Abstract
Causative genes in patients with idiopathic basal ganglia calcification (IBGC) (also called primary familial brain calcification (PFBC)) have been reported in the past several years. In this study, we surveyed the clinical and neuroimaging data of 70 sporadic patients and 16 families (86 unrelated probands in total) in Japan, and studied variants of PDGFB gene in the patients. Variant analyses of PDGFB showed four novel pathogenic variants, namely, two splice site variants (c.160 + 2T > A and c.457−1G > T), one deletion variant (c.33_34delCT), and one insertion variant (c.342_343insG). Moreover, we developed iPS cells (iPSCs) from three patients with PDGFB variants (c.160 + 2T > A, c.457−1G > T, and c.33_34 delCT) and induced endothelial cells. Enzyme-linked immunoassay analysis showed that the levels of PDGF-BB, a homodimer of PDGF-B, in the blood sera of patients with PDGFB variants were significantly decreased to 34.0% of that of the control levels. Those in the culture media of the endothelial cells derived from iPSCs of patients also significantly decreased to 58.6% of the control levels. As the endothelial cells developed from iPSCs of the patients showed a phenotype of the disease, further studies using IBGC-specific iPSCs will give us more information on the pathophysiology and the therapy of IBGC in the future.
Similar content being viewed by others
Introduction
Idiopathic basal ganglia calcification (IBGC), also known as Fahr’s disease1 or recently referred to as primary familial brain calcification (PFBC)2, is a rare and intractable disease. It is characterized by abnormal deposits of minerals including calcium (Ca) in the basal ganglia and other brain regions, such as the thalamus and cerebellum. Most cases are idiopathic in Japan. Regarding the diagnosis of IBGC, other secondary causes of calcification should be excluded1,2. The diverse clinical manifestations of IBGC include parkinsonism, cerebellar symptoms, cognitive impairment, psychosis, seizures, and chronic headache2. The following causative genes for familial IBGC (FIBGC) have been successively identified: SLC20A2 (IBGC1 [previously called IBGC3 and now referred to as IBGC1])3, PDGFRB (IBGC4)4, PDGFB (IBGC5)5, XPR1 (IBGC6)6 as autosomal dominant traits, and MYORG7 as an autosomal recessive trait. Myorg mRNA is expressed in astrocytes, and this may provide new insights on the mechanism underlying brain calcification6.
Several studies, including our studies, have shown that SLC20A2 variants are the most frequent in patients with IBGC in many countries2,8,9,10. PDGFRB variants encoding platelet-derived growth factor (PDGF) receptor-β (PDGFRβ), PDGFB encoding its ligand, and XPR-1 encoding a transporter which exports inorganic phosphorus (Pi) out of the cells, have been reported in the past several years. In this study, we conducted a nationwide survey for PDGFB variant in Japanese patients with IBGC.
PDGF is a dimeric glycoprotein which is composed of two subunits from the four components: A, B, C, and D. PDGFR, the receptor of PDGF, is classified as a receptor tyrosine kinase. PDGF-B is expressed in vascular endothelial cells and neurons in the brain5,11. PDGF-BB, a homodimer of PDGF-B, stimulates pericytes which are abundant in the brain12.
The specific treatment has not been found yet for patients with IBGC, including those with PDGFB variants. Mice models carrying hypomorphic human pdgfb alleles have been developed and showed calcium deposits in the brain5. However, the genetic pathophysiological mechanisms and the calcification sites in mice were different from those of humans5. We produced iPS cells (iPSCs) from a patient with SLC20A2 variant13. New models of IBGC, including iPSCs, should be developed for further investigation, especially for drug treatment. Then, we developed iPSCs from patients with PDGFB variants and induced the endothelial cells. They mainly produce PDGF-BB which stimulates the pericytes in the brain. The breakdown of the pathway due to the loss of function is thought to cause the disruption of pericytes14 and blood brain barrier (BBB)15,16,17,18. The decrease in the production of PDGF-BB in endothelial cells may be a target for the therapy for patients with PDGFB variants.
We have observed higher levels of Pi in CSF not only in patients with SLC20A2 variants but in other patients without variants than those of controls19. The role and presence of Pi is critical in the pathogenesis of IBGC in addition to Ca. This shows the disturbance in the intracellular uptake of Pi in patients with IBGC. PDGF-BB has been reported to stimulate the activation of a Pi transporter, PiT-1, which is encoded by SLC20A1, and increases the intracellular uptake of Pi in the vascular smooth muscle cells20. In this study we evaluated the level of PDGF-BB in sera and culture media of endothelial cells derived from iPSCs of humans.
