Abstract
Cadmium exposure induces nephrotoxicity by mediating oxidative stress, inflammation, and apoptosis. The purpose of this study was to examine the protective effect of royal jelly on Cd-induced nephrotoxicity. Adult male mice were distributed randomly into 4 clusters: untreated, royal jelly-treated (85 mg/kg, oral), CdCl2-treated (6.5 mg/kg, intraperitoneal), and pretreated with royal jelly (85 mg/kg) 2 h before CdCl2 injection (6.5 mg/kg, intraperitoneal) for seven consecutive days. Cd concentration in the renal tissue and absolute kidney weight of the Cd-treated mice were significantly higher than those of control group. The levels of kidney function markers, kidney injury molecules-1 (KIM-1), metallothionein, lipid peroxidation, nitric oxide, tumor necrosis factor-α, interleukin-1β, and the apoptosis regulators Bax and caspases-3 also increased significantly in the renal tissue of Cd-treated mice, whereas the levels of glutathione, antioxidant enzyme activities, and the apoptosis inhibitor Bcl-2 were significantly reduced in the renal tissue of Cd-treated group. Histopathological studies showed vacuolation and congested glomeruli in the kidney tissue of Cd-treated mice. However, all aforementioned Cd-induced changes were attenuated by pretreatment with royal jelly. We therefore concluded that royal jelly attenuated Cd-induced nephrotoxicity and it is suggested that this nephroprotective effect could be linked to its ability to promote the nuclear factor erythroid 2–related factor 2 (Nrf2)/antioxidant responsive element (ARE) pathway.
Similar content being viewed by others
Introduction
Cadmium is a reactive metal which negatively affects mammalian organs, such as the brain, liver, kidney, placenta, and testis1,2. In humans, occupational and environmental exposure to cadmium cause severe degeneration to the kidney. Contaminated air, soil, drinking water, and food, as well as cigarettes are the main sources of cadmium exposure3. The mechanisms underlying cadmium nephrotoxicity are not fully understood. However, metallothioneins (cysteine-rich low molecular weight proteins), Cd-binding proteins containing thiol (-SH) groups, and divalent metal-ion transporter-1 are playing a pivotal role in cadmium accumulation in the kidney tissue4. After long-term exposure to cadmium, the glomerular filtration rate decreases significantly, which eventually leads to kidney failure5. Cadmium may induce nephrotoxicity by generating reactive oxygen species (ROS), inflammation, and apoptosis in the kidney tissue1,6. Elkhadragy et al.2 reported that S1 and S2 segments of the proximal tubules are the main target site of cadmium nephrotoxicity.
It is important to counter the cadmium toxicity-induced generation of free with natural antioxidants, as synthetic chelating agents have showed several undesirable side effects. Royal jelly is a milky secretion of the hypopharingeal and mandibular glands of worker honey bees for feeding the developing queen bees. Royal jelly consists of proteins (such as royalactin), monosaccharides, lipids, fatty acids (such as 10-hydroxy-2-decenoic acid and 10-HDA), free amino acids (eight essential amino acids valine, leucine, isoleucine, threonine, methionine, phenylalanine, tryptophan, and lysine), minerals (such as Fe, Ca, K, Na, Mg, Zn, Cu, and Mn), and vitamins (A, B complex, E, and C)7. The biological benefits of royal jelly include antioxidant, anti-inflammatory, anti-aging, hypoglycemic, antitumor, and hypocholesterolemic activities8,9,10. Hence, this study aimed to explore the nephroprotective activity of royal jelly (RJ) against CdCl2-induced nephrotoxicity in male mice.
Material and Methods
Chemicals
Anhydrous CdCl2 was procured from Sigma-Aldrich (St. Louis, MO, USA). Lyophilized royal jelly powder 6% 10-HAD, 340 mg of each is equivalent to 1000 mg crude royal jelly, was purchased from Pharco Pharmaceuticals Inc. (Alexandria, Egypt). Kidney function assay kits were all purchased from BioDiagnostic Co. (Cairo, Egypt). TRIzol reagent and PCR primers were provided by Invitrogen (Carlsbad, CA, USA). Thermo Scientific First Strand cDNA Synthesis kit was acquired from Thermo Fisher Scientific Inc. (Waltham, MA, USA). All chemicals were of high quality and analytical grade, and used without further purification.
Dosage selection
The median lethal dose (LD50) of CdCl2 in mouth was 93.7 mg/kg11. In the present study, we have selected 6.5 mg/kg of CdCl2 which was 1/15 of the LD50 dose of CdCl2. Further, this dose of CdCl2 was used previously to produce nephrotoxicity in rats2. While, A pilot experiment was proceeded with three different doses of RJ (34, 85 and 170 mg/kg equivalent to 100, 250 and 500 crude RJs, respectively) to define the dose dependent effect of RJ in CdCl2-induced nephrotoxicity in mice. The pilot study reveled that RJ pretreatment at the doses of 34, 85 and 170 mg/kg markedly prevented pathological alternations in the kidney of CdCl2 intoxicated mice (data have not shown). However, 85 and 170 mg/kg of RJ showed the most protective effect than the other doses 34 mg/kg against Cd intoxication. Hence, we decided to choose the 85 mg/kg of RJ for our study.
