Abstract
Burkholderia seminalis strain TC3.4.2R3 is an endophytic bacterium isolated from sugarcane roots that produces antimicrobial compounds, facilitating its ability to act as a biocontrol agent against phytopathogenic bacteria. In this study, we investigated the thermoregulation of B. seminalis TC3.4.2R3 at 28 °C (environmental stimulus) and 37 °C (host-associated stimulus) at the transcriptional and phenotypic levels. The production of biofilms and exopolysaccharides such as capsular polysaccharides and the biocontrol of phytopathogenic fungi were enhanced at 28 °C. At 37 °C, several metabolic pathways were activated, particularly those implicated in energy production, stress responses and the biosynthesis of transporters. Motility, growth and virulence in the Galleria mellonella larvae infection model were more significant at 37 °C. Our data suggest that the regulation of capsule expression could be important in virulence against G. mellonella larvae at 37 °C. In contrast, B. seminalis TC3.4.2R3 failed to cause death in infected BALB/c mice, even at an infective dose of 107 CFU.mL−1. We conclude that temperature drives the regulation of gene expression in B. seminalis during its interactions with the environment.
Similar content being viewed by others
Introduction
Burkholderia species are widely distributed in the environment, including soil1, water2, plant tissues3, several animal species4 and in humans, particularly in hospital settings5. Members of the B. cepacia complex (Bcc) have been isolated as opportunistic pathogens that cause lung infections in immunocompromised patients with cystic fibrosis6. However, many species from this Bcc group have been isolated from plants and possess the ability to control plant diseases7 or to promote plant growth8. Root colonization ability does not depend on source or species in the Bcc group9, suggesting that members of the Bcc group can differentially regulate genes depending on their interactions with plant or animal hosts or in the soil environment where they compete with other microbial species.
Among Burkholderia species, Burkholderia seminalis was recently described10 and included in the Bcc group. This gram-negative, aerobic, non-sporulating, rod-shaped bacterium forms yellow-pigmented mucoid colonies on agar plates. B. seminalis has been found in several habitats, including water11, soil12, plants7 and the sputum of cystic fibrosis patients1. In plants, B. seminalis may be associated with growth promotion12, biocontrol of orchid necrosis7, root nodulation13 or phytopathogenesis, specifically peach rot14.
B. seminalis strain TC3.4.2R3 was isolated endophytically from sugarcane roots15 and was shown to be effective in the biocontrol of orchid necrosis caused by B. gladioli7. The TC3.4.2R3 strain produces pyochelin, a rhamnolipid, and other unidentified diffusible metabolites that have been associated with inhibition of Fusarium oxysporum16 and the cacao pathogens Moniliophthora perniciosa (fungus), Phytophthora citrophtora, P. capsici, and P. palmivora (oomycete)17. Therefore, Burkholderia spp. are considered effective biological control agents because they produce an array of antimicrobial compounds18. Several metabolites, including cepacins, pyrrolnitrins, cepaciamides, cepacidines, quinolones, phenazines, siderophores, lipopeptides, among others19,20 have been reported for Burkholderia species, and most of them present antifungal activity.
Many crops are infected by phytopathogenic fungi, and some of them, such as Fusarium oxysporum, may cause rot and vascular wilt in several cultures, including onion, tomato, lettuce, cotton, and brassicas, among others21,22. Colletotrichum spp. are other important phytopathogens that damage fruits and vegetables by causing blight and anthracnose spots in addition to postharvest rot23. Pineapple disease, wilt and postharvest black rot are plant diseases caused by Ceratocystis fimbriata and Ceratocystis paradoxa in economically important crops such as sugarcane24,25. Therefore, the use of Burkholderia species as a biocontrol agent may prevent economic losses in a diverse range of crops with important global implications. To date, the interactions of B. seminalis with its several hosts have been poorly studied.
Many Bcc members are human pathogens; therefore, testing the virulence of the TC3.4.2R3 strain is crucial to validate its possible use as an agricultural biocontrol agent. Larvae of the greater wax moth Galleria mellonella have been widely used as a non-mammalian model to investigate bacterial pathogenesis because the innate immune system of this insect is similar in many aspects to that of the mammalian innate system26. Comparison of bacterial pathogenicity in G. mellonella and mouse infection models is important to develop our understanding of the interactions of B. seminalis with different hosts.
Temperature is a central trigger for the control of gene expression in bacterial pathogens27. For example, differentially expressed virulence factors, such as toxins, are affected by temperature in Pseudomonas syringae28 and Yersinia entomophaga29. Motility, flagellar expression and biofilm production are often thermoregulated, for example, P. syringae28 and Campylobacter jejuni30. Furthermore, surfactant and exopolysaccharide (EPS) production appears to occur in a temperature-dependent manner in Pseudomonas putida31 and Erwinia amylovora32, respectively. Many genes involved in plant-microbe interactions28, insect-microbe interactions29, microbe-microbe interactions33 and biocontrol potential34 are also thermoregulated. Given that B. seminalis is a member of the Bcc that could have biocontrol applications, this study was undertaken to investigate the thermoregulation of B. seminalis strain TC3.4.2R3 in various hosts.
Results
Thermoregulation of biofilm formation, EPS and motility in B. seminalis TC3.4.2R3
To understand the role of temperature variation in strategies used by B. seminalis TC3.4.2R3 during ecological interactions, biofilm and EPS production, growth rate and swimming motility were evaluated (Fig. 1). We assessed the ability of B. seminalis TC3.4.2R3 to form biofilms using the MBEC (Minimum Biofilm Eradication Concentration) biofilm device of Innovotec Inc. (Edmonton, AB, Canada). Biofilm formation at 28 °C was 2.5-fold greater than that at 37 °C (Fig. 1a), and EPS production was 40% greater (p < 0.05) at 28 °C than at 37 °C (Fig. 1b). However, the swimming rate was significantly higher at 37 °C than at 28 °C (Fig. 1c, p < 0.0001), suggesting that biofilm production and motility are inversely related.
Comparative phenotypic profile of B. seminalis TC3.4.2R3 at 28 °C and at 37 °C. (a) Biofilm production; (b) EPS production in dry weight; (c) swimming motility; and (d) growth in TSB medium. For (a,d), the error bars represent the standard deviation of three technical replicates and eight biological replicates each; ***p < 0.0005. For (b), the error bars represent standard deviation; *p < 0.05 and (c) ***p < 0.0001.
The growth rate was evaluated, and the highest rate occurred at 37 °C (Fig. 1d) at p < 0.0001. Growth curves were constructed for the TC3.4.2R3 strain at 28 °C and 37 °C based on the OD595 values recorded at 2 h intervals during incubation (Supplementary Fig. S1). Growth curve analysis revealed that B. seminalis exhibited the highest growth rate at 37 °C. The stationary phase was reached before 20 h of incubation for B. seminalis cultured at 37 °C and was delayed until 26 h of incubation at 28 °C, showing that after this time, both cultures were in stationary phase. A linear regression analysis was used to establish growth equations: Y = 0.2931*X − 0.4318 for growth at 28 °C and Y = 0.2889*X + 0.2895 for 37 °C. The results for best fit comparisons between data sets revealed that the curves were parallel with significantly different intercepts (p = 0.0470).