Results
Variant analysis
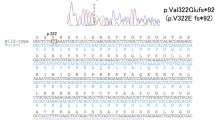
We sequenced the PDGFB gene in 70 sporadic patients with IBGC and 16 families (86 unrelated probands in total) in Japan, using the Sanger method sequencing of all coding regions. Four variants s were found in PDGFB: two variants at the splice junctions (Case 1: c.160 + 2T > A; Case 2: c.457−1G > T), one deletion variant (Case 3: c.33_34delCT), and one insertion variant (Case 4: c.342_343insG) (Table 1, Fig. 1). Electropherograms indicated that all variants were heterozygous (Supplementary Fig. 1). None of the variants were found in an in-house exome sequencing data set (358 Japanese control participants), dbSNP build 151 (https://www.ncbi.nlm.nih.gov/projects/SNP), Genome Aggregation Database (gnomAD) version 2.0 (http://gnomad.broadinstitute.org), Japanese Multi Omics Reference Panel (jMorp) (https://jmorp.megabank.tohoku.ac.jp), and the Human Gene Mutation Database (HGMD) professional 2018.2 (http://www.hgmd.cf.ac.uk). When confined to FIBGC, two of the 16 (12.5%) families showed PDGFB variants. In contrast, two of the 70 (2.9%) patients with sporadic IBGC carried PDGFB variants.
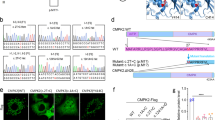
PDGFB variants and PDGF-B protein. (Upper) Schematic diagrams of PDGFB with variants. Four PDGF variants (one insertion variant in exon 4 [c.342_343insG], one deletion variant in exon 1 [c.33_34delCT], and two splice site variants in exon 2 [c.160 + 2T > A] and exon 5 [c.457−1G > T]) were found in the PDGFB gene. (Lower) Schematic structure of the PDGF-B protein with variants. aa = amino acid.
Clinical manifestations and variants in the probands and their families Familial cases
Case 1 (in family 1)
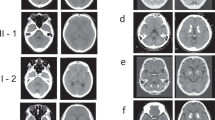
The proband (Fig. 2a; II-2) in the family was a 57-year-old man who was experiencing depression, anxiety, and mild cognitive impairment for 3 years. His neurologic examination findings revealed mild cerebellar ataxia (Table 1). Computed tomography (CT) images revealed marked calcification in the bilateral globus pallidus, caudate nuclei, pulvinar thalami, and dentate nuclei (Fig. 2b). His deceased father (Fig. 2a; I-1) had dementia. Moreover, CT images of his father showed similar findings to those of the proband (Fig. 2c). The son of the proband (Fig. 2a; III-1) had been treated for panic disorder since his teen years. He had the same variant as that of the proband. However, we have not confirmed brain calcification on CT images of III-1.
FIBGC (Case 1 in family 1). (a) Pedigree of family 1. The arrow indicates the index subject. Filled symbols represent patients with brain calcification. Participants’ ages are shown under the symbols in the pedigree of those whose data were available. The symbols + and − indicate variant carriers and noncarriers, respectively, as determined via genetic analysis. The striped symbol represents a variant carrier, although the CT image was not available for the study. (b) CT images of the proband (II-2). (c) CT images of the proband’s father with variant (I-1). Subdural hematoma was also identified on CT scan.
Case 2 (in family 2)
The proband (Fig. 3a; II-2) was a 16-year-old woman. She presented with headaches and had been refusing to attend school since 10 years old. Her neurological examination findings were normal (Table 1). CT images revealed spotty calcification in the bilateral globus pallidus and caudate nuclei and mild calcification in the thalamus, subcortical white matter, and dentate nuclei (Fig. 3b). Her mother (Fig. 3a; I-2) had the same variant, and she also presented with headaches and prominent calcification in the bilateral globus pallidus, caudate nuclei, thalamus, dentate nuclei, and subcortical white matter (Fig. 3c). On the basis of these results, the calcification was believed to progress with age. Although her third brother (Fig. 3a; II-5) was asymptomatic, he showed mild calcification in the globus pallidus on CT images obtained when he encountered a traffic accident (Fig. 3d). Considering his age, this calcification was pathologic (total calcification score = 6)21. The calcification in other regions of the brain, including the dentate nuclei of the cerebellum, could not be detected. DNA analysis revealed the same variant (Fig. 3a; II-5). Her eldest brother did not present with the variant. Thus, no calcification was observed on CT images (data not shown) (Fig. 3a; II-1). Her younger sister was in a nursing institution because of mental retardation, and a detailed clinical information about her sister was not available (Fig. 3a; II-4).