Animal treatment
Twenty-eight adult male Swiss mice (weighing 22–27 g, 10–12 weeks old) were procured from Vacsera (Cairo, Egypt). The mice were placed in the animal facility of the Zoology Department at Helwan University (Cairo, Egypt) at 22–25 °C with artificial 12-h light/dark cycle. The mice were supplied with pelleted rodent food and water ad libitum. For the evaluation of the nephroprotective effect of RJ on CdCl2-induced nephrotoxicity, mice were divided randomly into 4 groups (n = 7). Control mice received intraperitoneal (i.p.) injection of 0.9% NaCl (physiological saline) every day for 7 days. CdCl2 group received i.p. injection of 6.5 mg/kg CdCl2 each day for 7 days. RJ group was orally treated with RJ 85 mg/kg, which is equivalent to 250 crude RJs. RJ + CdCl2 group was orally administered with 85 mg/kg RJ 2 h prior to i.p. injection of 6.5 mg/kg CdCl2 daily for 7 days. CdCl2 was dissolved in NaCl solution. Before being sacrificed twenty-four h after the last treatment, they were housed separately for about 12 h for the collection of urine, and then the left kidney was immediately dissected, weighed, and directly homogenized in frozen 10 mM phosphate buffer (pH 7.4) to generate a 10% (w/v) homogenate for biochemical evaluation. Meanwhile, the right kidney was isolated for cadmium concentration determination and histopathological studies. All procedures were performed under anesthesia and all efforts were made to minimize suffering. All procedures involving animals were approved by the Committee of Research Ethics for Laboratory Animal Care, Department of Zoology, Faculty of Science, Helwan University (Approval No. HU2017/Z/03) in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, 8th edition (NIH Publication No. 85–23, revised 1985).
Concentration of Cd in the kidney tissue
The concentration of Cd in the kidney tissues were assessed using a standardized process2. In brief, kidney tissue samples were weighed and dried in an oven at 100 °C for 8 h. The dried samples were digested with 2 M nitric acid and 2 M hydrochloric acid at 150 °C for 5 h. The samples were diluted with deionized H2O to a final volume of 50 ml. Cadmium concentration was evaluated by graphite furnace atomic absorption spectrophotometry (Perkin-Elmer 3100) at 283.3 nm. Cd concentrations are expressed as µg/g of wet kidney tissue.
Kidney function assays
The serum level of kidney function markers was measured using specific commercial kits for uric acid, urea, and creatinine according to the manufacturer’s protocols.
Kidney injury molecule 1 assay
The renal concentration of kidney injury molecule 1 (KIM-1) was determined in kidney homogenates using enzyme-linked immunosorbent assay (ELISA) kit obtained from Abcam (Catalogue No. ab119597; Cambridge, UK). The assay procedures performed according to the ELISA kit instructions.
Metallothionein determination
Metallothionein level in the kidney homogenates were measured according to the methods of Linde and Garcia-Vazquez12. Briefly, the homogenates were centrifuged at 30,000 × g for 20 min to obtain a supernatant containing metallothionein. 1.05 ml of cold (−20 °C) absolute ethanol and 80 μl of chloroform per 1 ml of the resulting supernatant were added. The cold samples (at 0–4 °C) were centrifuged at 6000 × g for 10 min. 3 volumes of cold ethanol were added to the resulting supernatant and store at −20 °C for 1 h, and then, the samples were centrifuged at 6000 × g for 10 min. The resulting pellets were washed with ethanol:chloroform:homogenization buffer (87:1:12) and then were centrifuged again at 6000 × g for 10 min. The dried pellet was resuspended in 300 μl of 5 mM Tris‐HCl, 1 mM EDTA, pH 7. The resuspended metallothionein fraction was added to 4.2 ml of 0.43 mM 5,5′‐dithiobis(nitrobenzoic acid) in 0.2 M phosphate buffer, pH 8. After 30 min, the concentration of reduced sulfhydryl was determined by reading the absorbance at 412 nm in a spectrophotometer. The amount of metallothionein in the samples was determined from the equation x = (2.5–0.0524)/5.5553 = μmol.
Biochemical assays
Lipid peroxidation (LPO) was assessed as thiobarbituric acid reactive substances (TBARS) in terms of formed malondialdehyde (MDA) according to a method described by Ohkawa et al.13. Nitric oxide (NO) level was determined using Griess reagent14. Glutathione (GSH) content was assessed as according to a method illustrated by Ellman15. Superoxide dismutase (SOD) activity was valued using the nitroblue tetrazolium (NBT) method as defined by Nishikimi et al.16. Catalase (CAT) activity was determined according to a method by Aebi17, Glutathione peroxidase (GSH-Px) activity was determined utilizing the method of Paglia and Valentine18. Glutathione reductase (GSH-R) activity was analyzed utilizing the process described by De Vega et al.19.
