Abstract
Microalgae are the most abundant microorganisms in aquatic environments, and many possess the ability to remove organic contaminants. The presence of endocrine disruption compounds (EDCs) in many coastal marine systems and their associated risks have elicited great concern, especially in the case of nonylphenol (NP), which is classified as a priority contaminate by the U.S. EPA. In this context, batch experiments were conducted to investigate the intracellular absorption, extracellular adsorption and biodegradation of NP by four species of marine microalgae: Phaeocystis globosa, Nannochloropsis oculata, Dunaliella salina and Platymonas subcordiformis. The results showed a sharp reduction of NP in medium containing the four microalgal species during the first 24 h of incubation, and the four species exhibited the greatest capacity for NP adsorption and absorption within 24 h of culture. However, the amount of NP absorbed and adsorbed by all four microalgae decreased with increasing time in culture, and intracellular absorption was greater than extracellular adsorption. After 120 h of exposure to NP, the four species could biodegrade most of the NP in the medium, with efficiencies ranging from 43.43 to 90.94%. In sum, we found that the four microalgae have high biodegradation percentages and can thus improve the bioremediation of NP-contaminated water.
Similar content being viewed by others
Introduction
Alkylphenol polyoxyethylene ethers (APEs) are non-ionic surfactants that are used extensively in various industrial, agricultural and household applications. Many studies have shown that nonylphenol (NP), which is a major degradation product of alkylphenol ethoxylates under natural conditions, presents considerable residual and ecological risk; it is difficult to degrade and transform and is instead adsorbed to solid suspended objects or sediment1,2,3,4,5. NP is in the class of typical environmental hormones (EH) known as endocrine disruption compounds (EDCs); it is commonly identified in air, water, sediment and biota and represents a carcinogenic threat to humans6. However, persistent organic pollutants can be removed by artificial methods such as photodecomposition, electrochemical and bioactive carbon fibres7,8,9 that have the advantages of high removal and rapid reaction rates, but they may produce secondary pollution and are expensive. Therefore, bioremediation is considered a green and sustainable mitigation option.
Bioremediation technology allows the biological-specific decomposition of wastewater to provide complete degradation at low cost and without energy consumption, among other advantages10. In natural near-shore marine systems, microorganisms, plants and algae have the ability to purify water bodies contaminated with organic substances. As primary producers, microalgae play a vital role in aquatic ecosystems, and many species can degrade organic compounds. Microalgae have the advantages of adsorption capacity, a high surface area:volume ratio, wide distribution range, rapid metabolism, low cost, and abundant availability11,12,13. In a water body, microalgae directly contact and interact with pollutants, but some toxic pollutants can inhibit their growth14 and affect their physiological ecology15. Microalgae can also biodegrade or biotransform organic pollutants via metabolic action, and the mechanisms for removal include accumulation and degradation, which includes both transformation and mineralization16. Hendrik studied the degradation of the pesticide endosulfan by blue-green algae and found that the algae exhibited strong biodegradability17. Another researcher studied the adsorption, uptake and degradation effects of Scenedesmus obliquus on NP and octylphenol (OP) in water and reported that more than 89% of NP and 58% of octylphenol (OP) in the medium were removed by the microalgae after 5 days of incubation, and the highest removal efficiency was close to 100%18. In addition, studies of the adsorption, biotransformation and degradation of NP, bisphenol-A (BPA) and other environmental hormones have been reported in the microalgae of the genera Chlorella, Scenedesmus, and Fibrea as well as other species19. Microalgae can also absorb and biologically accumulate certain pollutants, which can then be transmitted up the food chain via biological amplification20. Cladophora glomerata can exert a bio-enrichment effect on NP so that the concentration in the organism is 10,000 times higher than the concentration in the environment21, and Isochrysis galbana can enrich soil NP at an initial concentration of 100 μg/L by 6490-fold, while 77% of the compound can be adsorbed and absorbed in one hour. In microalgae used as feed, the high enrichment of NP can impact organisms at higher trophic levels such as rotifers and zebrafish20. The above findings have led to research on the selection of microalgae species that are highly capable of degrading organic pollutants, and the use of microalgae for the bioremediation of contaminated water has been proposed as a beneficial strategy when implementing environmental bio-refineries10. However, there have been limited studies of the application of marine microalgae to degrade NP. The four microalgae examined in this study, Phaeocystis globosa, Nannochloropsis oculata, Dunaliella salina and Platymonas subcordiformis, are common and widely distributed species in aquatic ecosystems. In addition, since these four microalgae are rarely used for NP degradation, the data from this study can be used to supplement knowledge related to NP treatment. The purpose of this work was to study (1) the toxic effect of NP on four marine algae—P. globosa, N. oculata, D. salina and P. subcordiformis—and to assess whether they are NP-tolerant species; (2) the capability of these four species to remove and biodegrade NP from NP-contaminated aquatic environments; and (3) the mechanisms underlying NP bioaccumulation, biodegradation and removal capability of these four microalgae.