Effect of temperature on antagonism against plant pathogenic fungi
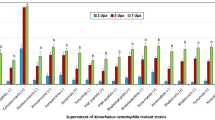
The ability of B. seminalis TC3.4.2R3 to inhibit the phytopathogenic fungi F. oxysporum, C. paradoxa, C. fimbriata and Colletotrichum spp. was evaluated at 28 °C and at 37 °C (Fig. 2). The ability of B. seminalis to inhibit in vitro phytopathogenic fungi was significantly higher at 37 °C than at 28 °C (Fig. 2a,b). In addition, we observed that B. seminalis produced less yellow pigment at 37 °C than at 28 °C (Fig. 2c) after 48 h of growth (Fig. 2a,b). Although fungal inhibition was observed when the bacteria were grown on plates for 24 h, the inhibition halo was twofold higher when grown for 48 h (Fig. 2a,b).
Antagonism of B. seminalis against phytopathogenic fungi at different temperatures. Bacteria were previously grown for (a) 24 h or (b) 48 h. The inhibition zone was measured in centimetres (cm). The error bars represent standard deviation; asterisks directly above columns indicate a significant difference compared to control plates; asterisks above lines indicate differences between temperature treatments: *p < 0.01 and ***p < 0.0001. (c) Antagonism of B. seminalis TC3.4.2R3 previously grown for 48 h at 28 °C and at 37 °C against C. paradoxa. Less yellow pigment was produced at 37 °C.
Effects of B. seminalis TC3.4.2R3 on seed germination
To verify whether temperature influences the interaction of B. seminalis with a plant host, we investigated the effects of B. seminalis inoculation on seed germination at 28 °C and 37 °C in maize, a monocotyledonous, and cotton, a dicotyledonous species (Supplementary Table S1). Seed germination was not affected by TC3.4.2R3 at any temperature.
Pathogenicity of B. seminalis TC3.4.2R3 infection is thermoregulated and host-dependent
The pathogenic potential of B. seminalis TC3.4.2R3 was evaluated in G. mellonella larvae (Fig. 3) and BALB/c mice. The B. seminalis TC3.4.2R3 pathogenic potential against G. mellonella was significant (p < 0.0001) and temperature-dependent. At 37 °C, by 24 h after inoculation with B. seminalis, all larvae had died. In contrast, at 28 °C, less virulence was observed; the larvae began to die after 48 h, and 100% death was observed after 65 h. In control experiments, no larvae death was observed for untreated larvae or larvae inoculated with PBS at both temperatures. Because strain TC3.4.2R3 showed higher virulence in the wax moth larvae infection model at 37 °C than at 28 °C, we proceeded to use the BALB/c mouse infection model to evaluate virulence in a mammalian model.
Demonstration of killing of G. mellonella larvae by B. seminalis TC3.4.2R3. Untreated: the larvae were not inoculated – line means the same results for both 28 °C and 37 °C; PBS: larvae inoculated with PBS - line means the same results for both 28 °C and 37 °C; B. seminalis 28 °C: larvae inoculated with B. seminalis and incubated at 28 °C; B. seminalis 37 °C: larvae inoculated with B. seminalis and incubated at 37 °C. Significance was determined using the log-rank (Mantel-Cox) test and Bonferroni correction; p < 0.0001.
The pathogenicity of B. seminalis to BALB/c mice was evaluated up to 30 days with intraperitoneal inoculations of 107, 106, 105, 104 or 103 CFU.mL−1 as the final concentration of bacteria. For all experiments, no signs of disease were observed, even at the highest bacterial inoculum.
RNAseq analysis of B. seminalis TC3.4.2R3 under different temperatures
We used RNA sequencing and comparative transcriptomic analysis to identify genes and their respective expression levels at 28 °C and 37 °C in B. seminalis TC3.4.2R3 and to identify genes that potentially contribute to temperature and phenotype changes in B. seminalis TC3.4.2R3. Although we used environmental (28 °C) and clinical (37 °C) temperatures, the gene expression should be more related to antifungal production than to pathogenesis, since after 48 h of growth, the bacterial culture was in stationary phase. As previously presented, although there was fungal inhibition by B. seminalis grown for 24 h, the higher antifungal production occurred after 48 h of growth (Fig. 2b) when the 28 °C and 37 °C cultures were in stationary stage. As a consequence of these observations, RNAseq was performed at these time points and temperatures.
Through the generation of more than 35 million reads per sample, we were able to map approximately 2 Gb of the TC3.4.2R3 genome sequence to the corresponding sequences in the B. seminalis TC3.4.2R3 genome (GenBank Accession Number LAEU01000000) that includes three genetic elements: chromosome 1 (3.50 Mb), chromosome 2 (3.05 Mb) and a mega-plasmid (1.09 Mb). Table 1 displays the details of the yield and quality of Illumina sequencing per sample. The average depth was 235X coverage for 28 °C samples and 233X coverage for 37 °C samples.
The RNA libraries were of high quality (Fig. 4), showing that 6912 (99.9%) of the 6917 genes were expressed. Only the pyochelin cluster was completely repressed at both 28 °C and 37 °C. To classify differentially expressed genes, we adopted a log2 fold-change (FC) above 1. Using this cutoff, 589 genes were differentially expressed, with 583 upregulated and six downregulated at 37 °C relative to 28 °C. Among these, 141 differentially regulated genes encoded hypothetical proteins, suggesting that these unknown genes could be related to adaptation to various environmental conditions. The downregulated genes encoded flagellin (Bsem_00099) and five hypothetical proteins (Supplementary Table S2). Functional classification of upregulated genes showed that these genes encode core functions such as energy metabolism, transport, regulatory proteins, cellular processes, and aromatic compound degradation and detoxification (Fig. 5).
The genes upregulated at 37 °C included 65 genes encoding translation and ribosomal proteins, 53 related to transcription regulation, 52 related to energy metabolism, 34 related to transport/secretion systems and 31 related to amino acid biosynthesis and metabolism. Growth at 37 °C also resulted in the upregulation of genes involved in responses to stress and detoxification (26 genes), DNA replication, repair and structure (20 genes), posttranslational modification and protein folding (19 genes), fatty acid biosynthesis and metabolism (15 genes), nucleotide biogenesis and metabolism (15 genes) and aromatic compound degradation (12 genes). Furthermore, 12 heat shock proteins and chaperones were upregulated at 37 °C, as well as genes encoding cofactors and vitamin metabolism (10 genes), terpenoids and polyketide metabolism (nine genes), virulence factors (eight genes), cold shock (six genes), signal transduction systems (six genes), toxin-antitoxin system and toxins (five genes), sulfur metabolism (four genes), flagella and pili (four genes), polyhydroxyalkanoate (PHA) biosynthesis (four genes), polysaccharide biosynthesis and metabolism (four genes), quorum-sensing (two genes) and lipopolysaccharide (LPS) biosynthesis (two genes) (Fig. 5). Furthermore, other genes, such as methyltransferases, cell division protein FtsB, GTP-binding proteins and AAA-ATPases, were also upregulated.
