Abstract
Stem cell competition could select the fittest stem cells and potentially control tumorigenesis. However, little is known about the underlying molecular mechanisms. Here, we find that ectopic Decapentaplegic (Dpp) signal activation by expressing a constitutively active form of Thickveins (TkvCA) in cyst stem cells (CySCs) leads to competition between CySCs and germline stem cells (GSCs) for niche occupancy and GSC loss. GSCs are displaced from the niche and undergo differentiation. Interestingly, we find that induction of TkvCA results in elevated expression of vein, which further activates Epidermal Growth Factor Receptor (EGFR) signaling in CySCs to promote their proliferation and compete GSCs out of the niche. Our findings elucidate the important role of Dpp signaling in regulating stem cell competition and tumorigenesis, which could be shed light on tumorigenesis and cancer treatment in mammals.
Similar content being viewed by others
Introduction
Tissue homeostasis is maintained by adult stem cells, which constantly divide and supply newly differentiated cells to replace dying or damaged cells. Increasing evidence shows that fittest stem cells are constantly selected through stem cell competition, which is critical for organ development and tissue homeostasis1,2,3,4,5,6. Moreover, stem cell competition is found to be implicated in tumorigenesis7,8. However, the underlying molecular mechanisms of stem cell competition are poorly understood.
The Drosophila testis is an ideal system to study stem cell maintenance, differentiation, and competition9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38. A group of non-dividing somatic cells, termed the hub, resides at the apex of the Drosophila testis14,22,26. About 5–9 GSCs closely attach to the hub via adhesion molecules. Another group of somatic stem cells, termed CySCs, attach to the hub by their cellular extensions10,12,13,26. The hub serves as the stem cell niche and expresses Unpaired (Upd), which activates the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signaling in GSCs and CySCs to control their maintenance3,32,39. GSCs undergo asymmetric divisions, producing new GSCs and differentiating gonialblasts (GBs). The GBs are engulfed by two somatic cyst cells, generated from asymmetric CySC divisions. The GBs undergo four rounds of mitotic division with incomplete cytokinesis before differentiation. The somatic cyst cells grow without further division to encapsulate the germline cells with their cellular extensions throughout spermatogenesis12,13,17,26,27,40,41. CySCs are also critical for GSC maintenance, therefore, CySCs together with the hub define the niche for GSCs3,32,33,42.
Bone Morphogenetic Protein (BMP) and Hedgehog (Hh) signaling play important roles in the maintenance of GSCs and CySCs20,21,23,24,29,30,43,44,45,46. The hub and the early cyst cells produce two BMP ligands, Glass bottom boat (Gbb) and Dpp43,44. Short-range BMP signaling is critical for GSC maintenance and differentiation. BMP production and diffusion within the niche must be tightly controlled to ensure localized BMP signaling inside the niche, while ectopic BMP signaling outside of the niche leads to aberrant GSC proliferation and differentiation45,47,48,49,50,51,52. Our recent study found that Tkv functions as ligand sink to spatially restrict Dpp signaling within the testis niche53. However, it remains unknown whether ectopic Dpp signaling in CySCs has any role in stem cell regulation.
CySCs and GSCs often compete for niche occupancy, making the Drosophila testis an excellent model to study the underlying mechanisms controlling stem cell competition. Stem cell competition selects fittest stem cells for tissue homeostasis, and is potentially implicated in tumorigenesis1,2,3,4,5. Previous studies found that CySCs compete with each other and with GSCs for niche occupancy. The mutant stem cell and its descendants with increased competitiveness will outcompete wild type stem cells4,6,15,16,19,24,46,54. In the Drosophila testis, CySC-GSC competition is first revealed in socs36E mutant, the negative regulator of JAK/STAT signaling16. Recent studies found that several signaling pathways, including Hh, Hippo (Hpo), and EGFR/Mitogen-activated protein kinase (MAPK), regulate stem cell competition15,19,24,46,54. However, the underlying mechanisms controlling stem cell competition are not fully understood.
In this study, we investigate whether additional factors regulate stem cell competition in the testis niche. Interestingly, we find that ectopic expression of tkvCA in CySCs results in competition between CySCs and GSCs for niche occupancy and GSC loss. We demonstrate that CySC-GSC competition observed in tkvCA-expressing testis is caused by enhanced expression of the EGF vein (vn), which in turn activates EGFR/MAPK signaling in CySCs to promote CySCs to outcompete GSCs. Our data elucidate a novel mechanism of stem cell competition, which may shed light into the development of potential clinical treatment for cancer.