Results
Growth of the four microalgae exposed to NP
The growth, according to changes in Chla content, and the effects of NP on the four microalgae showed a significant time-dose effect (Fig. 1). Statistically non-significant inhibition of the Chla concentration was observed at 0.5 mg/L compared to the control (p > 0.05), and a significant inhibition of Chla concentration was observed at 1.0 mg/L compared to the control (p < 0.05). The Chla concentration obviously decreased when NP concentrations were higher than 1.5 mg/L, and the inhibitory effects were significantly enhanced with increasing concentrations of NP after a 24-h exposure phase (Fig. 1). The 96-h EC50 of NP for the four microalgae was in the order of P. subcordiformis > P. globosa > D. salina > N. oculata. The EC50 values for the four microalgae were more than 1.0 mg/L; the EC50 of P. subcordiformis was the greatest at 1.497 mg/L, and the EC50 of N. oculata was the lowest at 1.004 mg/L (Table 1). There was no significant difference between D. salina and N. oculata under each treatment (p > 0.05). A linear relationship among algal density (cells/mL), dry weight and Chla content was established (Table S2). The results revealed a positive correlation between cell density and Chla content in the four microalgae and a positive correlation between microalgae biomass and Chla content.
Removal of NP by the four microalgae
The concentrations of NP in the incubation culture of the four microalgae is expressed in Fig. 2. The NP concentrations in the medium quickly declined in the treatment with algae. Within 24 h, NP was rapidly removed by the four microalgae, with removal efficiencies of 22.76–78.87% under the five concentration treatments. During the first 24 h, NP was rapidly removed by P. globosa, N. oculata, D. salina and P. subcordiformis from 1 mg/L to approximately 441.8 μg/L, 524.2 μg/L, 355.9 μg/L and 188.4 μg/L, respectively, which was equivalent to the removal of 43.14%, 34.01%, 52.43% and 70.75% of the initial amount of NP from the medium. The NP level in the medium decreased further to final concentrations of 235.1 μg/L, 257.4 μg/L, 192 μg/L and 62.3 μg/L NP, respectively, at the end of the 120-h period, and these values accounted for a 74.18%, 72.63%, 79.02% and 92.12% reduction of the initial amount of NP, respectively. After 5 days of culture, the NP removal efficiencies obtained with P. globosa, N. oculata, D. salina and P. subcordiformis from initial NP concentrations of 0.5–2.5 mg/L were 66.37%, 74.82%, 69.86%, and 82.38%, respectively. The NP concentrations in the control treatments showed little variation over 120 h, which indicated that photolysis played only a small role in the dissipation of NP.
The reduction of NP in the medium appeared to follow first-order kinetics. Table 2 shows the degradation kinetics equations and parameters of NP removal by the four microalgae. The NP removal statistics can be fitted better to the first-order model with half-lives of 2.188, 2.045, 1.488 and 1.285 days, respectively.
NP adsorbed onto the cell surfaces and absorbed into cells
NP was found to be adsorbed and absorbed by the four tested microalgae (Figs 3 and 4). The four species adsorbed and absorbed a large amount of NP within one day. The concentration of NP was 2.5 mg/L, and N. oculata had the highest intracellular absorption of 35.3 ± 3.0 × 10−8 μg/cell. With the increase in culture duration, the amount of NP adsorbed and absorbed by the four microalgae decreased (Figs 3 and 4), and the NP residues in the culture solutions of the four microalgae under different concentrations of NP showed a decreasing trend. The NP concentration was measured in algal cells under the different treatments, and Figs 3 and 4 show that the intracellular NP contents of the four microalgae were higher than those of the extracellular NP contents. The amounts of NP adsorbed and absorbed in algal cells declined with culturing time. The contents of NP accumulated in the cells of the four microalgae were higher in the first 24-h period than those after 120 h of culture (Fig. 5).