FIBGC (Case 2 in family 2). (a) Pedigree of family 2. The arrow indicates the index subject. Filled symbols represent patients with brain calcification. Participants’ ages are shown under the symbols in the pedigree. The symbols + and − indicate variant carriers and noncarriers, respectively. CT images of the proband (II-2) (b). Computed tomography (CT) image of her mother (I-2) with the variation (c). CT image of her younger brother (II-5) with the variation. The calcification of II-5 was mild (d). The calcification on CT images of the proband’s mother (I-2, C) was prominent compared to that of her children (II-2, B) and (II-5, D).
Sporadic cases
Case 3
The patient was a 71-year-old man who often presented with dizziness since 70 years old. He had mild cognitive impairment (mini-mental state examination [MMSE] score: 23) and mild bradykinesia based on his neurological examination (Table 1). Brain CT images showed calcified areas in the bilateral globus pallidus, caudate nuclei, pulvinar thalami, dentate nuclei, and subcortical and periventricular regions (Fig. 4a).
Case 4
The patient was a 60-year-old woman who had memory impairment accompanied with avolition since 50 years old. At age of 53, brain calcification was detected on CT images. She developed dementia at age 56 and underwent a medical examination at age 60. Her MMSE score was 13. She presented with impaired postural reflexes and signs of frontal lobe impairment, and she did not have any other neurological problems except for postural instability (Table 1). Brain CT images revealed calcification in the bilateral globus pallidus, caudate nuclei, pulvinar thalami, dentate nuclei, and subcortical white matter as well as atrophy in the medial temporal lobe (Fig. 4b). Furthermore, single photon emission computed tomography and positron emission tomography (PET) images showed decreased signals in the striatum and temporal and parietal lobes, and [11C] Pittsburgh compound B (PiB) retention was observed on [11C] PiB PET (data not shown), which suggests that she also had Alzheimer’s disease. Unfortunately, the patient had not come to our hospital in the past. Then, we could not measure the level of PDGF-BB in the serum and produce iPS cells from the patient.
Effects of variants on PDGFB mRNA splicing
To confirm the effect of PDGFB variants (Case 1, c.160 + 2T > A; Case 2, c.457−1G > T) on pre-mRNA splicing at the splice sites, the sequences between exons (Case 1, exons 2–3; Case 2, exons 4–6) were amplified via reverse transcription polymerase chain reaction (RT-PCR), followed by direct sequencing. The variant (c.160 + 2T > A) in Case 1 showed that the PCR products corresponded to the predicted lengths containing 172 bps of the unspliced intron between the exons (Fig. 5a,b). For the variant (c.457−1G > T) in Case 2, the proband (Fig. 3a; II-2) and her third brother (Fig. 3; II-5) had lower-shifted PCR products corresponding with the transcript that lacks exon 5. By contrast, the cDNA of the controls did not have the shifted PCR products (Fig. 5c).
Patients with FIBGC who presented with PDGFRB splicing site variant. (a) (Case 1) Splicing patterns of PDGFB mRNA with a splicing site variant. mRNA from the patients’ blood was reverse transcribed, and cDNA sequences between exons 2 and 3 (including c.160 + 2T > A) were amplified via polymerase chain reaction (PCR). (b) (Case 1) Sequence of PDGFB mRNA with intron sequence that was removed. PCR products were purified and sequenced. Uppercase and lowercase letters indicate exon and intron sequences, respectively. The boxes represent differentially spliced exons. The boldface in red indicates the splice site variant (c.160 + 2T > A). (c) (Case 2) Splicing patterns of PDGFB mRNA with splice site variant. The mRNA from the patients’ blood was reverse transcribed, and the cDNA sequences between exons 4 and 6 (including c.457−1G > T) were amplified via PCR. The sequence of PDGFB mRNA with intron sequence that was removed. PCR products were purified and sequenced. The upper bands indicate the wild-type products, and the lower bands indicate the variant products that lack exon 5. The exon 5 was removed from PDGFB mRNA.
