Abstract
The association between folic acid supplementation and birth defects other than neural tube defects remains unclear. We utilized data from a large population-based survey to examine the association between folic acid supplementation and birth defects in Northwestern China. A total of 29,204 women with infants born between 2010 and 2013 were surveyed in Shaanxi province, Northwestern China, using a stratified multistage sampling method. Propensity scores were used to match 9,293 women with optimal folic acid supplementation with 9,293 women with nonoptimal folic acid supplementation, and the effects of optimal folic acid supplementation on birth defects were assessed by a conditional logistic regression model. After propensity score matching, the overall birth defect rate, cardiovascular system defect rate and nervous system defect rate for the women with optimal folic acid supplementation were lower than those for the women with nonoptimal folic acid supplementation (overall birth defects: OR = 0.71, 95% CI = 0.57–0.89, P = 0.003; cardiovascular system defects: OR = 0.65, 95% CI = 0.44–0.96, P = 0.032; nervous system defects: OR = 0.13, 95% CI = 0.02–0.99, P = 0.049). Optimal folic acid supplementation was associated with a decreased prevalence of birth defects, especially in the cardiovascular system and nervous system. Our findings have important implications for birth defect intervention with folic acid supplementation for countries with a high prevalence of birth defects, such as China.
Similar content being viewed by others
Introduction
“Birth defect” is a general term for functional or structural abnormalities in a developing fetus. Some birth defects are caused by known genetic factors, such as chromosome aberrations or genetic mutations, environmental factors, or interactions between genetic factors and environmental factors. Birth defects are the leading cause of early miscarriage, stillbirth, perinatal death, and infant and child death1. Approximately 5.6% of pregnancies in China, including both live births and pregnancy losses, are affected by birth defects2. There are approximately 0.25 million births with major defects every year in China, which leads to a substantial disease burden2.
Both randomized controlled studies and observational studies have confirmed that periconceptional folic acid supplementation can reduce the prevalence of neural tube defects3,4,5,6. The WHO recommends that all women should take a folic acid supplement from the period before conception until 12 weeks of gestation7. In line with the WHO recommendation, China has issued a policy of folic acid supplementation (folic acid supplement ≥400 µg/d) during the 3 months before pregnancy and the first trimester of pregnancy. All Chinese women of childbearing age have access to free folic acid. The policy has played a very important role in significantly preventing neural tube defects (NTDs) in China2. However, the relationships between periconceptional folic acid supplementation and other birth defects, such as cardiovascular system defects, oral clefts, and urinary system defects, remain inconclusive. Interpretations of findings from observational studies are often limited by potential residual biases from measured confounders and possible biases resulting from unmeasured confounders. The propensity score (PS) technique has emerged as an effective tool to address selection and residual biases, and PS methods allow the transparent design and analysis of observational studies8,9,10. The objective of this study was to investigate the association between periconceptional folic acid supplementation and birth defects using PS methods in Shaanxi province, Northwest China.
Results
Participant characteristics
Among the birth defects surveyed, we identified 192.44 cases (per 10,000 infants) of overall birth defects before PS matching, including 6.85 cases (per 10,000 infants) of nervous system defects; 23.97 cases (per 10,000 infants) of eye, ear, face and neck defects;, 63.35 cases (per 10,000 infants) of cardiovascular system defects; 3.77 cases (per 10,000 infants) of respiratory system defects; 11.64 cases (per 10,000 infants) of oral clefts; 8.56 cases (per 10,000 infants) of digestive system defects; 5.82 cases (per 10,000 infants) of genital organ defects; 2.05 cases (per 10,000 infants) of urinary system defects; 34.58 cases (per 10,000 infants) of musculoskeletal system defects; 0.68 cases (per 10,000 infants) of chromosomal abnormalities; and 31.16 cases (per 10,000 infants) of other defects (Table 1).