The NP biodegradation, removal and biosorption ratios of the different species of microalgae
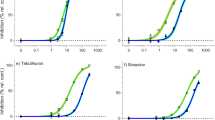
The NP biodegradation percentage changed with the NP concentration. As seen in Fig. 6d, P. subcordiformis had a strong biodegradation effect at low concentrations (0.5 mg/L), and the biodegradability of NP was significantly reduced with increasing NP concentrations (p < 0.05). P. globosa and P. subcordiformis exhibited the same biodegradation trend of a stronger biodegradation effect at low NP concentrations than at high NP concentrations (Fig. 6), and P. subcordiformis acclimated better than P. globosa. The N. oculata biodegradation ratio at low NP concentrations was significantly lower than that at high concentrations (p < 0.05). When the concentration of NP was 0.5–1.5 mg/L, the D. salina biodegradation percentage increased as the concentration increased, but when the NP concentration was greater than 2.0 mg/L, the D. salina NP biodegradation percentage significantly decreased (p < 0.05). The biosorption ratios of the four microalgae were higher in the first 24-h period than those after 120 h of culture. The removal of NP by the four species of microalgae was largely due to biodegradation or biotransformation by the algal cells rather than simple adsorption and absorption in the cells (Fig. 6).
The NP biodegradation, removal and biosorption ratios of the four species of microalgae. (a) P. globosa, (b) N. oculata, (c) D. salina, and (d) P. subcordiformis at the end of 120 h. (Values show the mean ± SD for three replicates.) Means followed by different concentrations are significantly different according to the LSD test at P < 0.05.
Discussion
The present study showed that the growth of the four marine microalgae showed differences after exposure to NP. At NP concentrations greater than 1.5 mg/L, the inhibitory effects measured after 24 h of culture were enhanced with increases in the NP concentration, and this finding agrees with the results of other studies on Microcystis aeruginosa, Chlorella species, Scenedesmus quadricauda, Ankistrodesmus acicularis and Chroococcus minutus exposed to NP22,23,24,25 and of Stephanodiscus hantzschii, Chlorella fusca and Monoraphidium braunii exposed to BPA26,27,28. The lowest Chla content was observed at higher concentrations of NP. A previous study showed that NP treatments induce the overproduction of reactive oxygen species (ROS) to cause oxidant damage, which might be one of the basic reasons for the inhibition of algal growth29. Higher concentrations of NP caused peroxidation of chloroplast membranes or increased production of ROS29,30. The 96-h EC50 value of NP was greater than 1 mg/L for all four microalgae species. For P. subcordiformis, a more tolerant species, the 96-h EC50 of NP was 1.497 mg/L, whereas P. subcordiformis exhibited higher NP biodegradation and biotransformation capabilities but lower adsorption and absorption in algal cells compared with those of the other three species. Torres31 suggested that biotransformation can improve the elimination, decontamination and redistribution of contaminants within an organism. Thus, we speculated that the strong tolerance of P. subcordiformis to NP might be due to its better acclimation, which allowed its photosynthetic activity to recover from the damage caused by NP.
We found that the residual NP concentrations in the medium after a 24-h exposure phase were clearly lower than the concentrations at the start of the experiment. The amounts of NP that accumulated in the cells of the four microalgae were higher in the first 24-h period than they were after 120 h of culture. We concluded that NP could be quickly removed from the medium and be biodegraded or biotransformed by the four microalgae. Stephanodiscus hantzschii had a high removal ability at low BPA concentrations, as BPA was bioaccumulated and biodegraded by the algal cells26. When BPA was added to the cultures of eight species of freshwater microalgae, a decrease in the concentration of BPA in the medium was observed in all the cultures32. Mafalda S. Baptista found that OP removal obviously increased in the presence of cyanobacteria, with a decrease in the half-life of the compound from 15 days in the absence to 9 days in the presence of cells33. In this study, the four microalgal species could adsorb, absorb and biodegrade NP. We determined that the removal of NP from the medium was largely due to biodegradation and biotransformation by the algae because the biodegradation ratio ranged from 43.43% to 90.94%. The capabilities of the four microalgae to biodegrade NP were higher than those of Cyclotella caspia, C. minutus and A. acicularis25,34. Differences in biodegradability can be due to the mechanism by which algae accumulate organic pollutants, which is analogous to the partitioning of organic compounds in lipid-water systems35. The bioaccumulation capability of algae is related to the lipid content in algal cells, which depends on the growth conditions36 and distribution in the cell.
Previous studies have identified the algal growth rate as a vital factor influencing the degradation of target compounds by algae37. A higher growth rate of microalgae leads to a higher algal biomass that can reduce the concentration of NP dispersed in the algal cells, thus reducing the NP-induced stress on the microalgae. Moreover, the higher algal biomass can enhance the NP biodegradation or biotransformation ability. We also found that the NP removal efficiency increased with the algal growth rate (Table S1 in the Supplementary Material). However, N. oculata displayed the highest biodegradation percentage and removal efficiency under the 2.0 and 2.5 mg/L NP treatments, which could have been due to the high adsorption and absorption in cells. Meanwhile, the higher adsorption and absorption by N. oculata with increasing NP indicates that the species could not recover from the NP-induced damage. Thus, N. oculata did not show significant growth under NP concentrations of 2.0 and 2.5 mg/L but showed the highest biodegradation percentage and removal efficiency. The biodegradation by the four microalgal species, especially P. subcordiformis, was mainly related to the microalgal growth rate. The P. subcordiformis has the advantages of strong NP tolerance, an efficient growth rate and high biodegradability. These advantages can be applied in the further study of the bioremediation of NP-contaminated water, and the species may be a good choice for the treatment of environmental hormones.