Inflammation marker assays
Renal levels of tumor necrosis factor-α (TNF-α; Cat. No. EZMTNFA, Millipore) and interleukin-1β (IL-1β; Cat. No. EM2IL1B, ThermoFisher Scientific) were measured using kits according to the manufacturer’s protocols.
Western blot analysis
We performed protein extraction and western blot analyses as described previously20. The utilized antibodies included mouse antibody to NQO1 (sc-376023, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Nrf2 (MAB3925, 1:500; R&D System), NFκB (p65) (sc-8008, 1:200; Santa Cruz Biotechnology), goat antibody to HO-1 (AF3169, 1:500; R&D System), β-actin (MAB8929, 1:500; R&D System), goat anti-mouse IgG (sc-2039, 1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and donkey anti-goat IgG (sc-2042, 1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The proteins were visualized using an enhanced chemiluminescence detection kit (Bio-Rad, USA) following the manufacturer’s protocol. Images were analyzed using the Kodak Image Station 2000R (Eastman Kodak Company, Rochester, NY, USA). Protein bands intensity were normalized to β-actin, and the data expressed in terms of percent relative to controls.
Quantitative RT-PCR
Total RNA was isolated from the kidney tissues using TRIzol reagent according to the instructions of the manufacturer. Approximately 5 µg of the total RNA were reverse transcribed to synthesis cDNA using a cDNA synthesis kit according to the manufacturer’s protocol. The sense/antisense primers for tested genes are listed in Supplementary Table 1. Real-time PCR analysis was performed using Power SYBR® Green Master Mix kit. The relative gene expression of Sod2, Cat, Gpx1, Gsr, Nfe2l2, Nos2, Il1β, Tnf, Bcl2, Bax and Casp3 was determined and expressed as proportional changes with respect to the control. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was employed as the internal control and was shown to be unaffected by the treatments.
Histological examination
Right kidney samples were fixed in 10% neutral formalin for 24 h at 25 °C, embedded in paraffin, and sliced into 4–5 μm–thick sections. The sections were stained with hematoxylin and eosin (H&E) for light microscopy. Nikon Eclipse E200-LED (Tokyo, Japan) was used to take images of the specimens with 400× magnification.
Immunohistochemistry analysis
For the detection of apoptosis-related proteins, the prepared kidney sections (4 μm thick) were blocked with 0.1% hydrogen peroxide containing methanol for 15 min to terminate endogenous peroxidase activity. Afterwards, the tissue slices were incubated with rabbit polyclonal Bcl-2, Bax, or caspase-3 antibody at 4 °C for 24 h. The tissues were washed with phosphate-buffered saline, incubated with biotinylated goat anti-rabbit immunoglobulins, and incubated with streptavidin–peroxidase complexes at 30 °C for 30 min. The peroxidase activity was developed with diaminobenzidine (DAB)-hydrogen peroxide. Nikon Eclipse E200-LED (Tokyo, Japan) was used to take microscopy images of the specimens at 400× magnification.
In the immunohistochemically-stained tissues, the color intensity of each protein was semi-quantitatively evaluated. The intensity was expressed as + (weak immunoreactivity), ++ (moderate immunoreactivity), +++ (strong immunoreactivity), or ++++ (very strong immunoreactivity).
Statistical analysis
One-way analysis of variance (ANOVA) was used for the statistical analysis with post-hoc Tukey’s test. Results are expressed as the mean ± SD (standard deviation). Differences were considered statistically significant at P values < 0.05.
Results
Mice intoxicated with Cd showed some clinical signs of cadmium toxicity including inappetence, increase in urination, slight decrease of the body weight and increase in respiratory (data not shown). However, mice pretreated with RJ showed less or no clinical signs of Cd toxicity.
Mice treated with CdCl2 showed a significant increase (P < 0.05) in Cd concentration in the kidney tissue compared with that of control group (Fig. 1). The daily injection of CdCl2 for 7 days caused significant decrease in absolute kidney weight compared with that of the control (Fig. 2). Furthermore, Cd accumulated in the kidney was concomitant with kidney function impairment, as indicated by the significant increase in creatinine, urea, and uric acid levels in Cd-injected mice (Fig. 3). RJ pretreatment abrogated all of those deterioration effects of Cd, as suggested by the significant decrease in Cd concentration, level of kidney function markers, and kidney weight compared with those of CdCl2-treated mice. No change in kidney weight and level of kidney function markers occurred in mice that were treated with RJ alone.
Metallothionein (MT) is characterized by their ability to bind metals such as Cd. Hence, the concentration of MT was determined in the present study, MT concentration in kidney of rats was shown in Fig. 4. The MT concentration was significantly increased in kidney of rats those exposed to Cd. On the other hand, RJ pretreatment minimized this increment in MT. however, compared with the control; MT concentration was also significantly increased in kidney tissue. As a result of MT induction, Cd was slowly released from the kidney and that causes nephrotoxicity evidenced by the significant increase in KIM-1 in kidney of the exposed rats (Fig. 5). However, RJ administration was significantly decreased KIM-1 concentration.