Among the matched participants, 20.79% were ≥30 years, 46.38% had ≥9 years of education, 0.43% were non-Han, and 35.95% were urban residents. Table 2 compares the participants’ baseline characteristics according to folic acid supplementation before and after PS matching. There were significant prematch imbalances in several important covariates, including multiple births and tobacco exposure, that were balanced after matching. Our PS matching reduced the standardized differences for all covariates below 10% in absolute value, demonstrating substantial improvement in covariate balance across the folic acid supplementation groups (Fig. 1).
Optimal folic acid supplementation and birth defects
After PS matching, there were 195.85 cases per 10,000 infants in the nonoptimal folic acid supplementation group and 138.89 cases per 10,000 infants in the optimal folic acid supplementation group (OR = 0.71; 95% CI = 0.57–0.89; P = 0.049). Compared with 8.61 nervous system defects per 10,000 infants in the nonoptimal folic acid supplementation group, there were 1.08 nervous system defects per 10,000 infants in the optimal folic acid supplementation group (OR = 0.13; 95% CI = 0.02–0.99; P = 0.049). Compared with 67.79 cardiovascular system defects per 10,000 infants in the nonoptimal folic acid supplementation group, there were 44.12 cardiovascular system defects per 10,000 infants in the optimal folic acid supplementation group (OR = 0.65; 95% CI = 0.44–0.96; P = 0.032) (Table 3). Among cardiovascular system defects, the pregnancy outcomes of the optimal folic acid supplementation group were less likely to involve ventricular septal defects, atrial septal defects and patent ductus arteriosus than those of the nonoptimal folic acid supplementation group (ventricular septal defect: OR = 0.58, 95% CI = 0.30–1.13, P = 0.109; atrial septal defect: OR = 0.83; 95% CI = 0.36–1.93, P = 0.672; patent ductus arteriosus: OR = 0.43, 95% CI = 0.17–1.12, P = 0.083). However, the relationships between ventricular septal defects, atrial septal defects, patent ductus arteriosus, pulmonary artery constriction, atrioventricular septal defects and tetralogy of fallot and folic acid supplementation were not statistically significant.
After PS matching, the pregnancy outcomes of the optimal folic acid supplementation group were less likely to include eye, ear, face and neck defects; respiratory system defects; oral clefts and digestive system defects than those of the nonoptimal folic acid supplementation group (eye, ear, face and neck defects: OR = 0.50, 95% CI = 0.25–1.01, P = 0.055; respiratory system: OR = 0.75, 95% CI = 0.17–3.35, P = 0.706; oral clefts: OR = 0.46, 95% CI = 0.18–1.21, P = 0.117; digestive system: OR = 0.67, 95% CI = 0.32–2.41, P = 0.796). However, the relationships between folic acid supplementation and respiratory system defects, oral clefts, digestive system defects, abnormalities of the genital organs, urinary system defects, musculoskeletal system defects, chromosomal abnormalities and other defects were not statistically significant.
Sensitivity analyses
In the full (prematched) group of participants (n = 29204), there were 216.00 cases of birth defects per 10,000 in the nonoptimal folic acid supplementation group, compared with 151.04 cases of birth defects per 10,000 in the optimal folic acid supplementation group (OR = 0.70; 95% CI = 0.58–0.84; P < 0.001). Compared with 9.67 cases of nervous system defects per 10,000 in the nonoptimal folic acid supplementation group, there were 1.89 cases of nervous system defects per 10,000 in the optimal folic acid supplementation group (OR = 0.20; 95% CI = 0.5–0.84; P = 0.028). Compared with 70.39 cases of cardiovascular system defects per 10,000 in the nonoptimal folic acid supplementation group, there were 50.98 cases of cardiovascular system defects per 10,000 in the optimal folic acid supplementation group (OR = 0.72; 95% CI = 0.53–0.99; P = 0.045). When we adjusted for PS, the association remained significant (birth defects: OR = 0.70, 95% CI = 0.58–0.89, P = 0.003; nervous system defects: OR = 0.20, 95% CI = 0.05–0.84, P = 0.030; cardiovascular system defects: OR = 0.72, 95% CI = 0.45–0.99, P = 0.032). There were no significant differences in other defects between the folic acid supplementation groups.