With initial NP concentrations lower than 0.5 mg/L, the normal and long-term growth of the microalgae are not problematic, but NP concentration that exceed 0.5 mg/L inhibit microalgal growth. At present, the actual concentration of NP in water bodies is less than 0.5 mg/L. Therefore, the four tested microalgae can be used as a component of the natural remediation system to achieve the dual purposes of NP pollution control and biomass culture. The addition of certain density of these microalgae to a bioreactor for the treatment of sewage would achieve the goal of removing NP; however, if the concentration of NP in the sewage is high, the growth of the microalgae could be inhibited and even prevented. The analysis of the degradation products revealed that different organisms utilize different pathways and mechanisms to degrade NP, and the obtained products show differences38,39,40. Therefore, our future research might focus on the NP biodegradation pathways used by different algae and their degradation products.
Materials and Methods
Microalgal species and culture conditions
P. globosa, N. oculata, D. salina and P. subcordiformis were obtained from the Hydrobiology Research Centre, Jinan University, Guangzhou, China. The four marine microalgae used in this study were axenically cultured following the method described by Su41; the growth medium was artificial seawater enriched with f/2 enrichment solution for microalgal cultures. The salinity of the artificial seawater was 30‰, and the primary pH of the medium was 6.5–7.0. The flasks were continuously shaken at 100 rpm. Throughout the experiments, f/2 medium42 was used, and the four microalgae were cultured in 2-L Erlenmeyer flasks containing 1 L of medium. The culture was illuminated with a cool, fluorescent light at an intensity of 150 μmol/m2·s at the surface of the medium (measured by a LI-250 Light meter, LI-COR, Inc., US) with a 12:12-h light:dark cycle in an environmental chamber at 23 ± 2 °C. The culture was aerated by a mechanical air pump with 0.2 μm filtered air at a rate of 35 mL/min, and it was maintained in an exponential growth phase through repeated sub-culturing with fresh medium every four days. Before the experiment, the microalgal cells were collected by centrifugation at 5000 g and 25 °C for 10 min at the exponential growth phase (around the mid-logarithmic phase), and the cell pellets were then washed twice with double-distilled, sterilized water.
NP treatments
NP obtained from Sigma-Aldrich (St. Louis, MO, USA) was dissolved in methanol as the stock solution at a concentration of 2000 mg/L. The stock solution was spiked into the culture at different initial NP concentrations: 0.5, 1.0, 1.5, 2.0 and 2.5 mg/L. Methanol at a concentration of 0.4% (v/v) was added to the culture (Fig. S1 in Supplementary Material) in each NP treatment to ensure that the spiked NP was fully dissolved in the medium. Each treatment, as well as the control, was performed in triplicate. The primary concentration of chlorophyll a (Chla) was maintained at 0.08 mg/L in a 250-mL Erlenmeyer flask by resuspending the appropriate amount of microalgal cell pellets in 100 mL of culture. Then, the cultures were incubated at 23 ± 2 °C on a rotary shaker at 100 rpm, and the cultures were illuminated with fluorescent lights at a light intensity of 150 μmol/m2·s under a 12:12-h light:dark cycle for 120 h.
Effects of NP on the growth of the four microalgae
Measurement of chlorophyll a content
The algal growth was measured by the daily changes in Chla content. First, 5 mL of microalgae culture was collected by centrifugation at 3000 g for 10 min. The supernatant was discarded, and the pellet was resuspended in 5 mL of extract (acetone: ethanol = 1:1), stored in a 4 °C refrigerator, and centrifuged at 3000 g for 10 min after 24 h. The absorbance of the supernatant was detected at wavelengths of 645 nm and 663 nm with an Agilent 2450 UV–visible spectrophotometer, and the Chla concentration of the extract was calculated using the following formula described by Dai43:
The Chla concentrations of the four microalgae were determined daily. The growth inhibition rates at different NP concentrations were calculated according to the following equation:
where I is the growth inhibition rate, μc is the algal growth rate of the control group, and μt is the algal growth rate at time t. Based on the NP concentration and inhibition rate, the 96-h EC50 was then calculated through the linear regression method.