Results
Ectopic expression of tkv CA in CySCs leads to CySC-GSC competition and GSC loss
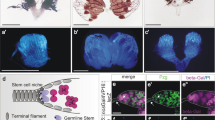
In order to search for new regulators of stem cell competition, we performed a large-scale screen using a c587ts driver (c587Gal4, UAS-GFP; esg-lacZ, tubGal80ts) (data not shown)53. c587Gal4 is strongly expressed in CySCs and somatic cyst cells of the Drosophila testis (Fig. 1a). Our recent data show that Tkv acts as receptor trap to restrain Dpp signaling within the niche53. Surprisingly, we found that when a constitutively active form of tkv (tkvCA) was expressed in CySCs (c587ts > tkvCA), all germline cells, including GSCs, were lost (Fig. 1b). The hub was tightly surrounded by a group of somatic cells, instead of GSCs (Fig. 1b–d). These data indicate that ectopic expression of tkvCA may cause CySC-GSC competition. The observed phenotype was resulted from systemic expression of tkvCA in all CySCs, we wondered whether ectopic expression of tkvCA in single CySC or only a portion of CySCs could cause the same defect. We explored this possibility by using MARCM technique to generate CySC clones expressing tkvCA. Compared with FRT control CySC clones, we found that tkvCA-expressing CySC clones tightly attached to the hub, and the number of GSCs per testis was significantly decreased (Fig. 1e–h). These data indicate that ectopic tkvCA expression in CySCs causes stem cell competition.
Ectopic expression of tkvCA in CySCs leads to GSC loss. (a) c587ts control testis. GSCs (white arrowheads) and CySCs (yellow arrowhead) are indicated. (b) c587ts > tkvCA testis. The hub is surrounded by CySCs (yellow arrowheads), and no germline cells can be observed (white arrowhead). (c) Quantification of the number of GSCs per testis in control and c587ts > tkvCA testes. n = 10–15 testes. (d) Quantification of the number of CySCs with their cell body attaching to the hub in control and c587ts > tkvCA testes. n = 10–15 testes. (e) CySC MARCM clones in FRT control. GSCs (white arrowheads) and GFP-marked CySCs (yellow arrowhead) are indicated. (f) tkvCA-expressing CySC MARCM clones. Some GFP-marked tkvCA CySCs (yellow arrowheads) tightly attach to the hub, and the number of GSCs per testis is greatly reduced (white arrowhead). (g) Quantification of the number of GSCs per testis in testes carrying FRT control and tkvCA MARCM clones. n = 10 testes. (h) Quantification of the number of CySCs with their cell body attaching to the hub in testes carrying FRT control and tkvCA MARCM clones. n = 10 testes. mean ± SEM is shown. **p < 0.01. GFP in green, Vasa in red, Fas3 in yellow, blue indicates DAPI staining for DNA. Scale bars: 10 μm.
Mad (Mothers against dpp), a transducer of Dpp signaling, is phosphorylated when the Dpp pathway is activated. Therefore, the accumulation of phosphorylated Mad (pMad) can be used as a read-out of Dpp pathway activation43,55,56. Consistent with previous reports that ectopic expression of tkvCA induces ectopic Dpp signaling activation43,55, we found that Dpp signaling activation was greatly increased in the cyst cell lineage of c587ts > tkvCA testis, using pMAD as a readout (Supplementary Fig. 1). As Dpp signaling is highly activated upon ectopic expression of tkvCA in CySCs (Supplementary Fig. 1), we examined whether the observed stem cell competition phenotype was a consequence of ectopic Dpp signaling. We used various functional RNAi lines to simultaneously deplete components downstream of Tkv in c587ts > tkvCA testes53,57. Mad and Med (Medea) are components downstream of Tkv in the Dpp signaling pathway. When these RNAi constructs were co-expressed with tkvCA in CySCs, we found that further removal of either Mad or Med could successfully suppress stem cell competition observed in c587ts > tkvCA testes (Supplementary Fig. 2). In these testes, GSCs were restored and resided around the hub, and differentiating spermatogonia could be observed (Supplementary Fig. 2). These data demonstrate that stem cell competition and GSC loss resulted from ectopic expression of tkvCA in CySCs is a consequence of ectopic Dpp signaling.
CySCs overproliferate and outcompete GSCs upon tkv CA expression
Next, we investigated the cell identity of the cells in c587ts > tkvCA testes. We first examined c587ts > tkvCA testes using the Zfh1 antibody, which labels CySCs and early cyst cells. The number of Zfh1+ cells was significantly increased compared to control testes, and CySCs tightly attached to the hub with their cell bodies, indicating that ectopic Dpp signaling in CySCs promotes CySC proliferation (Fig. 2a–c; data not shown). We then examined these c587ts > tkvCA testes using esg-lacZ, which was highly expressed in the hub and GSCs, and at low levels in CySCs (Fig. 2d). Interestingly, no germline cells were observed in these testes (Fig. 2e,f). esg-lacZ could only be observed in the hub and CySCs, and the number of esg-lacZ+ cells was dramatically increased (Fig. 2e,g). These data show that upon ectopic expression of tkvCA, CySCs continued to proliferate and occupied the whole niche, while the germline cells were completely lost.