Levels of PDGF-BB protein in the sera of patients with PDGFB variants, those with SLC20A2 variants, and healthy controls
PDGF-BB, a major dimeric glycoprotein, is composed of two subunits of PDGF-B. To determine the effect of the variants on PDGF-BB protein levels, we measured the PDGF-BB levels in the blood of patients with IBGC and healthy controls. Moreover, we measured those of patients with SLC20A2 variants to clarify the presence of convergence into a common pathogenic mechanism. Enzyme-linked immunoassay (ELISA) analysis showed the PDGF-BB protein levels in the blood sera of three patients except for Case 4 with PDGFB variants. Data of Case 4 were not available. The PDGF-BB protein levels of patients with PDGFB variants significantly decreased compared with those of the controls (34.0%) and those of patients with SLC20A2 variant (40.0%) (p < 0.01 in Student’s t-test) (Table 2).
Endothelial cell differentiation from the iPSCs of patients with PDGFB variants
The establishment of iPSCs had been successfully confirmed in in vitro differentiation study (Fig. 6a), genotyping analysis (Fig. 6b), and karyotype analysis (Fig. 6c). Differentiation into endothelial cells had been successfully performed from iPSCs according to the method with modified protocol22 (Fig. 7a). Their differentiation was identified via immunocytochemical study, and tubular formation was detected via morphological study (Fig. 7b). PDGF-BB protein levels in the culture media of the patients (Cases 1, 2, and 3) (22.9 ± 4.45 pg/μg protein) were significantly lower (58.6%) than those of healthy controls (39.1 ± 2.93 pg/μg protein) (p < 0.01 Student’s t-test). (Fig. 8).
Establishment of iPS cells obtained from patients. (a) Immunocytochemical study of iPS cells for the pluripotent marker NANOG, SSEA4, and differentiation markers for three germ layers, namely, βIII-Tubulin, αSMA, and Sox17. (b) Sequencing analysis of iPS cells. PDGF-B heterozygous base substitution resulted in variant c.33_34delCT, c.160 + 2T > A, and c.457 − 1G > T. (c) G-banded karyotype analysis of iPSCs. The scale bar shows 50 μm.
Measurement of PDGF-BB released in the supernatant of iPS-derived endothelial cells using ELISA. iPSC-derived ECs were plated on 96-well plates and cultured for 116 h. Supernatant from iPSC-derived endothelial cells were measured using ELISA. Data were presented as means ± SD. Statistical significance was determined using Student’s t-test (**p < 0.01).
Discussion
We have detected four novel variants of PDGFB in patients with IBGC. All variants are interpreted to be pathogenic according to ACMG/AMP variant classification guideline23,24 because they resulted in frameshifts or abnormal splicing leading to dynamic changes in protein translation. Several studies have reported pathogenic variants in PDGFB5,9,25,26,27,28. Most variants are thought to act as null variants. Some missense variants are predicted to act through loss of function mechanisms29. Species differences and region-specific susceptibility should be also examined in the future. In this study, in patients with PDGFB variants, PDGF-BB levels in the blood sera were down to less than half of those of controls and patients with SLC20A2 variants. The reduced PDGF-BB levels are assumed to be due to PDGF-B variant alleles which were expected as null variants.