Determination of the cell density and dry weight of the four microalgae
A haemocytometer (Marienfeld, Lauda-Königshofen, Germany) was used to determine the cell density. To measure the algal cell dry weight, a 20-mL aliquot of culture was sampled and then filtered through a pre-weighed 0.45-mm-pore Whatman GF/F glass-fibre filter. The filter with algal cells was dried overnight in an oven (101A–E, Shanghai Anting Scientific Instrument Co., Ltd.) at 60 °C until a constant weight was reached. The dry weight of the algal cells was the difference between the final weight after filtration and the initial weight before filtration.
Experimental determination of the biodegradation of NP by the four microalgae
Residual NP concentration in the medium
The concentrations of NP in the culture samples were examined at time intervals of 24, 48, 72, 96 and 120 h. First, 5 mL of medium was sampled from each flask, and the algal cultures were centrifuged at 5000 g for 15 min at 4 °C. The supernatant was extracted with dispersive liquid–liquid microextraction (DLLME), as described by Rezaee et al.44 with modifications reported by Luo et al.45. Briefly, 0.2 mL of the chlorobenzene and acetone mixture (1:2) was added to the sample in a 10-mL glass test tube with a conical bottom and screw cap. After gentle mixing, the glass tube was filled with a cloudy, milky solution composed of water/chlorobenzene. Then, the sample was centrifuged at 4500 g for 5 min, and the dispersed fine particles in the extraction phase that settled in the bottom of the tube were removed with a 50-μL microsyringe (zero dead volume, cone tip needle). This extraction procedure was repeated three times, and the sediment fractions were mixed together for further analysis by high-performance liquid chromatography (HPLC) (Agilent, Santa Clara, CA, USA). The entire extraction procedure was carried out at ambient temperature (23 ± 2 °C)44,45. The recovery rate of NP from the water was 90–94%.
Analysis of NP adsorption onto cell surfaces and absorption into cells
To determine the amount of NP adsorbed onto the surfaces of algal cells, the algal cell pellets from the above section were rinsed with 5 mL of 10% methanol and shaken for approximately 60 s. The NP present in the medium was viewed as surface-adsorbed NP46 and was extracted with DLLME following the steps described in section 2.4.1, then analysed via HPLC.
To determine the NP absorbed into the cells, the cell pellets obtained using the above-described procedure were added to an appropriate amount of anhydrous Na2SO4, sonicated (50% power, ice-bath) for 20 min and extracted with 3 mL of dichloromethane–methanol (1:2 v/v). The extract was centrifuged twice for 5 min at 3500 g, and the solvent fractions were ultimately mixed for further analysis20. The results regarding “NP adsorbed onto cell surfaces” and “NP absorbed into cells” were compared to those obtained for a control group without NP20.
According to the determined NP concentrations, the NP removal efficiency (R) and biodegradation percentage (BDP) of the algal biomass were calculated as previously described47, with minor modifications according to the following equations:
where R is the dissolved NP removal efficiency (%) and Ci and Cf are the initial and ultimate concentrations (mg/L) of NP in the solution, respectively. Then,
where Ci is the primary concentration (mg/L) of NP in the solution, Cr is the remaining concentration (mg/L) in the solution, Ca is the concentration of the abiotic removal (mg/L), Cd is the dry weight concentration (mg/L) of NP adsorbed on the surface of the cell walls, Cb is the concentration (mg/g dry weight) of NP absorbed in algal cells, and Wa is the dry weight of the algal biomass in g/L.
The intracellular NP content (μg/cell) and the extracellular NP content (μg/cell) were calculated according to the following equations:
where Cb is the dry weight concentration (mg/L) of NP absorbed in the cell and Cd is the dry weight concentration (mg/L) of NP adsorbed on the cell walls.
Kinetic equations for NP degradation by the four microalgae
First-order kinetic models can be used to describe algal degradation and are mainly used to describe degradation over time. The kinetic equation for algal degradation of NP can be expressed as follows:
where k is the kinetic constant in the first-order reaction, Ct is the NP concentration at time t, and C0 is the initial NP concentration. Parameter K was obtained through a linear regression between the removal rate (Ct/C0) and the treatment time (t).
Determination of NP
NP concentrations were analysed via an Agilent 1100 series high-performance liquid chromatograph (Agilent, Santa Clara, CA, USA) with a fluorescence detector. Each extracted sample was dried with N2 gas before the HPLC analysis. The elution was carried out with acetonitrile and Milli-Q water (80:20 v/v) as the mobile phase under isocratic conditions. An XDB-C18 RS column (4.6 × 250 mm, 5 μm) was used. The injection volume was 50 μL, and the flow rate was set as 1 mL/min. The fluorescence detector was set at an excitation wavelength of 230 nm and an emission wavelength of 305 nm. The retention time was 18 min, and the quantification limit for NP was 5 μg/L. The results were compared to those obtained with a control without NP20.