Ectopic expression of tkvCA in CySCs leads to CySC overproliferation and CySC-GSC competition. (a) CySCs (by Zfh1, red, white arrowheads) in c587ts control testis. (b) CySCs (white arrowheads) in c587ts > tkvCA testis. (c) Quantification of the number of Zfh1+ cells in control and c587ts > tkvCA testes. n = 10–15 testes. (d) esg-lacZ (by lacZ, red) in c587ts control testis. esg-lacZ is highly expressed in the hub (red arrowhead and asterisk) and the early germline cells (GSCs and GBs) (white arrowheads), and weakly expressed in CySCs (yellow arrowhead). (e) esg-lacZ (red) in c587ts > tkvCA testis. No germline cells can be observed. esg-lacZ can only be observed in the hub (red arrowhead and asterisk) and CySCs (yellow arrowheads). (f) Quantification of the number of esg-lacZ+ germline cells in control and c587ts > tkvCA testes. n = 10–15 testes. (g) Quantification of the number of esg-lacZ+ early cyst cells in control and c587ts > tkvCA testes. n = 10–15 testes. mean ± SEM is shown. **p < 0.01. GFP in green, blue indicates DAPI staining for DNA. Scale bars: 10 μm.
GSCs are competed out of the niche and undergo differentiation upon tkv CA expression
As no germline cells were observed in these testes, we examined the fate of the germline cells, especially GSCs. The complete disappearance of germline cells, especially GSCs, may be caused by differentiation or cell death. To distinguish these two possibilities, we first performed time chase experiments. No differences were observed between the control and c587ts > tkvCA testes at 6 hours and 24 hours after the flies were shifted from 18 °C to 29 °C (Fig. 3; Supplementary Fig. 3). However, by the 2nd day, we found that some CySCs closely attached to the hub with their cell bodies in the c587ts > tkvCA flies, and the number of GSCs per testis was decreased (Fig. 3; Supplementary Fig. 3). By the 3rd day after shifting, we found that the hub was closely associated by CySCs and all GSCs were competed out of the niche (Fig. 3; Supplementary Fig. 3). As time lapsed, GSCs were pushed further away from the hub by CySCs and underwent differentiation. By the 6th day, almost all germline cells were terminally differentiated, and fully differentiated spermatids could be observed at regions near the hub (Fig. 3; Supplementary Fig. 3). CySCs closely attached to the hub kept proliferating, resulting in accumulation of CySCs (Fig. 3; Supplementary Fig. 3). On the contrary, we did not find any significant increase of GSC/germline cell death (by active Caspase-3) in these testes (Supplementary Fig. 4). These data indicate that ectopic activation of Dpp signaling in CySCs outcompetes GSCs from the niche by CySCs, and the outcompeted GSCs are lost due to differentiation.
GSCs are competed out of the niche by CySCs and differentiated in c587ts > tkvCA testis. Time chase experiment is carried out to trace the fate of GSCs. Time points examined are indicated. The hub is marked by white asterisk, GSCs are indicated by yellow arrowheads, and CySCs by white arrowheads. CySCs begin to closely attach to the hub on the 2nd day at 29 °C from 18 °C. The hub is closely associated by CySCs on the 3rd day after shifting, and all GSCs are competed out of the niche by CySCs and undergo differentiation (white arrowhead). Germline cells move further away from the hub and undergo differentiation by the 6th day. Almost all the spermatogonia are terminally differentiated, and fully differentiated spermatids can be observed (yellow arrowhead). GFP in green, blue indicates DAPI staining for DNA. Scale bars: 10 μm.
Ectopic Dpp signaling in CySCs promotes Vn expression
Previous report found that increased expression of the adhesion protein integrin in socs36E mutant CySCs could promote CySC-GSC competition16. We examined whether CySC-GSC competition observed in c587ts > tkvCA testes was due to elevated expression of integrin. However, no obvious change in integrin levels was observed (by βPS-integrin), indicating that CySC-GSC competition observed in c587ts > tkvCA testes is unlikely mediated by integrin molecules (Supplementary Fig. 5).
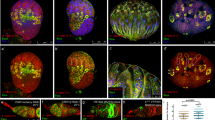
Previous studies found that elevated EGFR signaling in socs36E- and Madm-deficient CySCs was responsible for CySC-GSC competition15,19. Activation of EGFR by its extracellular ligands triggers a signal transduction cascade, mediated by the Ras/Raf/MEK cassette, which ultimately leads to dual phosphorylation and activation of the mitogen-activated protein kinase/extracellularly regulated kinase (MAPK/ERK), therefore, phosphorylated ERK (pERK) can be used as a read-out of EGFR pathway activation58. To investigate whether EGFR signaling is responsible for CySC-GSC competition observed in c587ts > tkvCA testes, we examined the activation of EGFR signaling by detecting the levels of pERK in tkvCA-expressing CySCs. Interestingly, we found the levels of pERK was significantly increased in tkvCA-expressing CySCs than those in the control, indicating that ectopic Dpp signaling promotes the activation of EGFR signaling (Fig. 4a–c). To further confirm this, we examined the expression of kekkon (kek), a primary downstream target of EGFR signaling. kek-lacZ is an enhancer trap that reflects endogenous kek expression59. We found that kek-lacZ was expressed in the early cyst cells and differentiated cyst cells in wild type testis (Fig. 4d). The expression pattern of kek-lacZ is similar to that of pERK, indicating that kek-lacZ could be used as a readout of EGFR activation in testis (Fig. 4a,d)60. We found tkvCA induction significantly enhanced the expression levels of kek-lacZ (Fig. 4e,f). These data show that ectopic Dpp signaling significantly promotes EGFR signaling in the somatic cyst cells.