Among the participants in PS tertiles two and three (n = 19936), we observed similar effects on optimal folic acid supplementation on birth defects, nervous system defects and cardiovascular system defects: PS-adjusted (birth defects: OR = 0.79, 95% CI = 0.65.−0.95, P = 0.013; nervous system defects: OR = 0.21, 95% CI = 0.05–0.87, P = 0.031; cardiovascular system defects: OR = 0.72, 95% CI = 0.52–0.99, P = 0.047).
Discussion
Shaanxi province is a high-prevalence area of birth defects in Northwest China. The overall prevalence of birth defects and cardiovascular system defects in Shaanxi province is higher than the national average and the averages for other provinces in Northwest China2. Thus, the prevention of birth defects and improvement of population health in Shaanxi province, Northwest China, are urgently necessary. This study investigated the association between folic acid supplementation and the birth defect spectrum, and our findings demonstrated that optimal folic acid supplementation was associated with a decreased risk of overall birth defects in Northwest China, especially nervous system and cardiovascular system defects. Optimal folic acid supplementation was not found to be associated with a significant decrease in other defects. These findings are very important because of the high prevalence of birth defects and the inconsistent rate of periconceptional folic acid supplementation in Northwest China.
Nervous system defects, especially NTDs, are common congenital anomalies in China. Nervous system defects always occur very early in pregnancy. The prevalence of NTDs was 13.8 per 1,000 births in 1999 in northern China, compared with less than 1 per 1,000 births in the USA11,12. A China-U.S. collaborative project for neural tube defect prevention conducted in northern and southern China revealed an 85% reduction in NTD risk in the northern region and a 40% reduction in NTD risk in the southern region6. Consequently, folic acid supplementation had a much greater effect on reducing the occurrence of NTDs in China. In our study, a lower prevalence of 6.85 per 10,000 infants was also observed. A large number of clinical trials and observational studies have shown that folic acid supplementation during pregnancy reduced the prevalence and recurrence rate of NTDs13,14,15,16, and an ecological study found that the prevalence of anencephaly and spina bifida decreased to 11% from 20% and to 21% from 34%, respectively, after folic acid fortification17. Our study was based on a birth defects survey in folate deficient areas in Northwestern China, and focused on the association between folic acid supplementation and birth defects. Although folic acid supplementation prevents NTDs, the dosage of folic acid supplementation and the duration of folic acid supplementation in practice are not consistent among different countries and regions of the world7, which are still of great impact on the prevention of NTDs. Our study provides additional evidence that optimal folic acid supplementation during the periconceptional period is associated with a reduction in the prevalence of nervous system defects (OR = 0.13; 95% CI = 0.02–0.99; P = 0.049). Therefore, our study further added to support for the reduction of nervous system defects by means of folic acid supplementation during the periconceptional period.
Cardiovascular system defects rank first among birth defects in China. The prevalence of cardiovascular system defects was 10~20 per 1,000 during the fetal period and 8~10 per 1,000 in live births18. From 2000 to 2011, the overall birth defect prevalence rates showed a downward trend. The National Stocktaking Report on Birth Defect Prevention (2012) (China) reported that the prevalence rate of cardiovascular system defects increased between 2000 and 20112. Some studies have confirmed that the use of vitamin supplements containing folic acid in early pregnancy was associated a reduced risk of cardiovascular system defects in offspring. Ionescuittu compared the prevalence of severe congenital heart disease before and after folic acid fortification in Quebec province, Canada19. The results showed that there was a significant 6% decrease per year (OR = 0.94; 95% CI = 0.90–0.97) in the seven years after folic acid fortification began. Liu carried out a population-based cohort study of all live births and stillbirths in Canada and found that folic acid fortification was associated with lower rates of nonchromosomal heart defects (OR = 0.89; 95% CI = 0.82–0.98)20. A 1984–1991 Hungarian randomized controlled trial (RCT) of periconceptional multivitamin supplementation containing folic acid (0.8 mg) showed a significant reduction in the first occurrence of cardiovascular abnormalities (OR = 0.60; 95% CI = 0.38–0.96), especially ventricular septal defects (OR = 0.26; 95% CI = 0.09–0.72)21. However, two case-control studies did not find a reduced risk of cardiovascular system defects as a result of folic acid supplementation22,23. In our study, after balancing all measured baseline covariates by PS matching, optimal folic acid supplementation may have reduced the risk of cardiovascular system defects by as much as 35% compared with the nonoptimal folic acid supplementation group. This finding implies the possibility of preventing cardiovascular system defects with folic acid supplementation in maternal and child health care practice during the periconceptional period. The association was analyzed further between the optimal folic acid supplementation and subtype of cardiovascular system defects. Because of lower prevalence, the association between the optimal folic acid supplementation and subtype of cardiovascular system defects and other birth defects were not significant statistically, but those results still were important clues for future study.