Genes encoding flagellin FliC (−2.17-fold) and the flagellar capping protein FliD (−0.72-fold) were downregulated at 37 °C. The flagellar transcriptional regulators FlhD and FlhC were upregulated (2.18- and 2.13-fold, respectively) at 37 °C, as was fliL (Bsem_03217), which encodes a flagellar basal body-associated protein and an OmpA/MotB domain-containing protein, which could be involved in forming the flagellar motor (Supplementary Table S3).
The capsule biosynthetic cluster wcb in B. seminalis is composed of 25 genes (Bsem_02937 to Bsem_02961) subdivided into six operons and three transcription units, of which 14 are significantly upregulated genes and three are log2(FC) > 1 (Fig. 6). Bsem_02945 encodes a phytanoyl-CoA dioxygenase (1.66-fold), the Bsem_02949 gene corresponds to a glucose-1-phosphate thymidylyltransferase (1.55-fold) and Bsem_02951 is a dTDP-glucose dehydratase (1.16-fold), suggesting that capsular polysaccharide production is stimulated at 37 °C.
We found that genes within the phenylacetic acid (PAA) catabolic locus were thermoregulated in B. seminalis (Supplementary Fig. S2). RNAseq analysis revealed that clusters paaABIJK and paaNJFDK (chromosome 1) were upregulated at 37 °C with FC values ranging from 1.11 (Bsem_00184) to 2.18 (Bsem_00388). On chromosome 2, the cluster paaHKF was also found to be induced (Supplementary Table S4). Furthermore, we found two genes involved in quorum sensing that were upregulated at 37 °C, Bsem_00682 (1.15-fold) and the autoinducer synthesis protein Bsem_05144 (1.18-fold).
Secondary metabolite clusters of B. seminalis and temperature-dependent expression
A homology search for genes encoding known proteins with motifs involved in secondary metabolism and antibiotic production in antiSMASH revealed four putative clusters in chromosome 1, including genes encoding a nonribosomal peptide synthetase (NRPS) cluster that shares 23% amino acid similarity to cupriachelin, a terpene cluster, an arylpolyene cluster and a type I polyketide synthase (T1PKS) cluster that shares 16% amino acid similarity to a galactoglucan protein. Expression of the NRPS cluster was not regulated by temperature. The terpene cluster had 40% of the upregulated genes (1.30- to 3.21-fold), arylpolyene and T1PKS clusters, present in the wcb gene cluster, had 10% (1.00 to 1.60-fold) and 20% (1.16- to 1.66-fold) of upregulated genes, respectively (Supplementary Table S5).
On chromosome 2, there were nine biosynthetic clusters, including a bacteriocin, a phosphonate, an ectoine, a homoserine lactone (hser-lactone), an NRPS and four terpene clusters. The hser-lactone cluster had 20% (1.04- to 1.96-fold) of upregulated genes, followed by the terpene cluster (Bsem_05163-05181) with 15% (1.44- to 2.19-fold), the ectoine cluster with 10% (1.19-fold) and the terpene cluster (Bsem_06110-06132) with 8% (1.08 to 1.69-fold) of upregulated genes (Supplementary Table S6).
The downregulated hypothetical protein Bsem_04337 composed an operon of five hypothetical proteins within the bacteriocin biosynthetic cluster. Inside the NRPS cluster, pyochelin biosynthetic genes, siderophores with antimicrobial activity were found, and their expression was not differentially regulated under the test conditions. Interestingly, five genes of the core pyochelin cluster were no-reads (Bsem_05509 to Bsem_05513), while the other six genes had no variation in expression level at the evaluated temperature (Supplementary Table S7).
Chromosome 3 presented five biosynthetic clusters, including two terpenes, a ketide synthase, a pyrrolnitrin and a bacteriocin cluster. Upregulated genes ranged from 5% for the terpene cluster (Bsem_06661-06679) (1.58-fold) to 2% for the pyrrolnitrin cluster (1.25-fold), an antibiotic with antifungal activity (Supplementary Table S8).
Some transcriptional regulators with the ability to repress antibiotic biosynthesis were found to be upregulated at 37 °C. A gene encoding the AbrB family transcriptional regulator (Bsem_03584) was 2.24-fold induced at 37 °C. Furthermore, four genes belonging to the TetR family of transcriptional regulators found on chromosomes 1 and 2 were also induced, namely, Bsem_01769 (1.35-fold), Bsem_02088 (1.06-fold), Bsem_04768 (1.05-fold) and Bsem_06238 (1.06-fold).
Discussion
Significant changes in gene expression in B. seminalis TC3.4.2R3 were observed in morphological traits comparing bacteria grown at 28 °C and 37 °C (Fig. 7). The bacteria increased biofilm formation, reduced motility, increased antimicrobial production and capsule biosynthesis and decreased virulence to G. mellonella at 28 °C, suggesting that the genes associated with these phenotypes could be important during interactions with the soil microbiota or during association with the plant host.
Schematic presentation of thermoregulation in B. seminalis TC3.4.2R3. At 28 °C, more EPS and biofilm are produced favoring the establishment of colonization. At 37 °C, energy pathways are induced, and more motility can be observed. Capsule biosynthesis is also upregulated leading to a higher virulence in larvae model.
In the present study, B. seminalis TC3.4.2R3 produced more biofilm at 28 °C than 37 °C (Fig. 1a). Previous studies have shown that biofilm formation precedes endophytic colonization in plant tissues35, which should occur at temperatures less than 30 °C. In contrast, Burkholderia species, such as B. pseudomallei strain 1026b, produce more biofilm at 37 °C in comparison to 30 °C36, suggesting that for pathogenic bacteria, biofilm formation should be induced close to host temperature. These results suggest that for the endophytic B. seminalis TC3.4.2R3, the increased biofilm production at 28 °C could have a beneficial role in plant host colonization but not in virulence against G. mellonella.