Ectopic Dpp signaling in CySCs promotes vn expression. (a) EGFR signaling activation (by pERK, red) in c587tscontrol testis. pERK signal is mainly observed in the early cyst cells (white arrowheads). (b) EGFR signaling is highly activated in the somatic cyst cells of c587ts > tkvCA testis. (c) Quantification of fluorescence intensity of pERK in control and c587ts > tkvCA testes. n = 10. (d) EGFR signaling activation (by kek-lacZ, red) in c587ts control testis. kek-lacZ is expressed in the somatic cyst cells (white arrowheads). (e) kek-lacZ is highly expressed in c587ts > tkvCA testis. (f) Quantification of the fluorescence intensity of kek-lacZ in control and c587ts > tkvCA testes. n = 10. (g) vn expression (by vn-lacZ, red) in c587ts control testis. vn-lacZ is expressed at low levels in the early cyst cells (white arrowheads). (h) vn-lacZ is highly expressed in c587ts > tkvCA testis. (i) Quantification of the fluorescence intensity of vn-lacZ in control and c587ts > tkvCA testes. n = 10. mean ± SEM is shown. **p < 0.01. GFP in green, blue indicates DAPI staining for DNA. Scale bars: 10 μm.
We then explored how EGFR signaling was activated by ectopic tkvCA expression. We reasoned that some components of the EGFR signaling pathway may be transcriptional upregulated by the activated MAD/MED complex, which in turn activate EGFR signaling. We thus investigated whether ectopic tkvCA expression promotes the transcription of EGFs. We examined the expression of EGFs (Spitz (Spi) and Vein (Vn)) in the testis using their enhancer traps. Consistently, we found that spi was expressed in the germline cells (by spi-lacZ) (Supplementary Fig. 6)61. While vn was expressed in the early somatic cyst cells, including CySCs (by vn-lacZ) (Fig. 4g)17,19. As tkvCA was ectopically expressed in CySCs, therefore, we focused on vn for further examination. To explore the relationship between ectopic Dpp signaling and vn expression, we examined the expression levels of vn in c587ts > tkvCA testes. We found vn expression was markedly increased upon tkvCA induction (Fig. 4h,i). These data suggest that ectopic Dpp signaling promotes vn expression, which in turn induces elevated EGFR signaling in the early cyst cells.
Ectopic Vn/EGFR/MAPK signaling is responsible for CySC-GSC competition
Therefore, we addressed whether elevated vn expression was responsible for CySC-GSC competition observed in c587ts > tkvCA testes. When ectopically expressed in CySCs (c587ts > vnEP), we found that vnEP overexpression resulted in CySC-GSC competition, which mimics c587ts > tkvCA testes (Fig. 5a–d). Consistently, the number of GSCs per testis was significantly reduced in c587ts > vnEP testes (Fig. 5a–c), and the number of CySCs tightly attaching to the hub was greatly increased in c587ts > vnEP testes compared with that of control testes (Fig. 5b,d). These data indicate that elevated vn expression promotes CySC-GSC competition. Furthermore, we found that expression of a constitutively active form of Ras (RasV12) also resulted in CySC-GSC competition and GSC loss, phenocopying tkvCA expression (data not shown). These data indicate that CySC-GSC competition observed in c587ts > tkvCA testes is likely a consequence of ectopic EGFR/MAPK signaling.
Ectopic Vn/EGFR/MAPK signaling is responsible for CySC-GSC competition and GSC loss in c587ts > tkvCA testes. (a) c587ts control testis. (b) c587ts > vnEP testis. Note that most GSCs (red, white arrowheads) are competed out of the niche by CySCs (yellow arrowheads). (c) Quantification of the number of GSCs per testis in the testis with indicated genotype. n = 10–15 testes. (d) Quantification of the number of CySCs with their cell body attaching to the hub per testis with indicated genotypes. n = 10–15 testes. (e) c587ts > tkvCA testes. (f) c587ts > vnRNAi testes (HMS00004). No obvious defects are observed when this shRNA is induced. (g) Stem cell competition observed in c587ts > tkvCA testis is almost completely suppressed by simultaneous expression of this shRNA against vn. (h) c587ts > egfrRNAi testes. The induction of this weak egfrRNAi line (JF01368) in CySCs does not cause any obvious defects. (i) Stem cell competition observed in c587ts > tkvCA testis is greatly suppressed by co-expression of this egfrRNAi. mean ± SEM is shown. **p < 0.01. GFP in green, Vasa in red, Fas3 in yellow, blue indicates DAPI staining for DNA. Scale bars: 10 μm.
