heat generated by the Sabatier reaction to compensate the water electrolysis thermal demand TΔS, the overall efficiency of the system can be improved. However, it is necessary to identify the conditions at which a maximum efficiency can be achieved. For this, an exergy analysis of the system is required to identify optimal operating conditions. Exergy valorization analysis is a useful technique to quantify the maximum work potential of a system. This thermodynamic analysis is also used to identify the factors or parameters that affect the efficiency of a process22,23,24.
Schematic illustration of the Power-to-Gas concept. (a) The system consists of supplying renewable energy to power a water electrolyzer to generate H2, then this H2 is used to hydrogenate CO2 and produce CH4 (this process is known as the Sabatier reaction). (b) Thermodynamic concept: the theoretical energy required to split liquid H2O into gaseous H2 and O2 is equal to the enthalpy change (ΔHelec) of H2O, which is the sum of the Gibbs free energy change (ΔG, electrical demand) and entropy change times the temperature (TΔS, thermal demand). By exchanging the heat generated by the Sabatier reaction to compensate the water electrolysis thermal demand TΔS, the overall efficiency of the system can be improved.
In this work, an exergy analysis of a water electrolyzer and CO2 hydrogenation tandem system is presented. Operating conditions at which high exergy efficiency can be achieved are identified.
Results and Discussion
Thermodynamic analysis of CO2 hydrogenation process
The equilibrium composition of the CO2 hydrogenation process was computed following the Gibbs energy minimization method, see Methods for more details. The main reaction to convert CO2 into methane is the Sabatier reaction:
Also during the CO2 hydrogenation process the following side reactions can take place:
To analyze the influence of temperature, pressure and feed H2/CO2 molar ratio on the CO2 hydrogenation products, equilibrium compositions were obtained at different conditions. Figure 2a shows the equilibrium composition of the CO2 hydrogenation process at H2/CO2 = 4, 0.1 MPa and different temperatures. It is observed that the largest mole fraction of CH4 and H2O products are obtained at low temperatures (100–300 °C), indicating that the Sabatier reaction is favored at low temperatures, where a high conversion of CO2 into CH4 can be achieved. At high temperatures, CH4 generation decreases and CO appears which means that the water gas shift reaction is favored. Figure 2b shows the thermodynamic conversion of CO2 at 0.1, 0.5 and 1.0 MPa, H2/CO2 = 4 and different temperatures. A high conversion is observed at low temperatures, it is also noticed that a high conversion can be maintained at higher temperatures by increasing the pressure. The effect of H2/CO2 molar ratio on the CO2 hydrogenation products and CO2 conversion is shown in Fig. 2c,d. Under these conditions, the CO2 conversion decreases with temperature and the generation of solid carbon (C) is favored. The presence of solid carbon affects the performance of catalyst, since the catalyst can be deactivated when solid carbon is deposited on it. To prevent the deactivation of catalysts, it is important to identify the conditions at which solid carbon can be generated. Figure 2e shows the carbon generation at 4 and 2 H2/CO2 molar ratios, and at different temperatures and pressures. It is observed that when the H2/CO2 molar ratio of 4 is not maintained, solid carbon is generated, while at a temperature above 590 °C this carbon generation is not observed under any conditions. This temperature coincides with the operating temperature of the Sabatier reactor used on the International Space Station11, probably as a measure to prevent the generation of carbon when the H2/CO2 ratio of 4 cannot be maintained.
Equilibrium composition of the CO2 hydrogenation products as a function of temperature computed following the Gibbs energy minimization method. (a) Molar fraction of possible products for a feed gas ratio H2/CO2 of 4. (b) CO2 conversion for a feed gas ratio H2/CO2 of 4. (c) Molar fraction of possible products for a feed gas ratio H2/CO2 of 2. (d) CO2 conversion for a feed gas ratio H2/CO2 of 2. (e) Effect of the feed gas ratio H2/CO2 on the carbon generation process.