In the survey, we identified four novel variants in PDGFB in 105 Japanese patients with IBGC. Among the 16 families with FIBGC, the variant frequency of PDGFB was 12.5% [2 of 16 families]. The variant frequency in this study is similar to the frequencies (19%, [6 of 32 families]) in the first report5. Presently, in Japan, the main causative gene for FIBGC is SLC20A2 (31.3%, [5 of 16 families]) and the second causative gene is PDGFB (12.5%). The frequency of SLC20A2 variants in FIBGC (31.3%) was lower than that (50.0%, [5 of 10 families]) in our previous study8 because the total number of familial patients has increased. Compared to the data reported by Ramos et al.10, Ramos et al., have screened 177 unrelated probands, and identified SLC20A2 variants at 16.9% (n = 30), followed by PDGFB at 3.4% (n = 6), PDGFRB at 1.7% (n = 3), and XPR1 at 3.4% (n = 6). In Japan, we identified 16 probands (18.6%) carrying either pathogenic or likely pathogenic variants (12.8%, n = 11). SLC20A2 provided the contribution 8.1% (n = 7), followed by PDGFB at 4.7% (n = 4). We have examined SLC20A2 in all patients enrolled in the study. We have not identified any pathogenic variants on PDGFRB in Japan as far as we examined and have not investigated the genetic variants on XPR1 and MYORG. Two patients with sporadic IBGC who had PDGFB variants were identified in our analysis (Case 3, c.33_34delCT; Case 4, c.342_343insC). As de novo variants have been reported26,30, we should examine the DNA of their parents. A large deletion in the SLC20A231, and a partial deletion in the PDGFB27 were identified. In addition, XPR1 has been identified as a new causative gene for FIBGC (IBGC6)6. Recently biallelic variants in MYORG have been reported to cause autosomal recessive IBGC7. Genetic studies have been very useful to find key molecules in validating the pathogenesis of the disease. Further genetic studies including other autosomal recessive genes, large deletion or insertion, and copy number variant in IBGC will be needed.
PDGFB variants has been reported only in patients with IBGC. As its receptor, PDGFRβ is expressed in the brain microvascular pericytes in the central nervous system. PDGF-B is secreted from the neurons and vascular endothelial cells in the brain11,32,33. PDGFRB variants cause autosomal-dominant infantile myofibromatosis34,35,36. A point variation in PDGFRB also causes autosomal-dominant Penttinen syndrome36. Recently, the overexpression of PDGFRB due to the PDGFRB variants causes Kosaki overgrowth syndrome37,38,39,40.
Mice that are deficient in Pdgfrβ or Pdgfb lack brain pericytes, and impaired maturation of the blood brain barrier is fatal11, although heterozygous Pdgfb or Pdgfb knockout mice, as well as double Pdgfb+/−; Pdgfrb+/− mice, did not develop brain calcification29. Pdgfb-null mice rescued by transgenic re-expression of one human Pdgfb allele in only endothelium developed substantial brain calcification at age 1 year5. This mouse model is supposed to correspond to the haplo-insufficiency of PDGF-B in patients with PDGFB variants. We observed decreased levels in the culture media of the endothelial cells differentiated from the iPS cells established from patients carrying the PDGFB variants, as similarly observed in the plasma of the patients. Although the causative role of low plasma PDGF-BB in development of brain calcification still remains unclear, the endothelial cells differentiated from the iPS cells may serve as a cellular model for screening of compounds that are capable of restoring plasma PDGF-BB levels.
Interestingly, PDGF-BB administration stimulates the Pi transport in the aortic vascular smooth muscle cells of rats19. Recently, we have reported that Pi levels in patients with IBGC, particularly those with SLC20A2 variants, are higher than those of the controls20. This indicates that the basic mechanism of brain calcification is due to Pi dyshomeostasis in the CSF and interstitial space in the brain. We speculate that the increased Pi levels in the interstitial space and the perivascular space may contribute to the formation of calcification, more precisely mineralization through the formation of complex with Ca and heavy metals19. In contrast, the decreased Pi levels in the cell cytoplasm, especially in neurons, is supposed to cause clinical symptoms. On such assumption, 5-ALA has neuroprotective effects against low Pi in neuronal cells41. Although some issues should be elucidated, further studies using IBGC-specific iPSCs will give us more information on the pathophysiological mechanism of IBGC in the future. Collectively, appropriate stimulation by PDGF-BB may have beneficial effects on patients with IBGC. The endothelial cells developed from iPSCs of the patients showed a phenotype of the disease and they give us a useful tool for the study on IBGC.