Statistical analysis
Each experiment was conducted in triplicate, and the mean values and standard deviations (SD) were calculated from the different replicates (n = 3). Statistical analysis was performed using the SPSS 16.0 package (SPSS Inc., Chicago, IL, USA). One-way ANOVA was followed by LSD multiple comparisons and the Wilcoxon test when nonparametric tests were necessary (p < 0.05 was considered significant, and p < 0.01 was considered highly significant).
Conclusions
We studied the influence of NP on the growth of the marine microalgae P. globosa, N. oculata, D. salina and P. subcordiformis and evaluated their ability to biodegrade NP in culture. P. subcordiformis has a stronger tolerance for NP than do the other three species. Moreover, the four microalgae could remove NP from the medium via biosorption, biodegradation or biotransformation. We conclude that P. subcordiformis is an excellent candidate species for the bioremediation of NP-polluted aquatic ecosystems.
References
Kannan, K. et al. Nonylphenol and nonylphenol ethoxylates in fish, sediment, and water from the Kalamazoo River, Michigan. Archives of Environmental Contamination & Toxicology 44, 77–82, https://doi.org/10.1007/s00244-002-1267-3 (2003).
Pybus, E. Nonylphenol, an integrated vision of a pollutant. Applied Ecology & Environmental Research 4, 1–25, https://doi.org/10.15666/aeer/0401-001025 (2004).
Soares, A., Jonasson, K., Terrazas, E., Guieysse, B. & Mattiasson, B. The ability of white-rot fungi to degrade the endocrine-disrupting compound nonylphenol. Appl Microbiol Biotechnol 66, 719–725, https://doi.org/10.1007/s00253-004-1747-7 (2005).
Venkatesan, A. K. & Halden, R. U. National inventory of alkylphenol ethoxylate compounds in U.S. sewage sludges and chemical fate in outdoor soil mesocosms. Environmental Pollution 174, 189–193, https://doi.org/10.1016/j.envpol.2012.11.012 (2013).
Ying, G. G., Williams, B. & Kookana, R. Environmental fate of alkylphenols and alkylphenol ethoxylates–a review. Environment International 28, 215–226, https://doi.org/10.1016/S0160-4120(02)00017-X (2002).
Omar, T. F. T., Ahmad, A., Aris, A. Z. & Yusoff, F. M. Endocrine disrupting compounds (EDCs) in environmental matrices: Review of analytical strategies for pharmaceuticals, estrogenic hormones, and alkylphenol compounds. TrAC Trends in Analytical Chemistry 85, 241–259, https://doi.org/10.1016/j.trac.2016.08.004 (2016).
Sasai, R. et al. The removal and photodecomposition of n -nonylphenol using hydrophobic clay incorporated with copper-phthalocyanine in aqueous media. Journal of Photochemistry & Photobiology A. Chemistry 155, 223–229, https://doi.org/10.1016/S1010-6030(02)00372-6 (2003).
Iwasaki, S. et al. Preparation of activated carbon from polyester floss and its application to the adsorptive removal of 4-nonylphenol in water. Carbon 48, 571–571, https://doi.org/10.1016/j.carbon.2009.09.046 (2010).
Armijos-Alcocer, K. G. et al. Electrochemical Degradation of Nonylphenol Ethoxylate-7 (NP7EO) Using a DiaClean® Cell Equipped with Boron-Doped Diamond Electrodes (BDD). Water, Air, & Soil Pollution 228, https://doi.org/10.1007/s11270-017-3471-9 (2017).
Delrue, F., Álvarez-Díaz, P., Fon-Sing, S., Fleury, G. & Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 9, 132, https://doi.org/10.3390/en9030132 (2016).
Escapa, C., Coimbra, R. N., Paniagua, S., García, A. I. & Otero, M. Comparison of the culture and harvesting of Chlorella vulgaris and Tetradesmus obliquus for the removal of pharmaceuticals from water. Journal of Applied Phycology, 1–15, https://doi.org/10.1007/s10811-016-1010-5 (2016).
Tikoo, V., Scragg, A. H. & Shales, S. W. Degradation of Pentachlorophenol by Microalgae. Journal of Chemical Technology & Biotechnology Biotechnology 68, 425–431, https://doi.org/10.1002/(SICI)1097-4660 (2010).
Escapa, C. et al. Valorization of Microalgae Biomass by Its Use for the Removal of Paracetamol from Contaminated Water. Water 9, 312, https://doi.org/10.3390/w9050312 (2017).