A drastic increase (P < 0.05) in NO along with LPO levels, coupled with a major reduction in GSH content, were observed in the kidney tissue of CdCl2-treated mice. RJ administration prior to CdCl2-treatment markedly restored the level of oxidative stress markers near that of the control group (Fig. 6).
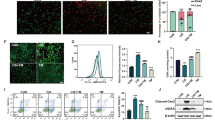
A major inhibition (P < 0.05) in enzymatic activity of antioxidants (SOD, CAT, GSH-Px, and GSH-R) was noticed in mice injected with CdCl2 (Fig. 7). The pretreatment with RJ along prior to CdCl2 injection ameliorated the adverse effects of Cd, as evidenced with the restoration of enzymatic activity of antioxidants. Consistent with these biochemical findings, qRT-PCR result revealed that mRNA expression of Sod2, Gpx1, Cat, and Gsr were prominently downregulated in CdCl2-treated mice and RJ pretreatment upregulated these genes (Fig. 7).Nuclear factor (erythroid-derived 2)-like 2 factor is the master regulator of antioxidant protein expression in the cell which protects it from oxidative damage triggered by injury and inflammation, whereas inducible nitric oxide synthase (iNOS) is responsible for producing large quantities of NO. Thus, high level of NO have increase the chance of it reacting with oxygen free radicals, which may lead to peroxynitrite formation and subsequently cell toxicity21. In the kidney of Cd-treated mice, the mRNA expression of Nfe2l2 was downregulated, whereas Nos2 expression was significantly upregulated (Fig. 8). However, RJ pretreatment alleviated the adverse effect of Cd. Taken together; the qRT-PCR results suggested a protective effect of RJ against Cd-induced oxidative stress. The study also examined nuclear factor kappa B (NF-κB) and Nrf2 and the expression of its down-stream target genes heme oxygenase 1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1). Cd exposure in rats induced a significant increase in NF-κB and a significant decrease in Nrf2 and its putative target genes, compared to the control group. However, RJ pretreatment abolished the Cd-induced impairment in the cellular detoxification system by enhancing Nrf2, HO-1, and NQO1 protein expressions and restraining NF-κB protein expression (Fig. 9).
Effects of royal jelly (RJ) on the enzymatic activity and expression of SOD, CAT, GSH-Px, and GSH-R in the kidney of CdCl2-treated mice. Antioxidant activities are expressed as the mean ± SD of 7 mice, whereas mRNA expression are expressed as the mean ± SD of triplicate assays and were normalized to GAPDH and expressed as fold induction (log 2 scale) relative to mRNA level in the control group. SOD2, superoxide dismutase 2; CAT, catalase; GSH-Px1, glutathione peroxidase 1; GSH-R, glutathione reductase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. ap < 0.05 vs. the control mice; bp < 0.05 vs. the CdCl2-treated mice analyzed using Tukey’s test.
Effects of royal jelly (RJ) on the gene expressions of Nrf2 and iNOS in the kidney of CdCl2-treated mice. Data (mean ± SD of triplicate assays) were normalized to those of GAPDH and expressed as fold induction (log 2 scale), relative to the mRNA level in the control. ap < 0.05 vs. the control mice; bp < 0.05 vs. the CdCl2-treated mice analyzed using Tukey’s test.
Western blot (WB) analysis and densitometric quantification of NF-κB and Nrf2 and its putative target proteins HO-1 and NQO1 in the kidney of mice intoxicated with CdCl2 and pretreated with royal jelly (RJ). Data (mean ± SD of triplicate assays). β-actin was used as the loading control. Molecular weight of proteins is indicated at the right-hand side. Full-length blots/gels are presented in Supplementary Fig. S1. ap < 0.05 vs. the control mice; bp < 0.05 vs. the CdCl2-treated mice analyzed using Tukey’s test.
The kidney levels of TNF-α and IL-1β were drastically (P < 0.05) increased in Cd-intoxicated mice in comparison with the untreated mice. RJ pretreatment substantially (P < 0.05) decreased the kidney levels of both TNF-α and IL-1β, compared with those of CdCl2 alone-treated mice (Fig. 10). In consistence with ELISA findings, qRT-PCR findings revealed that mRNA expressions of Tnf and Il1β were significantly upregulated in the kidney of mice treated with CdCl2. However, RJ pretreatment was able to downregulate these genes.
Effects of royal jelly (RJ) on the levels and gene expression of TNF-α and IL-1β in the kidney of CdCl2-treated mice. Data of ELISA findings are expressed as the mean ± SD of 7 mice, whereas data of mRNA expression (mean ± SD of triplicate assays) were normalized to those of GAPDH and expressed as fold induction (log 2 scale), relative to the mRNA level in the control. TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β. ap < 0.05 vs. the control mice; bp < 0.05 vs. the CdCl2-treated mice analyzed using Tukey’s test.