Two meta-analyses indicated that multivitamin supplementation with folic acid protected against oral clefts24,25. However, there is no strong evidence of an association between oral clefts and folic acid intake alone25. Some studies investigated the association between folic acid supplementation and the risk of eye, ear, face and neck defects, urinary system defects, musculoskeletal system defects and chromosomal abnormalities, but the results were inconsistent. Using the American National Birth Defects Prevention Network, Canfield investigated the change in the prevalence of urinary system defects after folic acid fortification and confirmed a reduction in the birth prevalence of renal agenesis, upper limb reduction defects, and omphalocele but an increase in obstructive genitourinary defects and Down syndrome17. Some studies have reported that multivitamin supplementation was associated with a reduced risk of limb reduction defects, while the associations were inconsistent for different limb reduction defect types17,26. A case-control study showed that a significant protective effect against Down’s syndrome was observed with large doses of folic acid (approximately 6 mg/d) during the first gestational month (OR = 0.4, 95% CI: 0.2 to 0.7)27. Unfortunately, these results were inconsistent among studies.
In our study, the prevalence of oral cleft, eye, ear, face and neck defects, respiratory system defects, digestive system defects and some specific birth defects (congenital hydrocephalus, ventricular septal defect, atrial septal defect and patent ductus arteriosus) in the optimal folic acid supplementation group was lower than in the nonoptimal folic acid supplementation group. However, there was no significant difference between the two groups. The lower prevalence of those specific defects could account for this lack of significance to some extent, and future studies with larger samples are required to further investigate these associations.
Previous observational studies usually used traditional regression methods to analyze the association between folic acid supplementation and birth defects20,21,22,23. However, selection bias and an imbalance of important variables between the groups were major problems in those observational studies28. Adjustment by specific covariates in regression model is adopted commonly but number of covariate, collinearity and sample size could influence the efficiency. The PS is a function of multiple covariates and represents the combined action of multiple covariates29. For an observational study, PS matching was effective in balancing confounding factors in a similar randomized treatment and reduced the selection bias8,9,10. When there were many covariates entering the model and relatively few events, such as the 17 covariates and 562 cases of birth defects (192.44 cases per 10,000 participants) in our study, PS matching was appropriate for providing an accurate estimated value compared with conventional multivariable methods30. Thus, the major strength of this study was the use of PS matching, which balanced optimal folic acid supplementation and nonoptimal folic acid groups on a large number of covariates by using a linear combination of covariates for a single score. To some extent, PS matching also reduced the confounding that may be present in our study. After matching, we further verified the consistency of the association between folic acid supplementation and birth defects by means of 3 models (including model 1, with no adjustment; model 2, with adjusted PS; and model 3, with adjusted covariates and PS). Our sample came from 10 urban districts and 20 rural counties in the province that were randomly selected according to the imbalanced population distribution and fertility level between rural and urban areas, and a stratified multistage sampling method was employed. Thus, our samples had relatively good representativeness and were suitable for evaluating the association between folic acid supplementation and birth defects in the population.