Staples, C. A., Weeks, J., Hall, J. F. & Naylor, C. G. Evaluation of aquatic toxicity and bioaccumulation of C8- and C9-alkylphenol ethoxylates. Environmental Toxicology & Chemistry 17, 2470–2480, https://doi.org/10.1002/etc.5620171213 (1998).
Kumaran, S. S., Kavitha, C., Ramesh, M. & Grummt, T. Toxicity studies of nonylphenol and octylphenol: hormonal, hematological and biochemical effects in Clarias gariepinus. Journal of Applied Toxicology 31, 752–761, https://doi.org/10.1002/jat.1629 (2011).
Storck, S. L. & Rittman, B. E. Using Attached Phototrophs To Detoxify Hazardous Organic Compounds. (1986).
Trekels, H., Meutter, F. V. D. & Stoks, R. Predator cues magnify effects of the pesticide endosulfan in water bugs in a multi-species test in outdoor containers. Aquatic Toxicology 138-139, 116–122, https://doi.org/10.1016/j.aquatox.2013.04.008 (2013).
Zhou, G. J., Peng, F. Q., Yang, B. & Ying, G. G. Cellular responses and bioremoval of nonylphenol and octylphenol in the freshwater green microalga Scenedesmus obliquus. Ecotoxicol Environ Saf 87, 10–16, https://doi.org/10.1016/j.ecoenv.2012.10.002 (2013).
Della, G. M. et al. Biotransformation of ethinylestradiol by microalgae. Chemosphere 70, 2047–2053, https://doi.org/10.1016/j.chemosphere.2007.09.011 (2008).
Correa-Reyes, G. et al. Nonylphenol algal bioaccumulation and its effect through the trophic chain. Chemosphere 68, 662–670, https://doi.org/10.1016/j.chemosphere.2007.02.030 (2007).
Ahel, M., Mcevoy, J. & Giger, W. Bioaccumulation of the lipophilic metabolites of nonionic surfactants in freshwater organisms. Environmental Pollution 79, 243, https://doi.org/10.1016/0269-7491(93)90096-7 (1993).
Gao, Q. T., Wong, Y. S. & Tam, N. F. Y. Removal and biodegradation of nonylphenol by different Chlorella species. Mar Pollut Bull 63, 445–451, https://doi.org/10.1016/j.marpolbul.2011.03.030 (2011).
Gao, Q. T., Wong, Y. S. & Tam, N. F. Y. Removal and biodegradation of nonylphenol by immobilized Chlorella vulgaris. Bioresource Technol 102, 10230–10238, https://doi.org/10.1016/j.biortech.2011.08.070 (2011).
Wang, J. & Xie, P. Antioxidant enzyme activities of Microcystis aeruginosa in response to nonylphenols and degradation of nonylphenols by M. aeruginosa. Environmental Geochemistry & Health 29, 375, https://doi.org/10.1007/s10653-007-9081-5 (2007).
He, N. et al. Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae. International Journal of Environmental Research & Public Health 13, https://doi.org/10.3390/ijerph13121239 (2016).
Li, R. et al. Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicology & Environmental Safety 72, 321, https://doi.org/10.1016/j.ecoenv.2008.05.012 (2009).
Gattullo, C. E., Bährs, H., Steinberg, C. E. & Loffredo, E. Removal of bisphenol A by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Science of the Total Environment 416, 501, https://doi.org/10.1016/j.scitotenv.2011.11.033 (2012).
Hirooka, T. et al. Biodegradation of bisphenol A and disappearance of its estrogenic activity by the green alga Chlorella fusca var. vacuolata. Environmental Toxicology &. Chemistry 24, 1896–1901, https://doi.org/10.1897/04-259R.1 (2005).
Qian, H. et al. Effect of nonylphenol on response of physiology and photosynthesis-related gene transcription of Chlorella vulgaris. Environ Monit Assess 182, 61–69, https://doi.org/10.1007/s10661-010-1858-9 (2011).
Gao, Q. T., Wong, Y. S. & Tam, N. F. Y. Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. Journal of Applied Phycology 29, 1317–1329, https://doi.org/10.1007/s10811-017-1065-y (2017).
Torres, M. A. et al. Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicology & Environmental Safety 71, 1, https://doi.org/10.1016/j.ecoenv.2008.05.009 (2008).
Nakajima, N. et al. Glycosylation of bisphenol A by freshwater microalgae. Chemosphere 69, 934–941, https://doi.org/10.1016/j.chemosphere.2007.05.088 (2007).