Exergy analysis of the Sabatier process
The exergy efficiency of the Sabatier process is defined by
where \({\eta }_{Sabatier}^{ex}\) is the Sabatier exergy efficiency, \(E{x}_{C{H}_{4}}\) and \(E{x}_{{H}_{2}}\) are the exergy of CH4 and H2, respectively. Details of the exergy calculations are described in the methods section. Figure 3 shows the exergy efficiency of the Sabatier process for H2/CO2 molar ratio of 4 (a) and 2 (b) at 0.1, 0.5 and 1.0 MPa as a function of temperature. In the case of the H2/CO2 molar ratio of 4 (Fig. 3a), the highest efficiency is observed in the temperature range of 100–150 °C and starts decreasing with temperature. In the case of the H2/CO2 molar ratio of 2 (Fig. 3b), the highest efficiency is observed at 340 °C for 0.1 MPa, while for 0.5 and 1.0 MPa it is observed at 400 °C and 420 °C, respectively. The exergy efficiency of the Sabatier process is proportional to the CH4 generation flow rate, and this rate has a high dependency on temperature and H2/CO2 molar ratio as can be observed in Fig. 2a,c.
Exergy analysis of the system
The overall exergy efficiency of the system is defined by the following equation:
where \({\eta }_{overall}^{ex}\) is the overall exergy efficiency, \(E{x}_{out}\) is the exergy output and \(E{x}_{in}\) is the exergy input. Here, \(E{x}_{out}\) is equal to \(C{H}_{4,out}\cdot (E{x}_{C{H}_{4}})\), while \(E{x}_{in}\) corresponds to the electrical energy required to generate hydrogen from water electrolysis, more details on the calculations are given in the methods section. Figure 4 shows the overall exergy efficiency of the system for H2/CO2 molar ratio of 4 (a) and 2 (b) as a function of Sabatier temperature. In the case of the H2/CO2 molar ratio of 4 (Fig. 4a), the overall exergy efficiency has a maximum at 200 °C. While in the case of the H2/CO2 molar ratio of 2 (Fig. 4b), the maximum is found at 340 °C. The maximum overall exergy efficiency is achieved when the thermal exchange between the water electrolyzer and CO2 hydrogenation reactor reaches its optimal point. This indicates that the thermal coupling within the system increases the exergy efficiency.
Our exergy valorization analysis identifies the three main parameters that contribute to achieve high exergy efficiencies for the water electrolyzer – CO2 hydrogenation system. The first parameter is the H2/CO2 feed gas molar ratio. When this molar ratio is lower than 4, the methane generation rate decreases and solid carbon is generated, Fig. 2c,e. The second parameter is the Sabatier reaction temperature. CH4 generation is favored in the temperature range of 100–300 °C, while at higher temperatures the water gas shift reaction takes, affecting the CH4 generation, Fig. 2a. The third parameter that contributes to the enhancement of the system is the thermal coupling of the water electrolyzer and Sabatier reactor. The heat generated by the Sabatier reaction compensates the water electrolysis thermal demand (TΔS), which contributes to increase the overall exergy efficiency of the system, Fig. 4a. In the case of co-electrolysis of CO2 in H2O, it has been shown that this system is also able to achieve high exergy efficiencies to generate H2 and CH4. However, the water gas shift reaction has a significant effect on the system efficiency16,17. As mentioned above, this reaction is favored at high temperatures and this type of CO2 co-electrolysis is carried out at high temperatures. In order to mitigate the effect of the water gas shift reaction and increase the efficiency of the system, CH4 has to be fed through the cell anode.
Since the focus of the present work is the exergy valorization of the water electrolysis-CO2 hydrogenation tandem system, a detailed cost analysis is not included. However, a few remarks can be made on the operating conditions that can have an impact on the system running costs. As mentioned above, maintaining a feed gas rate H2/CO2 of 4 avoids the generation of solid carbon. The generation of solid carbon affects the catalyst activity and decreases its lifetime, which directly impact the running cost of the system. Another factor that can impact the system running costs is high temperatures. Catalyst sintering is favored at high temperatures and this causes a loss in the catalyst activity12,18,19,20,21. So that, by operating the system at a relative low temperature (100–300 °C) helps to avoid catalyst sintering.
In summary, our thermodynamic analysis of the water electrolyzer-CO2 hydrogenation tandem system exposes the conditions at which high exergy efficiency can be achieved. The results also show that the thermal coupling within the CO2 hydrogenation and water electrolysis processes contributes to increase the overall exergy efficiency of the system.
Methods
In the analysis a steady state and steady flow are assumed. Chemical kinetics effects are not considered in the calculations. All values of enthalpy, Gibbs free energy and entropy for all the species were computed using Aspen Plus V8.8, Aspen TechTM.