Materials and Methods
Participants and samples
We collected clinical information on patients with IBGC in a nationwide study supported by a grant for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan. The diagnostic criteria were previously described7. Briefly, patients with causative biochemical abnormalities, such as high calcium levels, inorganic phosphate, and intact parathyroid hormone, were excluded. The genetic surveys were approved by the Ethics Committee of the Gifu University Graduate School of Medicine and the University of Tokyo. For genetic analysis, 105 patients from 87 hospitals provided written informed consent, and they were then enrolled in the project. Of these patients, 70 came from families with a single affected member, as shown in the available clinical data, and the other 35 patients came from 16 families with multiple members affected. We defined the former as patients with sporadic IBGC and the latter as patients with FIBGC. There were 51 male and 54 female patients. The patients’ mean age ± standard deviation (SD) was 46.7 ± 23.6 years at the time of registration. The 105 patients who were examined included 15 participants from 10 families with SLC20A2 variants, as revealed in a previous study. All experiments on human blood samples and iPS cells were approved by the Ethics Committees of Gifu University, Gifu Pharmaceutical University, the University of Tokyo and Kyoto University and performed in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, and Ethical Guidelines for Human Genome/Gene Analysis Research in Japan. After individual written informed consent was obtained, peripheral blood samples were collected. This study was registered to UMIN Clinical Trials Registry approved by International Committee of Medical Journal Editors (UMIN000030100).
Variant analysis
Genomic DNA was extracted from whole blood samples. In this study, variant analyses was performed by Sanger sequencing of all coding regions, and resequencing strategy was used on the basis of the VariantSEQr platform for the amplification of coding regions and their intron/exon junctions (Applied Biosystems). In the VariantSEQr resequencing system, each PCR primer was designed with priming sites tailed with either the M13 universal forward or reverse sequencing primer for PCR amplification and sequencing reaction under similar PCR and sequencing conditions, as described in detail in the supplementary data (Table e-1). The functional effects of the identified variants were predicted using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph/).
Transcript analysis
Total RNA was isolated from fresh blood using Tempus™ Blood RNA Tube and Tempus™ Spin RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA). For avoiding the amplification of genomic DNA, Absolute RNA Wash Solution (Thermo Fisher Scientific) was used during RNA isolation. cDNA was synthesized using SuperScript®III First-standard Synthesis Kit (Thermo Fisher Scientific). To determine the effect of splice site variants, RT-PCR was performed using primers spanning the exon–exon junctions (between exons 2 and 3; exons 4 and 6) and PrimeSTAR® HS DNA Polymerase (Takara Bio Inc.). PCR were separated using a 2% agarose gel. The corresponding bands were purified from the gel using Wizard® SV Gel and PCR Clean-Up System (Promega KK) and were confirmed via Sanger sequencing.
ELISA
The measurement of human PDGF-BB concentration in the blood was consigned to SRL, Inc. (Tokyo, Japan), using a Human PDGF-BB Quantikine ELISA kit (R&D Systems.), which does not recognize other human PDGFs, including the PDGF-AB heterodimer. iPSC-derived ECs were plated on 96-well plates and cultured for 116 h. Supernatants from iPSC-derived ECs were measured using commercially available ELISA kits (Human PDGF-BB Quantikine ELISA kit [R&D Systems]) in accordance with the manufacturer’s instructions.
Establishment of iPSCs
Peripheral blood mononuclear cells from patients with IBGC who have PDGF-B variant (c.33_34 delCT, c.160 + 2T > A, and c.457−1G > T) were reprogrammed by inducing the episomal vectors carrying OCT3/4, SOX2, KLF4, L-MYC, LIN28, EBNA1, and p53 carboxy-terminal dominant-negative fragment42. The obtained iPSCs were cultured on iMatrix-511 (Nippi, Tokyo, Japan)-coated plates with StemFit medium (Ajinomoto, Tokyo, Japan) supplemented with penicillin/streptomycin. Karyotyping was performed by LSI Medience Corporation (Tokyo, Japan). The genotyping of PDGF-B variant was performed by PCR amplification of genomic DNA and direct sequencing (3500xL Genetic Analyzer, Thermo Fisher Scientific). CTK solution was used for harvesting iPSCs, and an embryoid body (EB) was formed. Cell masses were cultured in DMEM/F12 (Thermo Fisher Scientific) comprising 20% knockout serum replacement (KSR, Thermo Fisher Scientific); 2 mM L-glutamine (Thermo Fisher Scientific); 0.1 M of nonessential amino acids (NEAA, Thermo Fisher Scientific); 0.1 M of 2-mercaptoethanol (Thermo Fisher Scientific); and 0.5% penicillin/streptomycin. The medium was replaced every other day, and after 8 days, the EB was cultured for another 8 days in DMEM comprising 10% fetal bovine serum (FBS) on a gelatin-coated coverslip.