Baptista, M. S., Stoichev, T., Basto, M. C., Vasconcelos, V. M. & Vasconcelos, M. T. Fate and effects of octylphenol in a Microcystis aeruginosa culture medium. Aquatic Toxicology 92, 59, https://doi.org/10.1016/j.aquatox.2008.12.005 (2009).
Liu, Y., Dai, X. & Wei, J. Toxicity of the xenoestrogen nonylphenol and its biodegradation by the alga Cyclotella caspia. Journal of Environmental Sciences 25, 1662–1671, https://doi.org/10.1016/s1001-0742(12)60182-x (2013).
Hung, W. N., Chiou, C. T. & Lin, T. F. Lipid-water partition coefficients and correlations with uptakes by algae of organic compounds. J Hazard Mater 279, 197–202, https://doi.org/10.1016/j.jhazmat.2014.06.071 (2014).
Sakurai, T. et al. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresource Technology 200, 861, https://doi.org/10.1016/j.biortech.2015.11.014 (2015).
Yan, H., Ye, C. & Yin, C. Kinetics of phthalate ester biodegradation by Chlorella pyrenoidosa. Environmental Toxicology & Chemistry 14, 931–938, https://doi.org/10.1002/etc.5620140602 (1995).
Zhang, Y., Liu, Y., Dong, H., Li, X. G. & Zhang, D. H. The nonylphenol biodegradation study by estuary sediment-derived fungus Penicillium simplicissimum. Environ Sci Pollut R 23, 15122–15132, https://doi.org/10.1007/s11356-016-6656-7 (2016).
Fujii, K., Urano, N., Ushio, H., Satomi, M. & Kimura, S. Sphingomonas cloacae sp nov., a nonylphenol-degrading bacterium isolated from wastewater of a sewage-treatment plant in Tokyo. Int J Syst Evol Micr 51, 603–610, https://doi.org/10.1099/00207713-51-2-603 (2001).
Dhooge, W. & Tanghe, T. Isolation of a bacterial strain able to degrade branched nonylphenol. Applied & Environmental Microbiology 65, 746, https://doi.org/10.1089/oli.1.1999.9.105 (1999).
Su, J., Yang, X., Zheng, T. & Hong, H. An efficient method to obtain axenic cultures of Alexandrium tamarense–a PSP-producing dinoflagellate. J Microbiol Methods 69, 425–430, https://doi.org/10.1016/j.mimet.2006.07.005 (2007).
Guillard, R. R. L. & Ryther, J. H. Studies on marine planktonic diatoms. 1. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. (1962).
Dai, R. J. et al. Determination of Algal Chlorophylls and Their Degradation Products. Journal of the Central University for Nationalities (2004).
Milani Hosseini, M. R. Determination of organic compounds in water using dispersive liquid-liquid microextraction. Journal of Chromatography A 1116, 1, https://doi.org/10.1016/j.chroma.2006.03.007 (2006).
Luo, S. et al. Determination of octylphenol and nonylphenol in aqueous sample using simultaneous derivatization and dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Journal of Chromatography A 1217, 6762–6768, https://doi.org/10.1016/j.chroma.2010.06.030 (2010).
Zhou, G. J., Peng, F. Q., Zhang, L. J. & Ying, G. G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environmental Science & Pollution Research International 19, 2918–2929, https://doi.org/10.1007/s11356-012-0800-9 (2011).
Liu, Y., Guan, Y., Gao, Q., Tam, N. F. & Zhu, W. Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 80, 592–599, https://doi.org/10.1016/j.chemosphere.2010.03.042 (2010).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 41476099 and No. 41676099) and the China Postdoctoral Science Foundation (55350257).
Author information
Authors and Affiliations
Contributions
L.Y.W. and S.S.D. conceived and designed the experiments; L.Y.W. and H.X. performed the experiments and analysed the data; N.H. and H.X. contributed reagents/materials/analytical tools; L.Y.W. contributed to the writing of the manuscript; and D.S., N.H. and S.S.D. contributed to the writing, reviewing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Xiao, H., He, N. et al. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci Rep 9, 5277 (2019). https://doi.org/10.1038/s41598-019-41808-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41808-8
This article is cited by
-
Innovative technologies to remove alkylphenols from wastewater: a review
Environmental Chemistry Letters (2022)
-
Endocrine Disrupting Chemicals in Aquatic Ecosystem: An Emerging Threat to Wildlife and Human Health
Proceedings of the Zoological Society (2021)
-
Toxic Impact of Alkylphenols on the Fish Reproduction and Endocrine Disruption
Proceedings of the Zoological Society (2021)
-
Effects of Environmental Pollutants on the Growth Characteristics, Oxidative Stress and Biochemical Composition of Nannochloropsis salina for Biodiesel Production
Iranian Journal of Science and Technology, Transactions A: Science (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.