The kidney tissues of control mice and RJ alone-treated mice showed normal morphology of glomeruli, proximal, and distal tubular cells (Fig. 11a,b, respectively). Mice injected with CdCl2 exhibited obvious damage with congested glomeruli, severe inflammatory leukocytes infiltration, cytoplasmic vacuolation, and tubular degeneration and desquamation (Fig. 11c). Several nuclei of the tubular epithelial cells were in various degrees of enlargement, the other nuclei were pyknotic. In contrast, the kidney of mice pretreated with RJ showed significant improvements in the kidney architecture (Fig. 11d).
Photomicrographs of mice kidney. Kidney tissues from the control and royal jelly-treated groups (a,b, respectively) shows normal kidney structure. In the cadmium chloride (CdCl2)-treated mice (c), severe inflammation, cytoplasmic vacuolation, severe tubular necrosis and apoptosis, and congested glomeruli are shown. Pretreatment with royal jelly (d) markedly attenuated all renal damages caused by cadmium. Hematoxylin and eosin (H&E), 400× magnification.
In the kidney CdCl2-treated mice, Bax and caspase 3 immunoreactivities were stronger than those of the control mice (Fig. 12), whereas the immunoreactivity of Bcl-2 was weaker than that of the control mice. Interestingly, RJ pretreatment markedly reduced the expression of Bax and caspase-3, and markedly increased Bcl-2 expression compared with the Cd-treated group (Table 1). In consistence with the immunohistochemistry findings, qRT-PCR results showed that the mRNA expression levels of Bax and casp3 were significantly higher in the kidney of CdCl2-treated mice, whereas Bcl2 expression was diminished. However, Cd-induced apoptosis in the kidney was blocked by RJ pretreatment (Fig. 13).
Effects of royal jelly (RJ) on the gene expressions of Bcl-2, Bax, and caspases-3 in the kidney of CdCl2-treated mice. Data (mean ± SD of triplicate assays) were normalized to those of GAPDH and expressed as fold induction (log 2 scale), relative to the mRNA level in the control. ap < 0.05 vs. the control mice; bp < 0.05 vs. the CdCl2-treated mice analyzed using Tukey’s test.
Discussion
Cadmium is considered as the most common environmental and accidental pollutant in developing countries that often leads to a serious threat to human and animals22. Cadmium accumulates mainly in metabolically active tissues, such as in the kidney. Repeated exposure to Cd leads to organ and systemic injuries, particularly the kidneys2. In the liver, Cd binds to metallothionein, a cysteine-rich protein with sulfhydryl group, to form Cd-MT complex. This complex reaches the kidney through circulation, where it is filtered through the glomeruli and reabsorbed by divalent metal-ion transporter-1 in the proximal tubular epithelial cells, in which proteases in the lysosomes degraded the complex to release Cd. However, the released Cd rebinds to newly synthesized MT, which causes kidney damage4. However, after the MT binding capacity is saturated, a large amount of Cd ion is liberated into the cytosol and Cd can directly affect and damage the integrity of plasma membranes and intracellular organelles causing cell death23,24. Accordingly, kidney concentrations of cadmium and metallothionein in Cd-treated mice in the current study were increased significantly, whereas the absolute kidney weight was decreased significantly. Kidney weight is presented either as absolute or relative (kidney to body) weight. In general, organ mass is balanced between differentiation, proliferation, and death, and a shift towards apoptosis in the equilibrium may lead to a reduction in organ mass. Therefore, the reported effect of Cd on kidney weights in the this study may be caused by Cd-induced oxidative injury in the kidney tissue25. However, Cd concentration and absolute kidney weight were maintained near normal level by RJ pretreatment as compared with those of Cd-treated mice. This suggested potential metal chelating and antioxidant activities of RJ26. The RJ chelation property may be due to the ability of RJ to (a) enhance MT synthesis and (b) facilitate Cd-MT execration. However, the ability of RJ to facilitate Cd removal from the kidney in the present study is still unclear and needs further study.
In the present study, Cd intoxication increased KIM-1 protein in the kidney tissue. Whilst, RJ pretreatment negated this. KIM-1 is a type I transmembrane protein molecule that is not detectable in healthy renal tissue, but is upregulated in de-differentiated proximal tubule epithelial cells after ischemic or toxic injury. KIM-1 is an early marker of Cd-induced proximal tubular injury27.
In the current study, increased level of kidney function markers reflected the deterioration of kidney function following Cd injection in mice. Kidney is vulnerable to cadmium toxicity because it receives 20% of cardiac output containing high amount excreted substances, which may accumulate in the tubular cells. Cd accumulation in the kidney leads to reduced glomerular filtration rate, polyuria, and severe tubular dysfunction. The decrease in glomerular filtration rate leads to accumulation of endogenous wastes, toxicants, and xenobiotics28. In consistence with the findings of the current study, histopathology of kidney tissue includes shrinkage and degradation of glomerulus, severe degradation of the tubular lining cells, and vascular congestion. In the present study, histopathological examinations showed that RJ pretreatment led to almost complete restoration of the CdCl2-induced renal damage and prevention against Cd-induced elevation in the level of kidney function markers. These results were consistent with those of Karadeniz et al.29, in which RJ restored kidney structure and function following cisplatin treatment.