To further confirm that elevated Vn/EGFR/MAPK signaling is responsible for CySC-GSC competition observed in c587ts > tkvCA testes, we performed suppression experiments. No obvious defects was caused when vn was depleted in CySCs using a shRNA (TH03149.N) (Fig. 5f). When vn was compromised in c587ts > tkvCA testes using this shRNA, the observed CySC-GSC competition and GSC loss defects were almost completely suppressed (Fig. 5e–g). The number of GSCs per testis and the number of CySCs tightly attaching to the hub were almost completely reverted by simultaneous knockdown of vn (Fig. 5a–d). These results indicate that ectopic vn expression is responsible for CySC-GSC competition observed in c587ts > tkvCA testes. To further confirm our conclusion, we targeted EGFR itself for suppression assay. As EGFR signaling is essential for spermatogonia differentiation, we selected a weak dsRNA against egfr (JF01368) to inhibit EGFR signaling17,37,62. Knockdown of egfr using this dsRNA resulted in no obvious defects (Fig. 5h). We found that the observed CySC-GSC competition and GSC loss defects were almost completely suppressed by simultaneous induction of this dsRNA in c587ts > tkvCA testes (Fig. 5e,h,i). Consistently, the number of GSCs per testis and the number of CySCs tightly attaching to the hub were almost completely suppressed by co-inhibition of egfr (Fig. 5c,d). Together, these data demonstrate that ectopic Vn/EGFR/MAPK signaling is responsible for CySC-GSC competition and GSC loss resulted from tkvCA expression in CySCs.
Discussion
Fittest stem cells are selected through stem cell competition in the niche to maintain tissue homeostasis. However, the mechanisms underlying stem cell competition remain largely unknown. Here, we reveal that cell-autonomous activation of Dpp signaling in CySCs results in CySC-GSC competition and GSC loss, which is mediated by elevated Vn/EGFR/MAPK signaling. The mechanism we uncovered may be general features of stem cell systems in regulating stem cell competition2,22,63.
Stem cell competition emerges as a mechanism to select fit stem cells and control tumorigenesis1,2,3,4,5. Stem cell competition takes place in three steps. The competitive stem cells first become more fit, before they move and anchor to a defined niche, followed by proliferation and outcompetition of neighboring stem cells. However, the detailed mechanisms underlying stem cell competition in the Drosophila testis are poorly understood. Elucidating the mechanisms controlling stem cell competition will help to develop potential clinic treatments for cancer. The testis niche supports two groups of stem cells: GSCs and CySCs, making it an excellent model to study stem cell competition regulation. Previous studies found that CySCs compete with each other and with GSCs for niche occupancy15,16,19,46. Mutations that confer increased competitiveness to CySCs result in outcompetition of wild type resident stem cells by the mutant stem cells and their descendants. The first identified regulator of niche competition is Socs36E, a negative feedback inhibitor of the JAK/STAT pathway. The competitive behavior of socs36E mutant CySCs was first attributed to increased JAK/STAT signaling16. However, it was recently found that the competitiveness of socs36E mutant CySCs is likely due to elevated MAPK signaling15. Stem cell competition also occurs among CySCs, it was reported that CySCs with increased Hh or Yorkie (Yki) activity displaced neighboring wildtype CySCs from the niche before they outcompeted neighboring wild type GSCs, indicating that both intra- (CySC-CySC) and inter-lineage (CySC-GSC) competitions take place in the testis46. It was recently reported that Slit-Robo signaling only regulates intra-lineage competition among CySCs54.
Ectopic Dpp signaling in CySCs results in CySC-GSC competition for niche anchoring and GSC loss (Fig. 1). We found that ectopic Dpp signaling leads to elevated Vn expression, which in turn activates EGFR/MAPK signaling in CySCs to promote their proliferation and ability to outcompete GSCs for niche occupancy (Figs 4 and 5). Ectopic expression of vn in CySCs results in CySC-GSC competition, which mimics c587ts > tkvCA. However, the GSC loss and the CySC overproliferation phenotype in c587ts > vnEP is not as severe as the latter. The differences may be caused by the vnEP line used in this study, which may not produce sufficient vn transcripts as that of tkvCA expression. Nevertheless, the observed CySC-GSC competition upon tkvCA expression is almost completely suppressed by compromising EGFR signaling (Fig. 5). Our study here demonstrate that the niche signals must be tightly controlled to prevent CySC-GSC competition, thereby maintaining niche homeostasis. Interestingly, a recent study found that the novel tumor suppressor Mlf1-adaptor molecule (Madm) regulates CySC-GSC competition19. They found that Madm regulates CySC-GSC competition by suppressing the expression of integrin and EGFR ligand Vn19. Although tkvCA induction promotes vn expression, we found that, unlike loss of madm, tkvCA induction does not affect integrin expression levels, suggesting that the downstream events regulating stem cell competition in tkvCA and madm−/− CySCs are not identical (Supplementary Fig. 5). It is established that EGFR/MAPK signaling is required in the somatic cyst cells for their proper differentiation and engulfment of the developing germline cells17,37,62. From recent studies on socs36E, Madm, and our study on tkvCA, we can conclude that EGFR/MAPK signaling in CySCs also plays a pivotal role in regulating CySC-GSC competition15,19. It will be interesting to investigate why BMP signaling is kept from being over-activated in CySCs under physiological conditions, and how different input signals are converged on the EGFR/MAPK signaling pathway to regulate CySC-GSC competition, which will help to understand the regulation of stem cell competition, tissue homeostasis, and tumorigenesis.