Water electrolysis
The theoretical energy required to split liquid H2O into gaseous H2 and O2 is equal to the enthalpy change (ΔHelec) of H2O, which is a function of temperature and pressure and can be calculated with the following equation:
where ΔG is the Gibbs free energy change and ΔS is the entropy change. These thermodynamic parameters are a function of the operative temperature (T) and pressure (P). The theoretical energy ΔH corresponds to the sum of electrical energy demand ΔG and thermal energy demand TΔS (Qdemand). ΔH, ΔG and ΔS were calculated using the following equations:
Where \({h}_{{H}_{2}(g)}\), \({h}_{{O}_{2}(g)}\) and \({h}_{{H}_{2}O(l)}\) are respectively the enthalpy values of H2 and O2 in gaseous phase, and H2O in liquid phase. \({g}_{{H}_{2}(g)}\), \({g}_{{O}_{2}(g)}\) and \({g}_{{H}_{2}O(l)}\) are respectively the Gibbs free energy of H2 and O2 in gaseous phase, and H2O in liquid phase. \({g}_{{H}_{2}(g)}\), \({g}_{{O}_{2}(g)}\) and \({g}_{{H}_{2}O(l)}\) are respectively the entropy of H2 and O2 in gaseous phase, and H2O in liquid phase. The enthalpy, Gibbs free energy and entropy values were computed using Aspen Plus V8.8, Aspen TechTM.
The exergy input for a water electrolysis process corresponds to the electrical energy demand, which is the minimum work required to split H2O into H2 and O2. Then, the exergy input is:
CO2 hydrogenation process
The equilibrium composition of the reactions was calculated using the Gibbs free energy minimization method, which is a widely used method to perform thermodynamic analysis of reacting systems20,21,25. The equilibrium composition and the enthalpy of reaction (ΔHr) for the CO2 hydrogenation possible products were computed using Aspen Plus V8.8, Aspen TechTM. The species considered in the analysis are: CH4, CO, CO2, H2O, H2 and C (solid carbon). All the species were considered in gas phase, with the exception of C, which was considered in solid phase. The computations were carried out in the temperature range of 100 °C–800 °C and at different pressures.
The thermodynamic conversion of CO2, CH4 generation and carbon generation were calculated from the equilibrium composition results using the following equations:
Where CO2,in and CO2,out are the molar flow rate of CO2 at inlet and outlet, respectively. CH4,out is the molar flow rate of CH4 at outlet. Cout is the molar flow rate of solid carbon at outlet.
Exergy calculation
The exergy coming out (Exout) of the system is calculated based on the CH4 generation molar rate:
The exergy of a specific gas (\(E{x}_{i}\)) is equal to the sum of physical exergy (\(E{x}_{i}^{Ph}\)) and chemical exergy (\(E{x}_{i}^{Ch}\)):
The physical exergy is calculated according the following equation:
where mi is the molar flow rate of the gas, hi is the enthalpy of the gas at the Sabatier reaction conditions, and h0 is the enthalpy at the gas at the dead state. T0 is the temperature at the dead state. Si is the entropy of the gas at the Sabatier conditions and S0 is the gas entropy at the dead state. The dead state conditions are assumed to be 298.15 K and 1 atm. The chemical exergy is calculated using the following equation:
where \(E{x}_{0,i}\) is the standard chemical exergy of the gas.
For the heat flow from the Sabatier reactor (Qsab) to the water electrolysis system, the exergy is calculated as follows:
where \({Q}_{Sab}\ge {Q}_{demand}\), otherwise, there is not heat transfer. Telec and Tsab are the temperatures of the electrolysis system and Sabatier reactor, respectively.
Calculation of the overall exergy efficiency of the water electrolyzer and CO2 hydrogenation tandem system:
References
The Greenhouse Gases Observing Satellite (GOSAT), Whole-atmosphere monthly CO2 concentration, http://www.gosat.nies.go.jp/en/recent-global-co2.html.
Dresselhaus, M. S. & Thomas, I. L. Alternative energy technologies. Nature 414, 332–337 (2001).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 294, 294–303 (2012).
Turner, J. A. Sustainable Hydrogen Production. Science 305, 972–974 (2004).