Cadmium-induced kidney dysfunction in our study was most likely the consequence of oxidant/antioxidant imbalance in the kidney, since it was parallel with elevated LPO and NO levels, which could be regarded as signals of increased generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). The reductions of enzymatic and non-enzymatic antioxidant molecules in the renal tissue of cadmium-exposed mice also supported these findings, suggesting that antioxidant defense system was unable to cope with the increase of ROS/RNS that resulted in cellular damage and death. In the current study, Cd intoxication caused lipid peroxidation that led to impairment of the structural and functional integrity of cell membrane. Furthermore, the increase in lipid peroxidation in the renal tissue causes the overconsumption and depletion of functional thiol (-SH) groups in several enzymatic and non-enzymatic molecules30. Cadmium injection increased iNOS expression, which led to more NO production. The deleterious effect of NO is observed once it reacts with superoxide anion, generating exceedingly reactive peroxynitrite anion (ONOO−). NO also has been known to exert pro-apoptotic effects via the c-Jun N-terminal kinase signaling pathway and caspases 3 and 631. However, pretreatment with RJ markedly restored the oxidative stress markers (LPO and NO) near the normal levels. This finding was in line with that of Ahmed et al.32, which revealed that RJ is able to reverse all deleterious effects of Cd.
The present study also showed a decrease in glutathione content and enzymatic activity of antioxidants, such as SOD, GSH-Px, CAT, and GSH-R, in renal tissue of CdCl2-injected mice. In the absence of cadmium, endogenous antioxidants are important scavenger of ROS. However, cadmium showed a high affinity for binding to sulfhydryl group, which is associated with the depletion of endogenous sulfhydryl compounds4. Cd-induced oxidative damage, which is further supported by the increase in LPO and NO levels as well as the downregulation of antioxidant and stress response genes, could therefore be associated with insufficient antioxidant activity and eventual cellular dysfunction and death33. Inhibition in the activities of SOD, CAT, GSH-Px, and GSH-R in the renal tissue of CdCl2-injected mice could be caused by the reduced synthesis of enzymes or oxidative inactivation of protein enzymes22. Our findings in this study were in agreement with other studies, which revealed that cadmium induced kidney toxicity by enhancing ROS formation, GSH consumption, and inhibiting antioxidant-mediated defense system34. However, this study also suggested that the pre-administration of RJ prior to cadmium injection prevented the alteration in enzymatic and non-enzymatic activity of antioxidant molecules, and the antioxidant and metal-chelating properties of RJ also prevented the generation of free radicals, further reducing the cadmium-induced oxidative damage. Furthermore, RJ contains free amino acids such as methionine, proline, cysteine and cystine might responsible for this effect by promoting biosynthesis of glutathione and scavenging free radicals, in addition to the ability of RJ to enhance the activity of antioxidant enzymes35.
In the current study, we found that CdCl2 injection induced the downregulation of Nrf2 expression in mice kidney. Nrf2 is a transcription factor that ameliorates in injury- and inflammation-induced damage by regulating the expression of antioxidant genes by binding to the antioxidant response element (ARE) in the upstream promoter region of many antioxidant genes like NQO1 and HO-1, initiating their transcription. However, Montes et al.36 found that Cd activates Nrf2 in rat kidney as a compensatory mechanism of oxidative stress to counteract the toxic effects of Cd and prevent apoptosis. In our present study, RJ pretreatment reversed this effect, indicating that RJ may be a potent antioxidant against Cd-induced nephrotoxicity by modulating the expression of antioxidant and detoxification enzymes.
Additionally, inflammation may complicate the pathologic processes of oxidative stress. In the current study, based on the elevated levels of IL-1β and TNF-α, Cd intoxication in mice caused inflammatory response. TNF-α is a cell signaling cytokine involved in systemic inflammation by firing up the acute phase reaction. TNF-α can activate three different pathways, namely mitogen-activated protein kinase (MAPK), NF-κB, and cell death signaling pathways. Mehaffey and Majid et al.37 found that TNF-α can promotes kidney dysfunction by direct cytotoxicity, vasoconstriction, and promoting inflammatory cells infiltration, which deteriorate both tubular function and viability. Wongmekiat et al.38 also showed that Cd-induced nephrotoxicity is associated with TNF-α. IL-1β is also an important mediator of the inflammatory response involved in cell proliferation, differentiation, and apoptosis39. Odewumi et al.40 found that Cd highly upregulated IL-1β. In the present study, the increases in TNF-α and IL-1β were regressed by RJ pretreatment, which were consistent with previous findings of Chen et al.41, in which RJ exhibited a strong inhibitory effect on Nos2, Il1β, and Tnf mRNA generation by suppressing the activity of NF-κB.