This study had some limitations. First, the recall bias existed possibly in this study because both the folic acid supplementation and risk factor information was obtained through the participant’s recall. Additionally, although we used the PS matching technique to balance selection bias between the two groups, the finding of our study might be potentially confounded by the biases related to unmeasured or hidden covariates because the covariates used for PS matching were limited, resulting in incomplete or inexact matching. In our study, it is unlikely that a hidden variable could be completely unrelated to any of the 17 covariates used in our PS analysis. Second, although the policy of folic acid supplementation has promoted universal periconception folate supplementation in China, the nonoptimal folic acid supplement group still may have been heterogeneous in that it included the women who used no or insufficient folic acid. Even though we tried our best to reduce the misclassification of birth defect diagnoses in our study by including a team of experts in the diagnosis of birth defects, a risk of misclassification of birth defect diagnoses still existed in the cases of deaths with birth defects. Finally, although our study included more than 29,000 women, there was limited power to detect associations due to the small number of women with pregnancies affected by birth defects in the study.
Conclusions
In conclusion, we observed that optimal folic acid supplementation was associated with a decreased prevalence of birth defects, especially of the cardiovascular system and nervous system, in Shaanxi province, Northwestern China. These findings may have important public health implications for the prevention of birth defects in Northwest China. Further prospective cohort and RCT studies are needed to confirm the preventive effects of folic acid supplementation.
Methods
Study design and participants
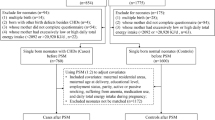
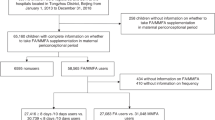
Data were collected from a large population-based survey conducted between August and November 2013 in Shaanxi province, Northwest China. The participants were women who were pregnant between August 2011 and August 2013 and gave birth at a gestational age of ≥28 weeks. A sample size of 30,000 was estimated assuming a prevalence of birth defects of 1.4%, a relative error of 15%, a design effect of 2.0, two-tailed α = 0.05, and an expected response rate of 80%. Considering the imbalanced population distribution and fertility level between rural and urban areas in the province, 10 urban districts and 20 rural counties were randomly selected, and a stratified multistage sampling method was employed to obtain the sample. First, six townships were randomly sampled from the chosen counties, and three streets were randomly sampled from the chosen districts. Second, six villages were randomly sampled from a chosen township, and six communities were randomly sampled from the chosen streets. Third, 30 participants were randomly sampled from the chosen villages, and 60 participants were randomly sampled from the chosen communities. Approximately 32,400 participants were expected to be sampled in the study. A total of 30,027 women were eventually enrolled in the survey (response rate: 92.68%). We excluded 823 women with unclear pregnancy outcomes or missing covariates, leaving a total of 29,204 women in this study. Written informed consent was obtained from each participant at the start of the survey.
Data collection
In the survey, all questionnaires, including the birth defects questionnaire and family questionnaire, and the field survey program were designed by Xi’an Jiaotong University Health Science Center. Face-to-face field surveys and data collection were carried out by uniformly trained field staff from Xi’an Jiaotong University Health Science Center. All the information was collected at the local village clinics and community health service centers. Our work was supported by local hospitals and health administration departments and the Ministry of Health in Shaanxi province.
A team of experts on the diagnosis of birth defects was established for this project. This team was composed of several senior medical technicians from the obstetrics and gynecology, ultrasound and pathology departments of the First Affiliated Hospital of Xi’an Jiaotong University. All cases of birth defects were diagnosed by the expert team using the ICD-10 to consistently and accurately code the diagnoses. For children with external anomalies, we collected their medical records and photographed the malformations for the final diagnosis. For children with congenital internal malformations, such as cardiovascular anomalies, we collected their medical records and conducted a free ultrasound examination at the First Affiliated Hospital of Xi’an Jiaotong University for the final diagnosis.
The family questionnaire was designed to collect information on maternal sociodemographic characteristics (including age, ethnicity, education, marital status, address of residence, occupation) and maternal exposure to periconceptional risk (including lifestyle, illnesses, periconceptional stress, exposure to harmful factors in the periconceptional environment, family history of diseases and periconceptional folic acid supplementation).