Crabtree, G. W., Dresselhaus, M. S. & Buchanan, M. V. The Hydrogen Economy. Physics Today 57(12), 39–44 (2004).
Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 38, 2039–2061 (2013).
Estermann, T., Newborough, M. & Sterner, M. Power-to-gas systems for absorbing excess solar power in electricity distribution networks. Int. J. Hydrog. Energy 41, 13950–13959 (2016).
Gotz, M. et al. Renewable Power-to-Gas: A technological and economic review. Renewable Energy 85, 1371–1390 (2016).
Walker, S. B., Mukherjee, U., Fowler, M. & Elkamel, A. Benchmarking and selection of Power-to-Gas utilizing electrolytic hydrogen as an energy storage alternative. Int. J. Hydrog. Energy 41, 7717–7731 (2016).
Simonis, B. & Newborough Sizing and operating power-to-gas systems to absorb excess renewable electricity. Int. J. Hydrog. Energy 42, 21635–21647 (2017).
Samplatsky, D. J., Grohs, K., Edeen, M., Crusan, J. & Burkey, R. Development and Integration of the Flight Sabatier Assembly on the ISS. AIAA Paper No. 2011-5151 (2011).
Shima, A., Sakurai, M., Sone, Y., Ohnishi, M. & Takayuki, A. Development of a CO2 Reduction Catalyst for the Sabatier Reaction. AIAA Paper No. 2012-3552 (2012).
Carmo, M., Fritz, D. L., Mergel, J. & Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 38, 4901–4934 (2013).
Gazey, R., Salman, S. K. & Aklil-D’Halluin, D. D. A field application experience of integrating hydrogen technology with wind power in a remote island location. J. Power Sources 157, 841–847 (2006).
Irvine, J. T. S. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 15014 (2016).
Ni, M. 2D thermal modeling of a solid oxide electrolyzer cell (SOEC) for syngas production by H2O/CO2 co-electrolysis. Int. J. Hydro. Energy 37, 6389–6399 (2012).
Xu, H., Chen, B., Irvine, J. & Ni, M. Modeling of CH4-assited SOEC for H2O/CO2 co-electrolysis. Int. J. Hydro. Energy 41, 21839–21849 (2016).
Brooks, K. P., Hu, J., Zhu, H. & Kee, R. J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 62, 1161–1170 (2007).
Lui, Z., Chu, B., Zhai, X., Jin, Y. & Cheng, Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor. Fuel 95, 599–605 (2012).
Kiewidt, L. & Thoming, J. Predicting optimal temperature profiles in single-stage fixed-bed reactors for CO2-methanation. Chem. Eng. Sci. 132, 59–71 (2015).
Nikoo, M. K. & Amin, N. A. S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol 92, 678–691 (2006).
Taner, T. Optimisation processes of energy efficiency for a drying plant: A case of study for Turkey. Energy 80, 247–260 (2015).
Topal, H. et al. Exergy analysis of a circulating fluidized bed power plant co-firing with olive pits: A case study of power plant in Turkey. Energy 140, 40–46 (2017).
Taner, T. & Sivrioglu, M. Energy-exergy analysis and optimisation of a model sugar factory in Turkey. Energy 93, 641–654 (2015).
Faungnawakij, K., Kikuchi, R. & Eguchi, K. Thermodynamic evaluation of methanol steam reforming for hydrogen production. J. Power Sources 161, 87–94 (2011).
Acknowledgements
This work was supported by CREST (Creation of Innovative Core Technology for Manufacture and Use of Energy Carriers from Renewable Energy) JST, Japan.
Author information
Authors and Affiliations
Contributions
Y.S., H.M. and T.A. designed the concept. Y.M. and O.S.M.-H. assisted with the design of the concept and carried out the calculations. A.S. and M.I. discussed the calculation results. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mendoza-Hernandez, O.S., Shima, A., Matsumoto, H. et al. Exergy valorization of a water electrolyzer and CO2 hydrogenation tandem system for hydrogen and methane production. Sci Rep 9, 6470 (2019). https://doi.org/10.1038/s41598-019-42814-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42814-6
This article is cited by
-
Hydrogen storage and geo-methanation in a depleted underground hydrocarbon reservoir
Nature Energy (2024)
-
Proposal and Investigation of a Novel Process Configuration for Heavy Fuel Oil Gasification and Light Hydrocarbon Production
Arabian Journal for Science and Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.