Bcl-2, Bax, and caspase 3 are involved in the mitochondria-mediated intrinsic apoptosis pathway. Bcl-2, located in the outer membrane of mitochondria, plays a pivotal task in inhibiting the actions of pro-apoptotic proteins and enhancing cellular survival. Bax is a cytosolic protein that increases the opening of the mitochondrial anion channel, which leads to the release of cytochrome c and loss of mitochondrial membrane potential. Caspase 3 is the final protease that activates apoptotic DNA fragmentation21. In the current study, immunohistochemistry analysis showed that Bax and caspase 3 expressions in Cd-treated mice kidney were markedly elevated, but Bcl-2 expression was decreased, suggesting that Cd initiated the mitochondria-mediated apoptotic pathway. These findings were consistent with those of Bao et al.42, in which chicken kidney exposed to Cd showed decreased Bcl-2 and increased Bax and caspase 3 levels. However, RJ pretreatment effectively reversed this Cd-induced process, suggesting that RJ resisted apoptosis by enhancing the expression level of anti-apoptotic protein Bcl-2. This finding was in accordance with that of Amiri et al.43, which found that RJ increased the expression of Bcl-2, but not Bax in mature oocytes, improved blastocyst formation, and decreased the apoptotic incidence during the in vitro development of sheep cumulus cells.
Conclusion
Our study evidently showed that RJ pretreatment exerted protective effect against Cd-induced nephrotoxicity in mice by facilitating Cd execration, restoring the oxidant/antioxidant balance and preventing inflammation and apoptosis. RJ could be a potent agent for renal protection against Cd-induced nephrotoxicity. Further studies on the Cd removal mechanism by RJ must be conducted to elucidate the main mechanism that responsible for RJ nephroprotective effect. The current study will provide a direction and basis for such further studies.
References
Kim, K. S. et al. Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem Toxicol 114, 34–40 (2018).
Elkhadragy, M. F., Al-Olayan, E. M., Al-Amiery, A. A. & Abdel Moneim, A. E. Protective Effects of Fragaria ananassa Extract Against Cadmium Chloride-Induced Acute Renal Toxicity in Rats. Biol Trace Elem Res 181, 378–387 (2018).
Elkhadragy, M. F. & Abdel Moneim, A. E. Protective effect of Fragaria ananassa methanolic extract on cadmium chloride (CdCl2)-induced hepatotoxicity in rats. Toxicol Mech Methods, 1–27 (2017).
Yang, H. & Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci 16, 1484–1494 (2015).
Dkhil, M. A. et al. The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem Toxicol 74, 98–106 (2014).
Ansari, M. A. et al. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-kappaB downregulation. Environ Toxicol Pharmacol 51, 100–107 (2017).
Xue, X., Wu, L. & Wang, K. In Bee Products - Chemical and Biological Properties (ed. José M. Alvarez-Suarez) 181–190 (Springer International Publishing, 2017).
Pan, Y. et al. Royal Jelly Reduces Cholesterol Levels, Ameliorates Abeta Pathology and Enhances Neuronal Metabolic Activities in a Rabbit Model of Alzheimer’s Disease. Front Aging Neurosci 10, 50 (2018).
Sun, Y., Han, M., Shen, Z., Huang, H. & Miao, X. Anti-hypertensive and cardioprotective effects of a novel apitherapy formulation via upregulation of peroxisome proliferator-activated receptor-alpha and -gamma in spontaneous hypertensive rats. Saudi J Biol Sci 25, 213–219 (2018).
Caixeta, D. C. et al. Adaptogenic potential of royal jelly in liver of rats exposed to chronic stress. PLoS One 13, e0191889 (2018).
Tarasenko, N. Y., Vorobeva, R. S., Spiridinova, V. S. & Shabalina, L. P. Experimental investigation of toxicity of cadmium and zinc caprylates. J Hyg Epidemiol Microbiol Immunol 18, 144–153 (1974).
Linde, A. R. & Garcia-Vazquez, E. A simple assay to quantify metallothionein helps to learn about bioindicators and environmental health. Biochemistry and Molecular Biology Education 34, 360–363 (2006).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95, 351–358 (1979).
Green, L. C. et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126, 131–138 (1982).
Ellman, G. L. Tissue sulfhydryl groups. Arch Biochem Biophys 82, 70–77 (1959).
Nishikimi, M., Appaji, N. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46, 849–854 (1972).
Aebi, H. Catalase in vitro. Methods Enzymol 105, 121–126 (1984).
Paglia, D. E. & Valentine, W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70, 158–169 (1967).
De Vega, L. et al. Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24, 421–432 (2002).
Fernandez-Gil, B. et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS One 12, e0174474 (2017).
Abdel Moneim, A. E. Indigofera oblongifolia Prevents Lead Acetate-Induced Hepatotoxicity, Oxidative Stress, Fibrosis and Apoptosis in Rats. PLoS One 11, e0158965 (2016).
Renugadevi, J. & Milton Prabu, S. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Experimental and Toxicologic Pathology 62, 471–481 (2010).