Materials and Methods
Fly lines and cultures
Flies were maintained on standard corn-meal cultural media at 25 °C. To inactivate Gal80ts, flies were shifted to 29 °C, and transferred to new vials every day and dissected at specific time points as indicated. Information about alleles and transgenes used can be found in FlyBase and as noted: c587Gal4, UAS-GFP, esg-lacZ, tubGal80ts (c587ts), UAS-tkvQ253D (tkvCA), UAS-madRNAi (GL01527, GLV21013, JF01263, JF01264, NIG 12399R-1, and 12399R-2), UAS-medRNAi (JF02218 and GL01313), kekBB142 (kek-lacZ, gift from Zhaohui Wang), spis3547 (spi-lacZ, gift from Rongwen Xi), vnp1749 (vn-lacZ, gift from Rongwen Xi), UAS-vnRNAi (TH03149.N, Tsinghua University), UAS-egfrRNAi (JF01368), vnEPg35521 (BL58498), UAS-wRNAi (BL33613 and HMS00004) (from TRiP at Harvard Medical School)64.
RNAi knock down and overexpression experiments
To examine gene function in CySCs, c587ts (c587Gal4, UAS-GFP, esg-lacZ, tubGal80ts) was used. Crosses were maintained at 18 °C. Progeny with the proper genotypes was collected 1–2 days after eclosion and maintained at 29 °C before examination. UAS-dsRNA and UAS-shRNA transgenic flies were used.
MARCM clone analyses
CySC MARCM clones were generated by heat shock treatment65. 1–3 days old adult flies were heat-shocked at 37 °C for 60 minutes for 2 consecutive days. Flies were maintained at 25 °C and transferred to new vials every day. The clones were assayed at indicated time points after clone induction (ACI).
Immunostainings and fluorescence microscopy
For fluorescent immunostainings, testes were dissected in 1 × PBS, and fixed in 4% paraformaldehyde for 25 min at room temperature. Testes were washed with 1 × PBT (0.1% Triton X-100 in 1 × PBS) for 3 times, 5 min each, and blocked with 3% BSA for 45 min. The samples were incubated with primary antibodies overnight at 4 °C. The following antibodies were used: mouse mAb anti-Fas3 (7G10, 1:50, developed by Corey S. Goodman, Developmental Studies Hybridoma Bank (DSHB)), mouse mAb anti-βPS-integrin (CF.6G11, 1:50, developed by D. Brower, DSHB), rabbit anti-Vasa (d-260, Santa Cruz, 1:200), rabbit anti-Zfh1 (1:5000, a generous gift from Ruth Lehmann, and 1:8000, generated in our lab)53, rabbit anti-β-galactosidase (1:5000, Cappel), mouse anti-β-galactosidase (1:1000, Cell Signaling), rabbit anti-pMAD3 (1:300, Epitomics), rabbit anti-active Caspase-3 (1;200, Abcam), and rabbit anti-pERK (p-p44/42, 1:200, Cell Signaling). Primary antibodies were detected by fluorescent-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Secondary antibodies were incubated at room temperature for 2 hrs. After secondary antibody staining, DAPI (0.1 μg/ml, Sigma-Aldrich) was added to the samples for 45 min at room temperature. Mounting medium (2.5% DABCO in 70% glycerol) was added to the samples. All images were captured under a Zeiss inverted confocal microscope (780) and were further processed using Adobe Photoshop and Illustrator.
Data Availability
The number of GSCs and CySCs was counted manually. For fluorescence intensity of pERK and lacZ, all images were taken under the same confocal settings. Image Pro Plus 5.0 software was used to measure fluorescence intensity of pERK and lacZ (using the measure/count function). Statistical analysis was performed using the Student’s t-test. PEMS 3.1 software was used for SEM analyses. The graphs were generated using SigmaPlot 10.0 software, and further modified using Adobe Photoshop and Illustrator.
References
Martins, V. C. et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 509, 465–470 (2014).
Jin, Z. et al. Differentiation-Defective Stem Cells Outcompete Normal Stem Cells for Niche Occupancy in the Drosophila Ovary. Cell Stem Cell 2, 39–49 (2008).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem Cell Self-Renewal Specified by JAK-STAT Activation in Response to a Support Cell Cue. Science 294, 2542–2545 (2001).
Sheng, X. R., Brawley, C. M. & Matunis, E. L. Dedifferentiating Spermatogonia Outcompete Somatic Stem Cells for Niche Occupancy in the Drosophila Testis. Cell Stem Cell 5, 191–203 (2009).
Stine, R. R. & Matunis, E. L. Stem cell competition: finding balance in the niche. Trends in Cell Biology 23, 357–364 (2013).
Amoyel, M. & Bach, E. A. Cell competition: how to eliminate your neighbours. Development 141, 988–1000 (2014).
Bondar, T. & Medzhitov, R. p53-Mediated Hematopoietic Stem and Progenitor Cell Competition. Cell Stem Cell 6, 309–322 (2010).