Classification of birth defects
Birth defects were classified according to the International Classification of Diseases Clinical Modification Codes, tenth edition, (ICD-10) as congenital malformations, deformations, and chromosomal abnormalities (Codes Q00–Q99), which include nervous system defects (Q00–Q07); eye, ear, face and neck defects (Q10–Q18); cardiovascular system defects (Q20–Q28); respiratory system defects (Q30–Q34); oral clefts (Q35–Q37); digestive system defects (Q38–Q45); defects of the genital organs (Q50–Q56); urinary system defects (Q60–Q64); musculoskeletal system defects (Q65–Q79); other defects (Q80–Q89); and chromosomal abnormalities (Q90–Q99).
Definitions of main variables
The information on folic acid supplementation and covariates was collected from the participants through face-to-face interviews conducted by well-trained interviewers via standard family questionnaires. The folic acid supplementation information included dosage per day, timing of use, and the period of regular use before and during pregnancy. This information was further confirmed by family members.
Optimal folic acid supplementation was defined regularly taking folic acid only or multiple micronutrients (≥400 µg folic acid per day) from 3 months before pregnancy through the first trimester of pregnancy for a duration of more than 3 consecutive months. Nonoptimal folic acid supplementation was defined as nonfolic acid supplementation, folic acid supplementation during the second and third trimesters of pregnancy, or folic acid supplementation for less than 3 months between the 3 months before through the 3 months after conception.
Other important covariates were defined. Infectious disease was defined as febrile episodes (>38 °C), influenza/common cold (influenza-like symptoms, seasonal influenza), infections of the kidney, bladder, urinary tract and genital tract and other infections affecting multiple organ systems (e.g., HIV infection, chicken pox) during pregnancy. Tobacco exposure was defined as active smoking (≥one cigarette per week for 3 consecutive months during pregnancy) or passive smoking (≥15 minutes of smoke inhalation per day for 1 consecutive month during pregnancy). Environmental risk factor exposure was defined as living within one kilometer of a coal mine, paper factory, cement factory, or power plant during pregnancy.
Quality control
All of the interviewers were trained with the same guidelines before the study. A quality control system was constructed during the study, and the interviewers checked themselves and were checked by other interviewers and by a supervisor. When errors and missing values were detected, participants were reinterviewed immediately.
Statistical analysis
We categorized 29,204 participants into optimal folic acid supplementation and nonoptimal folic acid supplementation groups. Of the 29,204 participants, 10,593 (36.27% of 29,204) had optimal folic acid supplementation. The PS was a function of multiple covariables and represents the combined action of multiple covariates29. Given the imbalance of baseline characteristics between the two groups, PS matching was used; PS matching can balance potential confounders between two groups with a similar randomized treatment and can reduce selection bias8,9,10. Then, we estimated the PS for each participant using a multivariable logistic regression model29, in which the level of folic acid supplementation was modeled using all the baseline participant characteristics shown in Table 2. We used the nearest neighbor within the caliper to match each participant with optimal folic acid supplementation with a participant with nonoptimal folic acid supplementation; thus, 9,293 (87.73% of the 10,593) participants with optimal folic acid supplementation were matched to 9,293 participants with nonoptimal folic acid supplementation and a similar estimated PS31. In our matching algorithm, we matched each participant with optimal folic acid supplementation with a participant with nonoptimal folic acid supplementation who had a similar PS to five decimal places. The nearest neighbor within caliper matching function was as follows:
Pi: propensity score of the optimal folic acid supplementation group; Pj: propensity score of the nonoptimal folic acid supplementation group; I0: the set of the nonoptimal folic acid supplementation group; C(Pi): the matching nonoptimal folic acid supplementation participant for the optimal folic acid supplementation participant; ε: tolerance for matching (caliper). The absolute standardized difference based on the chi-square and Student’s t-tests was used to compare the baseline characteristics of the participants with optimal folic acid supplementation and those with nonoptimal folic acid supplementation before and after matching10,32. The prematch mean PS for the participants with optimal folic acid supplementation and nonoptimal folic acid supplementation were 0.39809 and 0.34259, respectively (absolute standardized difference = 70.58%, P < 0.001). After matching, the mean PS for the higher of the participants with optimal folic acid supplementation and nonoptimal folic acid supplementation were 0.38425 and 0.38425, respectively (absolute standardized difference = 0.01%, P = 0.999).