Sabolić, I., Breljak, D., Škarica, M. & Herak-Kramberger, C. M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 23, 897–926 (2010).
Lee, Y. K. et al. Evaluation of cadmium-induced nephrotoxicity using urinary metabolomic profiles in sprague-dawley male rats. J Toxicol Environ Health A 77, 1384–1398 (2014).
Sakr, S. A., Bayomy, M. F. & El-Morsy, A. M. Rosemary extract ameliorates cadmium-induced histological changes and oxidative damage in the liver of albino rat. The Journal of Basic & Applied Zoology 71, 1–9 (2015).
Zhai, Q., Narbad, A. & Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 7, 552–571 (2015).
Prozialeck, W. C. et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney international 72, 985–993 (2007).
Orr, S. E. & Bridges, C. C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int J Mol Sci 18 (2017).
Karadeniz, A. et al. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev 2011, 981793 (2011).
Akinyemi, A. J. et al. Curcumin inhibits adenosine deaminase and arginase activities in cadmium-induced renal toxicity in rat kidney. J Food Drug Anal 25, 438–446 (2017).
Liu, L., Yang, B., Cheng, Y. & Lin, H. Ameliorative Effects of Selenium on Cadmium-Induced Oxidative Stress and Endoplasmic Reticulum Stress in the Chicken Kidney. Biol Trace Elem Res 167, 308–319 (2015).
Ahmed, M. M., El-Shazly, S. A., Alkafafy, M. E., Mohamed, A. A. & Mousa, A. A. Protective potential of royal jelly against cadmium‐induced infertility in male rats. Andrologia 0, e12996 (2018).
Rani, A., Kumar, A., Lal, A. & Pant, M. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24, 378–399 (2014).
Shen, R. et al. Protective effect of Potentilla anserina polysaccharide on cadmium-induced nephrotoxicity in vitro and in vivo. Food Funct 8, 3636–3646 (2017).
Almeer, R. S. et al. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother 106, 1490–1498 (2018).
Montes, S. et al. Immunohistochemical Study of Nrf2-Antioxidant Response Element as Indicator of Oxidative Stress Induced by Cadmium in Developing Rats. Oxid Med Cell Longev 2015, 570650 (2015).
Mehaffey, E. & Majid, D. S. A. Tumor necrosis factor-alpha, kidney function, and hypertension. Am J Physiol Renal Physiol 313, F1005–F1008 (2017).
Wongmekiat, O., Peerapanyasut, W. & Kobroob, A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn-Schmiedeberg’s Archives of Pharmacology 391, 385–394 (2018).
Almeer, R. S., Mahmoud, S. M., Amin, H. K. & Abdel Moneim, A. E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem Toxicol 115, 49–62 (2018).
Odewumi, C. O. et al. Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. Environ Toxicol 31, 1612–1619 (2016).
Chen, Y. F., Wang, K., Zhang, Y. Z., Zheng, Y. F. & Hu, F. L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediators Inflamm 2016, 3583684 (2016).
Bao, R. K., Zheng, S. F. & Wang, X. Y. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ Sci Pollut Res Int 24, 20342–20353 (2017).
Amiri, M. V., Deldar, H. & Pirsaraei, Z. A. Impact of supplementary royal jelly on in vitro maturation of sheep oocytes: genes involved in apoptosis and embryonic development. Syst Biol Reprod Med 62, 31–38 (2016).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-180).
Author information
Authors and Affiliations
Contributions
R.S. Almeer research concept and design, writing, editing and critical revision of the manuscript, and accountability towards work submitted. G.I. AlBasher: contributed reagents/materials/analysis tools and accountability towards work submitted. S. Alarifi contributed reagents/materials/analysis tools and accountability towards work submitted. S. Alkahtani contributed reagents/materials/analysis tools and accountability towards work submitted. D. Ali contributed reagents/materials/analysis tools and accountability towards work submitted. A.E. Abdel Moneim research design, collection and/or assembly of data, data analysis and interpretation, writing, editing and critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almeer, R.S., AlBasher, G.I., Alarifi, S. et al. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci Rep 9, 5825 (2019). https://doi.org/10.1038/s41598-019-42368-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42368-7
This article is cited by
-
Reversal Effects of Royal Jelly and Propolis Against Cadmium-Induced Hepatorenal Toxicity in Rats
Biological Trace Element Research (2024)
-
Candesartan Protects Against Cadmium-Induced Hepatorenal Syndrome by Affecting Nrf2, NF-κB, Bax/Bcl-2/Cyt-C, and Ang II/Ang 1–7 Signals
Biological Trace Element Research (2023)
-
Alleviative Effect of Threonine on Cadmium-Induced Liver Injury in Mice
Biological Trace Element Research (2023)
-
Activation of Nrf-2 Transcription Factor and Caspase Pathway with Royal Jelly Reduces Fluoride Induced Testicular Damage and Infertility in Rats
Reproductive Sciences (2023)
-
Renal-protective effect of Asparagus officinalis aqueous extract against lead-induced nephrotoxicity mouse model
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.