The synthesis of EPS by B. seminalis TC3.4.2R3 also occurred in a temperature-dependent manner (Fig. 1b) and was greater at 28 °C than 37 °C. EPS is a major component of the biofilm matrix in many bacteria. EPS production was consistent with greater biofilm production at 28 °C. The production of EPS by B. kururiensis is regulated in response to growth and external conditions37. Similar results were observed for B. tropica, with biofilm formation affected in a temperature-dependent manner up to 24 h38. Interestingly, even though the amount of biofilm was greater at 28 °C, bacterial growth was higher at 37 °C in B. seminalis strain TC3.4.2R3 (Fig. 1d), indicating no association between higher growth and increased biofilm formation.
Motility can be beneficial for bacteria in terms of nutrient acquisition, translocation in specific hosts to access optimal colonization sites, avoidance of noxious agents and dispersal in the environment39. We found that B. seminalis was more motile at 37 °C than at 28 °C, suggesting that flagellar synthesis or function was thermoregulated (Fig. 1c). The flagellar apparatus presents a complex modulation, as suggested for B. thailandensis, which contains flagellar genes up- and downregulated at 37 °C27. In B. seminalis TC3.4.2R3, the flagellin gene fliC was downregulated, while the flhD, flhC and fliL genes were upregulated at 37 °C, suggesting differential regulation of these genes. Similar results were found in B. pseudomallei strain 153: more motility was observed at 37 °C than at 25 °C and at 30 °C more so than at 42 °C, although fliC was downregulated (−2.60-fold)40.
Increased flagellar activity and adaptations to warmer temperatures require a significant amount of energy to support bacterial growth and function. Interestingly, not only energy production under aerobic conditions was favoured, characterized by the over-expression of the F0/F1 ATP synthase but also anaerobic pathways, such as alcohol fermentation. Since cytochrome genes are induced, it is expected that a microaerobic environment is created because cytochromes have high affinity for oxygen, triggering oxygen depletion in Burkholderia41. For B. seminalis TC3.4.2R3 grown at 37 °C, genes encoding chaperons and heat-shock proteins, universal stress proteins and several genes involved in cellular detoxification were upregulated to protect and ensure cell survival, as also observed in B. pseudomallei strain 153 at increased temperature40.
Furthermore, we found a cluster of PHA biosynthesis upregulation (Bsem_01251 to Bsem_01254) at 37 °C. Polyhydroxyalkanoates (PHA) are energy-reserve polymers produced by bacteria for surviving periods of starvation in natural habitats and possibly confer a competitive advantage by allowing storage and mobilization of carbon in the face of environmental changes41,42. This result, in combination with the energy generation, motility and growth curve (Fig. S1), suggests that at 37 °C, B. seminalis TC3.4.2R3 generates more energy to sustain increased motility, but as the growth rate remains similar, the surplus energy could be stored in the form of PHA.
The increased expression of genes involved in primary metabolism, including nucleotide biosynthesis, cell division, RNA polymerases, ribosomal proteins and protein processing, indicates the high activity and energy-requiring cellular processes needed at 37 °C. Due to the accelerated activity, it is possible that cells enter a starvation period when grown at 28 °C; therefore, protein synthesis may be required during the starvation period to mediate nutrient scavenging, heat-shock response and recovery from starvation43. The rapid response to the availability of nutrients requires that the starved cells have an efficiently expressed transport system for nutrient uptake44, a result in agreement with our finding that many transmembrane and outer membrane transporters that are highly expressed at 37 °C. Moreover, the production of cell envelope structures, such as peptidoglycan, LPS and lipoproteins, was induced at 37 °C. This may be anticipated as they could confer protection to the cell during adaptation phases45.
Non-mammalian infection models have become particularly attractive because they are cheap, easy to assay, not limited by ethical concerns and can survive at 37 °C, thus enabling high-throughput in vivo screening. In this context, G. mellonella larvae have been used as a model organism to study bacterial pathogenicity, including members of the Bcc, because of the similarities between the innate immune systems of insects and mammals46,47,48. B. seminalis strains isolated from water, fruits and the rhizosphere are considered weak pathogens or non-virulent to G. mellonella in comparison with clinical isolates49. B. seminalis TC3.4.2R3 killed 100% of larvae in 24 h at 37 °C with 107 CFU.mL−1, but the virulence was attenuated at 28 °C, and all larvae died in 65 h (Fig. 3). The results showed that although B. seminalis TC3.4.2R3 can kill wax moth larvae, this virulence is significantly increased at 37 °C. For B. cenocepacia, the largest number of virulence genes was induced at 37 °C in comparison to 20 °C, suggesting that temperature adaptation during host infection is associated with pathogenesis50.
However, in the BALB/c model, B. seminalis TC3.4.2R3 was not virulent, even at high concentrations (107 CFU.mL−1), indicating that this strain is not pathogenic to mice and that the results for G. mellonella and mice infection models were not equivalent. Similar results were observed for B. thailandensis, which is avirulent in mouse infection models51 but is highly virulent in insects and nematoid infection models52. These results suggest that for some environmental strains, such as B. seminalis TC3.4.2R3, temperature plays a role mainly in the interaction with soil- and plant-associated microbiota, including bacteria, fungi and insects, increasing the capability of this bacterium to compete with other microorganisms and to colonize the host plant.
Many factors may be implicated in virulence, depending on the infection model. For B. cenocepacia, AHL-mediated quorum sensing, siderophores and LPS were found to be important virulence factors in mammals and G. mellonella; however, several virulence factors were host-specific for these bacteria53,54. In addition, it is likely that in G. mellonella, other virulence factors, such as flagellar motility, facilitate disease progression. Flagellar motility may be related to increased virulence in B. pseudomallei55, but pathogenic Burkholderia species appear to have adopted very different strategies in terms of the regulation of flagellar motility during host infection27.
Among the probable virulence factors, we found that capsular polysaccharide production was significantly induced at 37 °C. Capsular polysaccharides are often associated with host colonization and virulence56. In B. seminalis TC3.4.2R3, the genes Bsem_02944 to Bsem_02953, present in the central part of the WCB cluster, were upregulated at 37 °C (Fig. 6), suggesting that the capsule could be a key factor associated with virulence in G. mellonella but has minor roles in soil and plant interactions. A previous study showed that these genes were variably expressed in Burkholderia, which were associated with different lifestyles7.
Phenylacetic acid (PAA) is a signal molecule that could be involved in microbial interactions with the host57. The phenylacetic acid (PAA) catabolic pathway was shown to be required for the full pathogenicity of B. cenocepacia in a Caenorhabditis elegans nematode infection model58. Nevertheless, B. seminalis TC3.4.2R3 does not possess all genes of the PAA pathway. Clusters paaGHIJK and paaABCE are the two core functional units of the PAA catabolic pathway common in many organisms59. The paaE gene is absent in TC3.4.2R3 and has been shown to be necessary for the survival of B. cenocepacia in rats60. Therefore, the pathway for PAA degradation present on chromosomes 1 and 2 of B. seminalis TC3.4.2R3 may allow the partial degradation of PAA, and the accumulation of intermediates might have toxic effects on G. mellonella larvae, but the lack of paaE could explain the non-virulence in mice.