Then, we used univariate conditional logistic regression models to estimate the association of optimal folic acid supplementation with various birth defects and overall birth defects. We conducted sensitivity analyses using two approaches to assess the robustness of our findings regarding the effect of folic acid supplementation on birth outcomes to changes in the analytic approach. To address concerns about incomplete matching, we analyzed data from all 29,204 participants using GEE adjustment for PS and all baseline covariates and subclassification based on tertiles of PS.
The data were managed by a database using EpiData Version 3.02 (The EpiData Association, Odense, Denmark), and data entries were duplicated. All of the analyses were performed with STATA version 12.0 software (STATA Corporation, College Station, TX, USA). The level of significance was set at P < 0.05.
Ethics statement
The Human Research Ethics Committee of the Xi’an Jiaotong University Health Science Center approved this study (No. 2012008). Written informed consent was obtained from all adult participants after a detailed explanation of the research. All research was performed in accordance with relevant guidelines and regulations.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Matthews, T. J., MacDorman, M. F. & Thoma, M. E. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 64, 1–30 (2015).
Office of maternal and child health surveillance (China). National Stocktaking Report on Birth Defect Prevention (2012) (China). National Health and Family Planning Commission of thePeople’s Republic of China, http://www.moh.gov.cn/wsb/pxwfb/201209/55840.shtml (2012).
Viswanathan, M. et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 317, 190, https://doi.org/10.1001/jama.2016.19193 (2017).
Bibbins-Domingo, K. et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. Jama 317, 183–189, https://doi.org/10.1001/jama.2016.19438 (2017).
Czeizel, A. E., Istvan, D., Attila, V. & Ferenc, B. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 5, 4760, https://doi.org/10.3390/nu5114760 (2013).
Berry, R. J. et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. The New England journal of medicine 341, 1485–1490, https://doi.org/10.1056/nejm199911113412001 (1999).
Gomes, S., Lopes, C. & Pinto, E. Folate and folic acid in the periconceptional period: recommendations from official health organizations in thirty-six countries worldwide and WHO. Public health nutrition 19, 176–189, https://doi.org/10.1017/s1368980015000555 (2016).
Rosenbaum, P. R. & Rubin, D. B. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American statistical association 79, 516–524, https://doi.org/10.1080/01621459.1984.10478078 (1984).
D’Agostino, R. B. Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine 17, 2265–2281, https://doi.org/10.1002/(SICI)1097-0258(19981015)17:193.0.CO;2-B (1998).
Austin, P. C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 46, 399–424, https://doi.org/10.1080/00273171.2011.568786 (2011).
Honein, M. A., Paulozzi, L. J., Mathews, T. J., Erickson, J. D. & Wong, L. Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 285, 2981–2986, https://doi.org/10.1001/jama.285.23.298 (2001).
Li, Z. et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol 76, 237–240, https://doi.org/10.1002/bdra.20248 (2006).
Venn, B. J. et al. Assessment of three levels of folic acid on serum folate and plasma homocysteine: a randomised placebo-controlled double-blind dietary intervention trial. European journal of clinical nutrition 56, 748–754, https://doi.org/10.1038/sj.ejcn.1601388 (2002).
Moore, L. L., Bradlee, M. L., Singer, M. R., Rothman, K. J. & Milunsky, A. Folate intake and the risk of neural tube defects: an estimation of dose-response. Epidemiology 14, 200–205, https://doi.org/10.1097/01.ede.0000040253.12446.b2 (2003).
Osterhues, A., Ali, N. S. & Michels, K. B. The role of folic acid fortification in neural tube defects: a review. Critical reviews in food science and nutrition 53, 1180–1190, https://doi.org/10.1080/10408398.2011.575966 (2013).