Marusyk, A., Porter, C. C., Zaberezhnyy, V. & DeGregori, J. Irradiation Selects for p53-Deficient Hematopoietic Progenitors. PLOS Biology 8, e1000324 (2010).
Li, L. & Xie, T. Stem Cell Niche: Structure and Function. Annual Review of Cell and Developmental Biology 21, 605–631 (2005).
Decotto, E. & Spradling, A. C. The Drosophila Ovarian and Testis Stem Cell Niches: Similar Somatic Stem Cells and Signals. Developmental Cell 9, 501–510 (2005).
Yamashita, Y. M., Fuller, M. T. & Jones, D. L. Signaling in stem cell niches: lessons from the Drosophila germline. Journal of Cell Science 118, 665–672 (2005).
Davies, E. L. & Fuller, M. T. Regulation of Self-renewal and Differentiation in Adult Stem Cell Lineages: Lessons from the Drosophila Male Germ Line. Cold Spring Harb. Symp. Quant. Biol. 73, 137–145 (2008).
de Cuevas, M. & Matunis, E. L. The stem cell niche: lessons from the Drosophila testis. Development 138, 2861–2869 (2011).
Fuller, M. T. & Spradling, A. C. Male and Female Drosophila Germline Stem Cells: Two Versions of Immortality. Science 316, 402–404 (2007).
Amoyel, M., Anderson, J., Suisse, A., Glasner, J. & Bach, E. A. Socs36E Controls Niche Competition by Repressing MAPK Signaling in the Drosophila Testis. PLOS Genetics 12, e1005815 (2016).
Issigonis, M. et al. JAK-STAT Signal Inhibition Regulates Competition in the Drosophila Testis Stem Cell Niche. Science 326, 153–156 (2009).
Kiger, A. A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000).
Eun, S. H., Shi, Z., Cui, K., Zhao, K. & Chen, X. A Non–Cell Autonomous Role of E(z) to Prevent Germ Cells from Turning on a Somatic Cell Marker. Science 343, 1513–1516 (2014).
Singh, S. R., Liu, Y., Zhao, J., Zeng, X. & Hou, S. X. The novel tumour suppressor Madm regulates stem cell competition in the Drosophila testis. Nature. Communications 7, 10473 (2016).
Michel, M., Kupinski, A. P., Raabe, I. & Bökel, C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development 139, 2663–2669 (2012).
Michel, M., Raabe, I., Kupinski, A. P., Perez-Palencia, R. & Bokel, C. Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche. Nat. Commun. 2, 415 (2011).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Zhang, Z., Lv, X., Jiang, J., Zhang, L. & Zhao, Y. Dual roles of Hh signaling in the regulation of somatic stem cell self-renewal and germline stem cell maintenance in Drosophila testis. Cell Res. 23, 573–576 (2013).
Amoyel, M., Sanny, J., Burel, M. & Bach, E. A. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development 140, 56–65 (2013).
Eun, S. H., Shi, Z., Cui, K., Zhao, K. & Chen, X. A Non–Cell Autonomous Role of E(z) to Prevent Germ Cells from Turning on a Somatic Cell Marker. Science 343, 1513–1516 (2014).
Fuller, M. T. Spermotogenesis. In The Development of Drosophila melanogaster, M. Bate and A. Martinez-Aries, eds, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press (1993).
Gonczy, P. & DiNardo, S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437–2447 (1996).
Gregory, L., Came, P. J. & Brown, S. Stem cell regulation by JAK/STAT signaling in Drosophila. Semin. Cell Dev. Biol. 19, 407–413 (2008).
Inaba, M., Buszczak, M. & Yamashita, Y. M. Nanotubes mediate niche–stem-cell signalling in the Drosophila testis. Nature 523, 329–332 (2015).
Inaba, M., Sorenson, D. R., Kortus, M., Salzmann, V. & Yamashita, Y. M. Merlin is required for coordinating proliferation of two stem cell lineages in the Drosophila testis. Scientific Reports 7, 2502 (2017).
Le Bras, S. & Van Doren, M. Development of the male germline stem cell niche in Drosophila. Developmental Biology 294, 92–103 (2006).
Leatherman, J. L. & DiNardo, S. Zfh-1 Controls Somatic Stem Cell Self-Renewal in the Drosophila Testis and Nonautonomously Influences Germline Stem Cell Self-Renewal. Cell Stem Cell 3, 44–54 (2008).
Leatherman, J. L. & DiNardo, S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12, 806–811 (2010).
Losick, V. P. et al. Drosophila Stem Cell Niches: A Decade of Discovery Suggests a Unified View of Stem Cell Regulation. Dev. Cell 21, 159–171 (2011).
Zoller, R. & Schulz, C. The Drosophila cyst stem cell lineage: partners behind the scenes? Spermatogenesis 3, 145–157 (2012).
Voog, J., D’Alterio, C. & Jones, D. L. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454, 1132–1136 (2008).
Tran, J., Brenner, T. J. & DiNardo, S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754–757 (2000).
Matunis, E. L., Stine, R. R. & De Cuevas, M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis 3, 137–144 (2012).
Tulina, N. & Matunis, E. Control of Stem Cell Self-Renewal in Drosophila Spermatogenesis by JAK-STAT Signaling. Science 294, 2546–2549 (2001).