Indole-acetic acid (IAA) and phosphate solubilization are important characteristics in plant growth-promoting bacteria. IAA is an auxin that promotes cell expansion and plant growth. B. seminalis TC3.4.2R3 is able to produce IAA and solubilize phosphate15, but the expression of these genes was not thermoregulated. Additionally, in this study, B. seminalis had no effect on cotton and maize seed germination, regardless of the temperature.
B. seminalis TC3.4.2R3 presents the ability to control orchid necrosis7, which seems to be temperature dependent, since we observed less antimicrobial activity at 37 °C. However, the expression of the pyochelin and rhamnolipid gene clusters was not affected by temperature shift. Indeed, previous studies showed that the production of these antimicrobial compounds is induced by the presence of phytopathogenic fungi and oomycetes16,17. Interestingly, transcriptional factors related to repression of antibiotic biosynthesis were upregulated at 37 °C. The AbrB family transcriptional regulator inhibited bacitracin61 and lantibiotics62 biosynthesis in Bacillus. The TetR family of transcriptional regulators can also repress antibiotic biosynthesis, such as kanamycin63, auricin64 and the peptidyl nucleoside antibiotic, gougerotin65. In this work, we found four TetR genes upregulated at 37 °C, which could be associated with repression of antibiotic production in B. seminalis TC3.4.2R3. Additionally, less pigment was produced at 37 °C, which could also be related to a secondary metabolite.
The behaviour and pathogenesis of B. seminalis TC3.4.2R3 are strongly influenced by temperature. At 28 °C, the temperature that reflects conditions that the bacterium encounters in the environment, more biofilm and EPS were produced, as well as potential biocontrol capabilities such as inhibition of phytopathogen growth. In terms of colonization and plant protection, this points to optimum adaptation at 28 °C. At 37 °C, the temperature reflecting infection of a mammalian host, the bacterium was more motile and produced fewer antimicrobial compounds, suggesting that a set of genes are regulated in a temperature-specific way, which could be related to environmental/host conditions. We observed that genes that encode EPS are induced at 37 °C and that this capsular polysaccharide is an important virulence factor for larvae infection, but this bacterium failed to induce mouse death, which could be associated with the lack of genes associated with virulence. In fact, the Type 3 (T3SS), T4 (T4SS) and T6 (T6SS) secretion systems gene clusters were not completely identified in the genome of this TC3.4.2R3 strain7. Although B. seminalis TC3.4.2R3 is within the Bcc group, it does not cause disease in the murine model, which is an important consideration given its biocontrol potential in crops. However, to corroborate the biosafety of this strain as a biocontrol agent, intranasal inoculations in mice and other mammalian models should be performed to confirm the avirulence of this endophytic strain.
Materials and Methods
Bacterial strain and growth conditions
B. seminalis strain TC3.4.2R3 was isolated from inside sugarcane root tissues15 where it resides as an endophytic bacterium. The whole genome of the strain was previously sequenced and described7. B. seminalis was maintained at −80 °C in Luria-Bertani (LB) broth (Difco Laboratories, Sparks, USA) and 20% glycerol and was recovered on Luria-Bertani agar or broth (Difco Laboratories) with incubation for 24 h at 28 °C or 37 °C.
Swimming motility assay
An overnight bacterial culture was harvested and resuspended in phosphate buffered saline (PBS) to an OD600 of 1.0. Bacterial suspensions were inoculated on the centre of LB with 0.3% w/v agar and incubated at 28 °C and 37 °C. Motility was evaluated by the appearance of growth rings around the inoculum area, and the halo diameter (mm) was measured after 24, 48 and 120 h66. The experiment was performed in triplicate.
Biofilm assays
Biofilm formation was quantified using a 96-well plate and accompanying peg-lid of the MBEC (Minimum Biofilm Eradication Concentration) assay device (Innovatech, Edmonton, Canada). Wells received 150 µL bacterial suspension of B. seminalis standardized to 107 CFU.mL−1 in Tryptone Soya Broth (TSB) (Difco Laboratories). Eight wells were inoculated in at least three independent experiments. The peg-lid was placed on the plate and incubated at 28 °C or 37 °C for 24 h. The peg-lid was transferred to a fresh 96-well plate containing pre-warmed TSB and incubated for more than 24 h. The peg-lid was rinsed with PBS and then baked at 60 °C for 20 minutes, followed by staining with crystal violet 0.1% (w/v) (200 µL per well) and incubation for 30 minutes at room temperature. Three wash plates containing PBS (200 µL per well) were used to rinse the pegs following staining; subsequently, the crystal violet was solubilized with 95% ethanol prior to measurement at OD59567. In addition, a growth curve was prepared. Flasks containing 100 mL of TSB were each inoculated with a bacterial suspension to reach OD595 equal to 0.05. Then, flasks were shaken at 150 rpm at 28 °C or 37 °C. Aliquots were removed at periods of 2 h until the stationary phase, and OD595 readings were measured.
Measurement of exopolysaccharide (EPS) weight
EPS was purified from cell culture media68 with modifications. Briefly, B. seminalis was grown for 5 days at 28 °C or at 37 °C in 10 mL of mannitol medium (0.2% yeast extract and 2% mannitol). The bacterial cultures were vortexed and centrifuged at 2,300 × g for 10 minutes. The supernatant was transferred to new tubes, and phenol was added at a 10% final concentration and then incubated at 4 °C for 5 h and further centrifuged for 15 minutes. The water phase was collected, 4 volumes of isopropanol was added and incubated at −20 °C for 18 h. The precipitated exopolysaccharide was centrifuged at 9,100 × g for 15 minutes and suspended in 150 µL of distilled water. The extract was lyophilized, and the dry weight was measured.
Antagonism against plant pathogenic fungi
An overnight B. seminalis culture was adjusted to 107 CFU.mL−1 and 10 µL was cultivated for 48 h at 28 °C or at 37 °C on potato dextrose agar (PDA) (Difco Laboratories). After bacterial growth, disks of phytopathogenic fungi (6 mm) were transferred to the centre of PDA medium and incubated at 28 °C. After fungi growth, the inhibition zone was measured and compared with the control, which consisted of fungi plates without B. seminalis. The antagonism was evaluated against Ceratocystis fimbriata, C. paradoxa, Colletotrichum falcatum and Fusarium oxysporum. The experiment was performed in triplicate69. Detection of putative secondary metabolism and antibiotic genes in the three chromosomes of B. seminalis was performed by antiSMASH version 3.070.