Immunocytochemistry
The cells were fixed with 4% paraformaldehyde and blocked with PBS containing 5% FBS. DAPI (4′,6-diamidino-2-phenylindole) (Thermo Fisher Scientific) was used for labelling the nuclei. Fluorescence imaging was performed using IN CELL Analyzer 6000 (GE Healthcare). The following primary antibodies were used: NANOG (ReproCELL, Yokohama, Japan) (RCAB0003P, 1/500), SSEA-4 (Millipore) (MAB4304, 1/1,000), βIII-tubulin (Millipore) (CBL412, 1/500), SOX-17 (R&D Systems) (AF1924, 1/300), αSMA (DAKO, Glostrup Denmark) (M0851, 1/100), CD31 (Dako) (M023, 1/100), and vWF (Abcam, Cambridge, MA) (Ab6994, 1:400).
Endothelial cell differentiation
Regarding endothelial differentiation, on day 0, iPSC colonies were dissociated into single cells with Accutase (Sigma). Cells were resuspended in basic medium containing StemPro34 (Invitrogen), L-glutamine (Invitrogen), transferrin (Roche), monothioglycerol (Sigma), and ascorbic acid (Sigma), and Y-27632 (naclai tesque), BMP-4 (Peprotech), and Matrigel (BD) were added to form EBs. On day 1, the medium was supplemented with Activin A (R&D Systems) and BMP4. On day 4, EBs were seeded in Matrigel-coated dishes, and the medium was supplemented with VEGF (R&D Systems) for EC expansion. On day 14, the EBs were harvested using Accutase (Sigma-Aldrich), and CD31 positive cells were positively sorted using magnetic-activated cell sorting using anti-CD31 (Miltenyi) (Fig. 7a).
References
Manyam, B. V. What is and what is not ‘Fahr’s disease’. Parkinsonism. Relat. Disord. 11, 73–80 (2005).
Ramos, E. M., Oliveira, J., Sobrido, M. J. & Coppola, G. Primary Familial Brain Calcification. GeneReviews® NIBI Booshelf [online] (1993). https://www.ncbi.nlm.nih.gov/books/NBK1421/ (Last Update: August 24, 2017).
Wang, C. et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 44, 254–256 (2012).
Nicolas, G. et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 80, 181–187 (2013).
Keller, A. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 (2013).
Legati, A. et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 47, 579–581 (2015).
Yao, X. P. et al. Biallelic Mutations in MYORG Cause Autosomal Recessive Primary Familial Brain Calcification. Neuron 98, 1116–1123 (2018).
Yamada, M. et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology 82, 705–712 (2014).
Nicolas, G. et al. Brain calcification process and phenotypes according to age and sex: Lessons from SLC20A2, PDGFB, and PDGFRB mutation carriers. Am. J. Med. Genet. B. Neuropsychiatr. Genet 168, 586–594 (2015).
Ramos, E. M. et al. Primary brain calcification: an international study reporting novel variants and associated phenotypes. Eur. J. Hum. Genet. 26, 1462–1477 (2018).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 (2008).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 21, 193–215 (2011).
Sekine, S. et al. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a mutation in SLC20A2 gene. Stem Cell Res 24, 40–43 (2017).
Lindblom, P. et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835–1840 (2003).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–27 (2010).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med 19, 1584–1596 (2013).
Hozumi, I. et al. Inorganic phosphorus (Pi) in CSF is a biomarker for SLC20A2-associated idiopathic basal ganglia calcification (IBGC1). J. Neurol. Sci. 388, 150–154 (2018).
Kakita, A. et al. Stimulation of Na-dependent phosphate transport by platelet-derived growth factor in rat aortic smooth muscle cells. Atherosclerosis 174, 17–24 (2004).
Nicolas, G. et al. Phenotypic spectrum of probable and genetically-confirmed idiopathic basal ganglia calcification. Brain. 136, 3395–3407 (2013).
Funakoshi, S. et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 6, 19111, https://doi.org/10.1038/srep19111 (2016).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Amendola, L. M. et al. Performance of ACMG-AMP Variant-Interpretation Guidelines among Nine Laboratories in the Clinical Sequencing Exploratory Research Consortium. Am. J. Hum. Genet. 98, 1067–1076 (2016).
Hayashi, T., Legati, A., Nishikawa, T. & Coppola, G. First Japanese family with primary familial brain calcification due to a mutation in the PDGFB gene: an exome analysis study. Psychiatry Clin. Neurosci. 69, 77–83 (2015).