Issigonis, M. & Matunis, E. SnapShot: Stem Cell Niches of the Drosophila Testis and Ovary. Cell 145, 994–994 (2011).
Cheng, J., Tiyaboonchai, A., Yamashita, Y. M. & Hunt, A. J. Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development 138, 831–837 (2011).
Flaherty, M. S. et al. Chinmo is a Functional Effector of the JAK/STAT Pathway that Regulates Eye Development, Tumor Formation, and Stem Cell Self-Renewal in. Drosophila. Dev. Cell 18, 556–568 (2010).
Kawase, E., Wong, M. D., Ding, B. C. & Xie, T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365–1375 (2004).
Shivdasani, A. A. & Ingham, P. W. Regulation of Stem Cell Maintenance and Transit Amplifying Cell Proliferation by TGF-β Signaling in Drosophila Spermatogenesis. Current Biology 13, 2065–2072 (2003).
Zheng, Q., Wang, Y., Vargas, E. & DiNardo, S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Developmental Biology 357, 202–210 (2011).
Amoyel, M., Simons, B. D. & Bach, E. A. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. The EMBO Journal 33, 2295–2313 (2014).
López-Onieva, L., Fernández-Miñán, A. & González-Reyes, A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development 135, 533–540 (2008).
Huang, J., Reilein, A. & Kalderon, D. Yorkie and Hedgehog independently restrict BMP production in escort cells to permit germline differentiation in the Drosophila ovary. Development 144, 2584–2594 (2017).
Wang, L., Li, Z. & Cai, Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. The Journal of Cell Biology 180, 721–728 (2008).
Hayashi, Y., Kobayashi, S. & Nakato, H. Drosophila glypicans regulate the germline stem cell niche. The Journal of Cell Biology 187, 473–480 (2009).
Liu, M., Lim, T. M. & Cai, Y. The Drosophila Female Germline Stem Cell Lineage Acts to Spatially Restrict DPP Function Within the Niche. Sci. Signal. 3, ra57–ra57 (2010).
Guo, Z. & Wang, Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136, 3627–3635 (2009).
Xu, R. et al. Self-restrained regulation of stem cell niche activity by niche components in the Drosophila testis. Dev. Biol. 439, 42–51 (2018).
Stine, R. R., Greenspan, L. J., Ramachandran, K. V. & Matunis, E. L. Coordinate Regulation of Stem Cell Competition by Slit-Robo and JAK-STAT Signaling in the Drosophila Testis. Plos Genetics 10, e1004713 (2014).
Lecuit, T. et al. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381, 387–393 (1996).
Persson, U. et al. The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434, 83–87 (1998).
Li, Z., Zhang, Y., Han, L., Shi, L. & Lin, X. Trachea-derived Dpp controls adult midgut thomeostasis in Drosophila. Dev. Cell 24, 133–143 (2013).
Yung, Y. et al. Detection of ERK activation by a novel monoclonal antibody. FEBS Lett. 408, 292–296 (1997).
Pai, L.-M., Barcelo, G. & Schüpbach, T. D-cbl, a Negative Regulator of the Egfr Pathway, Is Required for Dorsoventral Patterning in Drosophila Oogenesis. Cell 103, 51–61 (2000).
Tang, Y. et al. Germline Proliferation Is Regulated by Somatic Endocytic Genes via JNK and BMP Signaling in Drosophila. Genetics 206, 189–197 (2017).
Sarkar, A. et al. Antagonistic Roles of Rac and Rho in Organizing the Germ Cell Microenvironment. Current Biology 17, 1253–1258 (2007).
Schulz, C., Wood, C. G., Jones, D. L., Tazuke, S. I. & Fuller, M. T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–4534 (2002).
Luo, L., Wang, H., Fan, C., Liu, S. & Cai, Y. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. The Journal of Cell Biology 209, 595–608 (2015).
Ni, J.-Q. et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Meth. 8, 405–407 (2011).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 (2001).
Acknowledgements
We thank Yu Cai, Ruth Lehmann, Zhaohui Wang, Rongwen Xi, Developmental Studies Hybridoma Bank (DSHB), Bloomington stock center, and TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947)/Tsinghua University for reagents and stocks. This study was supported by grants from the National Natural Science Foundation of China (Nos 31471384, 31872081, and 31271582), Beijing Natural Science Foundation (5162004), Scientific Research Improvement Project of Beijing University of Agriculture (GZL2014003), and Beijing Municipal Commission of Education (Nos 010135336400 and 010155310500).
Author information
Authors and Affiliations
Contributions
Y.Y. and Z.L. initiated the project and designed the experiments. Y.L. and Z.L. performed most of the experimental work. Y.L. and Z.L. financed the project. Z.L. directed the project and wrote the manuscript, which was approved by all authors prior to submission.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Y., Yao, Y. & Li, Z. Ectopic Dpp signaling promotes stem cell competition through EGFR signaling in the Drosophila testis. Sci Rep 9, 6118 (2019). https://doi.org/10.1038/s41598-019-42630-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42630-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.