Effects of Burkholderia on seed germination
B. seminalis was grown in LB broth at 28 °C or at 37 °C, shaking at 180 rpm for 48 h. Bacteria were harvested, and the absorbance was adjusted to OD600 1.0 with sterile distilled water. Seeds of maize (Zea mays) and cotton (Gossypium hirsutum) were surface sterilized in 70% alcohol for 1 minute and 3% sodium hypochlorite for 1 minute and rinsed twice in sterile water. Subsequently, the seeds were immersed in the B. seminalis suspension at 28 °C or at 37 °C under agitation at 150 rpm for 1 h. Control seeds were treated with sterile water (untreated control). A replicate consisting of 50 seeds was placed on moist filter paper and incubated in a germination chamber at 28 °C or at 37 °C and a 12 h photoperiod for five days. During the experiment, filter paper was maintained humid, adding a suitable volume of sterile water daily and wrapping containers in transparent plastic bags. The experiment was carried out in triplicate. The mean germination percentage was calculated at the end of the experiment71.
Galleria mellonella infection model
Larvae were obtained from LiveFoods UK and stored in woodchips at 10 °C prior to use. B. seminalis TC3.4.2R3 was inoculated in the hindmost larvae proleg using a 25 μL 22s-gauge gas-tight Hamilton syringe with 10 μL of 108 CFU.mL−1 and incubated at 28 °C or at 37 °C. PBS and non-inoculated larvae were used as controls and were incubated at the same temperature as the treatments. For each strain and treatment, ten larvae were inoculated per experiment in two independent experiments. The larval survival was monitored up to 72 h48.
BALB/c mouse infection model
To assess the pathogenic potential of B. seminalis TC3.4.2R3 in a mammalian host, six- to eight-week-old female BALB/c mice were infected. Experiments were approved by the Ethics Committee on the Use of Animals (CEUA) from University of Campinas under Registration No. 4305-1 and were conducted according to institutional standards. B. seminalis TC3.4.2R3 used for challenge was grown in LB broth at 37 °C under agitation (180 rpm) for 18 h. After growth, 1 mL of bacterial suspension was transferred to 50 mL of LB and incubated under the same conditions until OD600 1.0 was reached. The culture was harvested for 15 minutes, and the pellet was washed with 10 mL of cold PBS. After centrifugation, the pellet was resuspended in PBS. Groups of three BALB/c mice were challenged by an intraperitoneal route with 100 µL of B. seminalis suspensions (109, 108, 107, 106 or 105 CFU.mL−1). PBS was used as a control. The delivered doses of bacterial cells were then verified by plate counts on TSA. Mice were monitored for clinical signs and symptoms for 30 days.
RNA extraction and sequencing
B. seminalis was grown for 48 h with shaking (180 rpm) at 28 °C or at 37 °C in TSB in triplicate. These conditions were set up based on the time (48 h) where the greatest difference in the ability to inhibit fungi between the two evaluated temperatures was observed, in which temperature was correlated with the environmental (28 °C) or clinical (37 °C) conditions.
For RNA extractions, after growth, samples were frozen with liquid nitrogen and macerated. Total RNA was extracted using the Illustra RNAspin Mini Kit (GE Healthcare, Chicago, United States) according to the manufacturer’s instructions. Samples were tested for quality in agarose gel, and 60 ng/mL of each sample was dissected with RNAstable (Biomatrica, San Diego, United States). RNA quality was evaluated by Qubit and Bioanalyzer, and only samples with intact RNA were processed. Removal of rRNA was performed using the Ribozero Bacteria (Gram-Negative) kit. The sequencing library was prepared according to the Illumina Truseq Stranded RNAseq protocol and was sequenced on the Illumina HiSeq 2000/2500 platform46 to generate single-end reads with 50 bp. Reads were mapped on B. seminalis TC3.4.2R3 chromosomes (GenBank Accession Number LAEU01000000).
Mapping and analysis of Illumina reads
Sequence reads from each sample had rRNA and tRNA subtracted with Bowtie72. The reference sequences used were E. coli and Burkholderia. The resulting sequences were mapped on the B. seminalis TC3.4.2R3 genome (GenBank Accession Number LAEU01000000) with BWA software73. The Samtools package74 was used to convert sam into bam format files, sorted and indexed. To determine the differential expression of known transcripts, the resulting aligned reads were analysed by TopHat75 and Cuffdiff package76. Transcripts mapping to the B. seminalis TC3.4.2R3 genome with a P-value of ≤0.05 were considered differentially expressed transcripts, and those with log2(FC) above 1 were analysed further. Relative expression graphs and tables were assembled using the R program and the CummeRbund library77. Volcano plots were made in R. Functional and Gene Ontology annotations of the genome were performed with Blast2GO software78. Furthermore, transcripts were analysed by dbCAN (carbohydrate-active enzyme annotation)79, SignalP for the presence and location of signal peptide80, EffectiveDB for prediction of bacterial protein secretion81 and PSORTb for bacterial protein subcellular localization prediction82.
Statistical analysis
All in vitro assays were performed at least in triplicate, with subsequent statistical analysis by one-way ANOVA with Tukey’s test (GraphPad Prism 7.0) and multiple comparisons. For growth curves, linear regression was used to calculate line equations, and curves were compared by the F test. P < 0.05 was deemed statistically significant. A Kaplan-Meier survival plot with log-rank (Mantel-Cox) test and Bonferroni correction (GraphPad Prism 7.0) was used to compare survival of G. mellonella.
References
Vanlaere, E. et al. Burkholderia sartisoli sp. nov., isolated from a polycyclic aromatic hydrocarbon-contaminated soil. Int. J. Syst. Evol. Microbiol. 58, 420–423 (2008).
Lim, J. H., Baek, S. H. & Lee, S. T. Burkholderia sediminicola sp. nov., isolated from freshwater sediment. Int. J. Syst. Evol. Microbiol. 58, 565–569 (2008).
Aizawa, T., Vijarnsorn, P., Nakajima, M. & Sunairi, M. Burkholderia bannensis sp. nov., an acid neutralizing bacterium isolated from torpedo grass (Panicum repens) growing in highly acidic swamps. Int. J. Syst. Evol. Microbiol. 61, 1645–1650 (2011).
Elschner, M. C. et al. Isolation of the highly pathogenic and zoonotic agent Burkholderia pseudomallei from a pet green Iguana in Prague, Czech Republic. BMC Vet. Res. 10, 283 (2014).
Khosravi, Y., Vellasamy, K. M., Mariappan, V., Ng, S. L. & Vadivelu, J. Antimicrobial susceptibility and genetic characterisation of Burkholderia pseudomallei isolated from Malaysian patients. Sci. World J. 2014, 132971 (2014).
Martinucci, M. et al. Accurate identification of members of the Burkholderia cepacia complex in cystic fibrosis sputum. Lett. Appl. Microbiol. 62, 221–229 (2016).
Araújo, W. L. et al. Genome Sequencing and Transposon Mutagenesis of Burkholderia seminalis TC3.4.2R3 Identify Genes Contributing to Suppression of Orchid Necrosis Caused by B. gladioli. Mol. Plant. Microbe. Interact. 29, 435–446 (2016).