Nicolas, G. et al. A de novo nonsense PDGFB mutation causing idiopathic basal ganglia calcification with laryngeal dystonia. Eur. J. Hum. Genet. 22, 1236–1238 (2014).
Nicolas, G. et al. PDGFB partial deletion: a new, rare mechanism causing brain calcification with leukoencephalopathy. J. Mol. Neurosci. 53, 171–175 (2014).
Koyama, S. et al. Clinical and radiological diversity in genetically confirmed primary familial brain calcification. Sci. Rep. 7, 12046, https://doi.org/10.1038/s41598-017-11595-1 (2017).
Vanlandewijck, M. et al. Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification. PLoS One 10, e0143407, https://doi.org/10.1371/journal.pone.0143407 (2015).
Ferreira, J. B. et al. First report of a de novo mutation at SLC20A2 in a patient with brain calcification. J. Mol. Neurosci. 54, 748–751 (2014).
Baker, M. et al. SLC20A2 and THAP1 deletion in familial basal ganglia calcification with dystonia. Neurogenetics 15, 23–30 (2014).
Funa, K. & Sasahara, M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J. Neuroimmune Pharmacol 9, 168–181 (2014).
Cheung, Y. H. H. et al. A Recurrent PDGFRB Mutation Causes Familial Infantile Myofibromatosis. Am. J. Hum. Genet. 92, 996–1000 (2013).
Lee, J. W. Mutations in PDGFRB and NOTCH3 are the first genetic causes identified for autosomal dominant infantile myofibromatosis. Clin. Genet. 84, 340–341 (2013).
Martignetti, J. A. et al. Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am. J. Hum. Genet. 92, 1001–1007 (2013).
Johnston, J. J. et al. A point mutation in PDGFRB causes autosomal-dominant Penttinen syndrome. Am. J. Hum. Genet. 97, 465–474 (2015).
Takenouchi, T. et al. Novel Overgrowth Syndrome Phenotype Due to Recurrent De Novo PDGFRB Mutation. J. Pediatr. 166, 483–486 (2015).
Minatogawa, M. et al. Expansion of the phenotype of Kosaki overgrowth syndrome. Am. J. Med. Genet. A 173, 2422–2427 (2017).
He, C. et al. STAT1 modulates tissue wasting or overgrowth downstream from PDGFRβ. Genes Dev. 31, 1666–1678 (2017).
Gawliński, P. et al. Phenotype expansion and development in Kosaki overgrowth syndrome. Clin. Genet. 93, 919–924 (2018).
Takase, N. et al. Neuroprotective effect of 5-aminolevulinic acid against low inorganic phosphate in neuroblastoma SH-SY5Y cells. Sci. Rep. 7, 5768, https://doi.org/10.1038/s41598-017-06406-6 (2017).
Okita, K. et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31, 458–466 (2013).
Acknowledgements
This work was supported by grants from the Ministry of Health, Labour and Welfare of Japan (H26-Nanbyotou (Nan)-Ippan-001, H26-Nanchitoh (Nan)-Ippan-085), the Ministry of Education, Culture, Sports, Science and Technology of Japan (Basic Research (B) (17H04198)) and Japan Agency for Medical Research and Development (AMED) (18ek0109313h0001 and 18ek0109372h0001) in Japan.
Author information
Authors and Affiliations
Contributions
I. Hozumi designed and organized the project. T. Shoji and H. Inoue designed the research. S. Sekine, M. Kaneko, M. Tanaka, Y. Ninomiya, H. Kurita, M. Inden, M. Yamada, Y. Hayashi, J. Mitsui, H. Ishiura, and T. Kondo performed the experiments. H. Fujigasaki, H. Tamaki, R. Tamaki, S. Kito, and Y. Taguchi collected and analyzed the clinical data. T. Inuzuka, A. Iwata, K. Tanaka, N. Atsuta, and G. Sobue analyzed the data and supervised the project. S. Sekine, M. Kaneko, M. Tanaka, and I. Hozumi wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sekine, Si., Kaneko, M., Tanaka, M. et al. Functional evaluation of PDGFB-variants in idiopathic basal ganglia calcification, using patient-derived iPS cells. Sci Rep 9, 5698 (2019). https://doi.org/10.1038/s41598-019-42115-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42115-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.