Young, L. S., Hameed, A., Peng, S. Y., Shan, Y. H. & Wu, S. P. Endophytic establishment of the soil isolate Burkholderia sp. CC-Al74 enhances growth and P-utilization rate in maize (Zea mays L.). Appl. Soil Ecol. 66, 40–47 (2013).
Vidal-Quist, J. C. et al. Arabidopsis thaliana and Pisum sativum models demonstrate that root colonization is an intrinsic trait of Burkholderia cepacia complex bacteria. Microbiol. (United Kingdom) 160, 373–384 (2014).
Vanlaera, E. et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov., and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int. J. Syst. Evol. Microbiol. 58, 1580–1590 (2008).
Fang, Y., Xie, G. L., Lou, M. M., Li, B. & Muhammad, I. Diversity analysis of Burkholderia cepacia complex in the water bodies of West Lake, Hangzhou, China. J. Microbiol. 49, 309–314 (2011).
Panhwar, Q. A. et al. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. PLoS One 9 (2014).
Huang, K. H., Chen, B. Y., Shen, F. T. & Young, C. C. Optimization of exopolysaccharide production and diesel oil emulsifying properties in root nodulating bacteria. World J. Microbiol. Biotechnol. 28, 1367–1373 (2012).
Lou, M. M. et al. Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr. Res. 346, 1294–1301 (2011).
Luvizotto, D. M. et al. Genetic diversity and plant-growth related features of Burkholderia spp. from sugarcane roots. World J. Microbiol. Biotechnol. 26, 1829–1836 (2010).
Araújo, F. D., da, S., Araújo, W. L. & Eberlin, M. N. Potential of Burkholderia seminalis TC3.4.2R3 as Biocontrol Agent Against Fusarium oxysporum Evaluated by Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom. 28, 901–907 (2017).
Araujo, F. D. S. et al. Desorption electrospray ionization mass spectrometry imaging reveals chemical defense of Burkholderia seminalis against cacao pathogens. RSC Adv. 7, 29953–29958 (2017).
Parke, J. L. & Gurian-Sherman, D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 39, 225–58 (2001).
Huang, Y. & Wong, P. T. W. Effect of Burkholderia (Pseudomonas) cepacia and soil type on the control of crown rot in wheat. Plant Soil 203, 103–108 (1998).
Mao, S. et al. Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr. Microbiol. 53, 358–364 (2006).
Armitage, A. D. et al. Characterisation of pathogen-specific regions and novel effector candidates in Fusarium oxysporum f.sp. cepae. Sci. Rep. 8, 13530 (2018).
Leslie, J. F. & Summerell, B. A. The Fusarium Laboratory Manual. 388p (Blackwell Publishing, 2006).
Dean, R. et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430 (2012).
Zhang, M. et al. Perillaldehyde Controls Postharvest Black Rot Caused by Ceratocystis fimbriata in Sweet Potatoes. Front. Microbiol. 9, 1102 (2018).
Zhang, Z. P., Li, Q., Luo, L. X., Li, J. Q. & Hao, J. J. First Report of Mango Wilt Caused by Ceratocystis fimbriata in Mangifera indica in China. Plant Dis. 101, 1042–1042 (2017).
Browne, N., Heelan, M. & Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 4, 597–603 (2013).
Peano, C. et al. Gene and protein expression in response to different growth temperatures and oxygen availability in Burkholderia thailandensis. PLoS One 9 (2014).
Hockett, K. L., Burch, A. Y. & Lindow, S. E. Thermo-Regulation of Genes Mediating Motility and Plant Interactions in Pseudomonas syringae. PLoS One 8 (2013).
Hurst, M. R. H. H. et al. Temperature-dependent Galleria mellonella mortality as a result of Yersinia entomophaga infection. Appl. Environ. Microbiol. 81, 6404–6414 (2015).
Teh, A. H. T., Lee, S. M. & Dykes, G. A. The Influence of Prior Modes of Growth, Temperature, Medium, and Substrate Surface on Biofilm Formation by Antibiotic-Resistant Campylobacter jejuni. Curr. Microbiol. 73, 859–866 (2016).
Dubern, J. F. & Bloemberg, G. V. Influence of environmental conditions on putisolvins I and II production in Pseudomonas putida strain PCL1445. FEMS Microbiol. Lett. 263, 169–175 (2006).
Wei, Z. M., Sneath, B. J. & Beer, S. V. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J. Bacteriol. 174, 1875–1882 (1992).
Mannaa, M. & Kim, K. D. Effect of Temperature and Relative Humidity on Growth of Aspergillus and Penicillium spp. and Biocontrol Activity of Pseudomonas protegens AS15 against Aflatoxigenic Aspergillus flavus in Stored Rice Grains. Mycobiology 46, 287–295 (2018).
Landa, B. B., Navas-Cortés, J. A. & Jiménez-Díaz, R. M. Influence of temperature on plant-rhizobacteria interactions related to biocontrol potential for suppression of fusarium wilt of chickpea. Plant Pathol. 53, 341–352 (2004).
Meneses, C. H. S. G., Rouws, L. F. M., Simoes-Araujo, J. L., Vidal, M. S. & Baldani, J. I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol. Plant. Microbe. Interact. 24, 1448–1458 (2011).
Plumley, B. A. et al. Thermoregulation of biofilm formation in Burkholderia pseudomallei is disrupted by mutation of a putative diguanylate cyclase. J. Bacteriol. 199 (2016).
Hallack, L. F. et al. Structural elucidation of the repeat unit in highly branched acidic exopolysaccharides produced by nitrogen fixing Burkholderia. Glycobiology 20, 338–347 (2009).
Serrato, R. V. et al. Culture conditions for the production of an acidic exopolysaccharide by the nitrogen-fixing bacterium. Burkholderia tropica. Can. J. Microbiol. 52, 489–493 (2006).
Rashid, M. H. & Kornberg, a. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97, 4885–4890 (2000).
Paksanont, S. et al. Effect of temperature on Burkholderia pseudomallei growth, proteomic changes, motility and resistance to stress environments. Sci. Rep. 8, 9167 (2018).
Paungfoo-Lonhienne, C. et al. Crosstalk between sugarcane and a plant-growth promoting Burkholderia species. Sci. Rep. 6 (2016).
Gasser, I., Müller, H. & Berg, G. Ecology and characterization of polyhydroxyalkanoate-producing microorganisms on and in plants. FEMS Microbiol. Ecol. 70, 142–150 (2009).
Watson, S. P., Clements, M. O. & Foster, S. J. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol. 180, 1750–1758 (1998).
Kolter, R., Siegele, D. A. & Tormo, A. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 47, 855–874 (1993).
Pletnev, P., Osterman, I., Sergiev, P., Bogdanov, A. & Dontsova, O. Survival guide: Escherichia coli in the stationary phase. Acta Naturae 7, 22–33 (2015).
Denman, C. C., Robinson, M. T., Sass, A. M., Mahenthiralingam, E. & Brown, A. R. Growth on mannitol-rich media elicits a genomewide transcriptional response in Burkholderia multivorans that impacts on multiple virulence traits in an exopolysaccharide-independent manner. Microbiology 160, 187–197 (2014).
Pereira, M. F. et al. Galleria mellonella is an effective model to study Actinobacillus pleuropneumoniae infection. Microbiology 161, 387–400 (2015).
Seed, K. D. & Dennis, J. J. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect. Immun. 76, 1267–1275 (2008).
Ibrahim, M. et al. Diversity of potential pathogenicity and biofilm formation among Burkholderia cepacia complex water, clinical, and agricultural isolates in China. World J. Microbiol. Biotechnol. 28, 2113–2123 (2012).
Sass, A. M. et al. The unexpected discovery of a novel low-oxygen-activated locus for the anoxic persistence of Burkholderia cenocepacia. ISME J. 7, 1568–81 (2013).
Sim, B. M. Q. et al. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 11, 1–17 (2010).
Wand, M. E., Müller, C. M., Titball, R. W. & Michell, S. L. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol. 11, 11 (2011).
Schwager, S. et al. Identification of Burkholderia cenocepacia strain H111 virulence factors using nonmammalian infection hosts. Infect. Immun. 81, 143–153 (2013).
Uehlinger, S. et al. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect. Immun. 77, 4102–4110 (2009).
Chua, K. L., Chan, Y. Y. & Gan, Y. H. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71, 1622–1629 (2003).
Reckseidler-Zenteno, S. L. et al. Characterization of the type III capsular polysaccharide produced by Burkholderia pseudomallei. J. Med. Microbiol. 59, 1403–14 (2010).
Lightly, T. J., Phung, R. R., Sorensen, J. L. & Cardona, S. T. Synthetic cystic fibrosis sputum medium diminishes Burkholderia cenocepacia antifungal activity against Aspergillus fumigatus independently of phenylacetic acid production. Can. J. Microbiol. 63, 427–438 (2017).
Pribytkova, T. et al. The attenuated virulence of a Burkholderia cenocepaciapaaABCDE mutant is due to inhibition of quorum sensing by release of phenylacetic acid. Mol. Microbiol. 94, 522–536 (2014).
Navarro-Llorens, J. M. et al. Phenylacetate catabolism in Rhodococcus sp. strain RHA1: A central pathway for degradation of aromatic compounds. J. Bacteriol. 187, 4497–4504 (2005).
Hunt, T. A., Kooi, C., Sokol, P. A. & Valvano, M. A. Identification of Burkholderia cenocepacia genes required for bacterial survival in vivo. Infect. Immun. 72, 4010–22 (2004).
Shu, C. C. et al. Analyzing AbrB-knockout effects through genome and transcriptome sequencing of Bacillus licheniformis DW2. Front. Microbiol. 9 (2018).
Bochmann, S. M., Spieß, T., Kötter, P. & Entian, K. D. Synthesis and succinylation of subtilin-like lantibiotics are strongly influenced by glucose and transition state regulator AbrB. Appl. Environ. Microbiol. 81, 614–622 (2015).
Bunet, R. et al. Characterization and manipulation of the pathway-specific late regulator AlpW reveals Streptomyces ambofaciens as a new producer of kinamycins. J. Bacteriol. 193, 1142–1153 (2011).
Novakova, R., Kutas, P., Feckova, L. & Kormanec, J. The role of the TetR-family transcriptional regulator Aur1R in negative regulation of the auricin gene cluster in Streptomyces aureofaciens CCM 3239. Microbiology 156, 2374–83 (2010).
Wei, J., Tian, Y., Niu, G. & Tan, H. GouR, a TetR Family Transcriptional Regulator, Coordinates the Biosynthesis and Export of Gougerotin in Streptomyces graminearus. Appl. Environ. Microbiol. 80, 714–22 (2014).
Flannagan, R. S., Linn, T. & Valvano, M. A. A system for the construction of targeted unmarked gene deletions in the genus. Burkholderia. Environ. Microbiol. 10, 1652–1660 (2008).
Denman, C. C. & Brown, A. R. Mannitol promotes adherence of an outbreak strain of Burkholderia multivorans via an exopolysaccharide-independent mechanism that is associated with upregulation of newly identified fimbrial and afimbrial adhesins. Microbiol. (United Kingdom) 159, 771–781 (2013).
Kim, J. K. et al. Purine biosynthesis, biofilm formation, and persistence of an insect-microbe gut symbiosis. Appl. Environ. Microbiol. 80, 4374–4382 (2014).
de Fávaro, L. C. L., de Sebastianes, F. L. S. & Araújo, W. L. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS One 7, 1–10 (2012).
Weber, T. et al. AntiSMASH 3.0-A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43, W237–W243 (2015).
Kunova, A. et al. Selection of Streptomyces against soil borne fungal pathogens by a standardized dual culture assay and evaluation of their effects on seed germination and plant growth. BMC Microbiol. 16, 272 (2016).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25 (2009).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–60 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Goff, L. A., Trapnell, C. & Kelley, D. CummeRbund: visualization and exploration of Cufflinks high-throughput sequencing data. R Packag. Version 2, 2 (2012).
Conesa, A. et al. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–6 (2005).
Yin, Y. et al. DbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 40, W445–51 (2012).
Petersen, T. N., Brunak, S., Von Heijne, G. & Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nature Methods 8, 785–786 (2011).
Eichinger, V. et al. EffectiveDB-updates and novel features for a better annotation of bacterial secreted proteins and Type III, IV, VI secretion systems. Nucleic Acids Res. 44, D669–74 (2015).
Yu, N. Y. et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–15 (2010).
Acknowledgements
This research was supported by grant from FAPESP (2012/24217-6, 2013/03426-9, 2014/24375-6, 2017/12510-4 and 2017/50447-2).
Author information
Authors and Affiliations
Contributions
W.L.A. designed the study. P.J.R.O.G. prepared experiments and analyzed the data. C.C.D.H. assisted in biofilm, motility and Galleria experiment. These experiments were supported by B.W.W. A.J.F. analyzed transcriptomic data and prepared Figure 4. S.T. made germination tests and prepared Figure 6. M.B. conducted mice experiments. Other figures and tables were prepared by P.J.R.O.G. The manuscript was written by P.J.R.O.G., B.W.W. and W.L.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonçalves, P.J.R.d., Hume, C.C., Ferreira, A.J. et al. Environmental interactions are regulated by temperature in Burkholderia seminalis TC3.4.2R3. Sci Rep 9, 5486 (2019). https://doi.org/10.1038/s41598-019-41778-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41778-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.