Wolff, T., Witkop, C. T., Miller, T. & Syed, S. B. Folic acid supplementation for the prevention of neural tube defects: an update of the evidence for the U.S. Preventive Services Task Force. Annals of internal medicine 150, 632–639, https://doi.org/10.7326/0003-4819-150-9-200905050-00010 (2009).
Canfield, M. A. et al. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth defects research. Part A, Clinical and molecular teratology 73, 679–689, https://doi.org/10.1002/bdra.20210 (2005).
WHO.Marth of dimes:Management of birth defects and haemolglobin disdorders. WHO (2006).
Ionescu-Ittu, R., Marelli, A. J., Mackie, A. S. & Pilote, L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ (Clinical research ed.) 338, b1673, https://doi.org/10.1136/bmj.b1673 (2009).
Liu, S. et al. Effect of Folic Acid Food Fortification in Canada on Congenital Heart Disease Subtypes. Circulation 134, 647–655, https://doi.org/10.1161/circulationaha.116.022126 (2016).
Czeizel, A. E., Dobo, M. & Vargha, P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth defects research. Part A, Clinical and molecular teratology 70, 853–861, https://doi.org/10.1002/bdra.20086 (2004).
Werler, M. M., Hayes, C., Louik, C., Shapiro, S. & Mitchell, A. A. Multivitamin supplementation and risk of birth defects. American journal of epidemiology 150, 675–682, https://doi.org/10.1093/oxfordjournals.aje.a010070 (1999).
Scanlon, K. S. et al. Preconceptional folate intake and malformations of the cardiac outflow tract. Baltimore-Washington Infant Study Group. Epidemiology 9, 95–98, https://doi.org/10.1097/00001648-199801000-00019 (1998).
Johnson, C. Y. & Little, J. Folate intake, markers of folate status and oral clefts: is the evidence converging? International journal of epidemiology 37, 1041–1058, https://doi.org/10.1093/ije/dyn098 (2008).
Badovinac, R. L., Werler, M. M., Williams, P. L., Kelsey, K. T. & Hayes, C. Folic acid-containing supplement consumption during pregnancy and risk for oral clefts: a meta-analysis. Birth defects research. Part A, Clinical and molecular teratology 79, 8–15, https://doi.org/10.1002/bdra.20315 (2007).
Czeizel, A. E. The primary prevention of birth defects: Multivitamins or folic acid? International journal of medical sciences 1, 50–61, https://doi.org/10.7150/ijms.1.50 (2004).
Czeizel, A. E. & Puho, E. Maternal use of nutritional supplements during the first month of pregnancy and decreased risk of Down’s syndrome: case-control study. Nutrition 21, 698–704, https://doi.org/10.1016/j.nut.2004.10.017 (2005).
Sturmer, T. et al. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59, 437–447, https://doi.org/10.1016/j.jclinepi.2005.07.004 (2006).
Rosenbaum, P. R. & Rubin, D. B. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55, https://doi.org/10.2307/2335942 (1983).
Cepeda, M. S., Boston, R., Farrar, J. T. & Strom, B. L. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. American journal of epidemiology 158, 280–287, https://doi.org/10.1093/aje/kwg115 (2003).
Ahmed, A. et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 27, 1431–1439, https://doi.org/10.1093/eurheartj/ehi890 (2006).
Normand, S. T. et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 54, 387–398, https://doi.org/10.1016/S0895-4356(00)00321-8 (2001).
Acknowledgements
We thank the local hospitals and health administration departments and the Ministry of Health in Shaanxi province. We thank all the participants and investigators in this study. We also thank the staff from Xi’an Jiaotong University for their assistance with the data collection. This work was supported by the National Natural Science Foundation of China (No. 81230016 and No. 81771657) and the Project of Birth Defect Control and Prevention in Shaanxi (No. Sxwsjswzfcght2016–013).
Author information
Authors and Affiliations
Contributions
The authors’ contributions are as follows: P.Q., S.D., L.Z. and H.Y. conceived and designed the study; P.Q., S.D., D.W., W.S. and J.S. drafted and revised the manuscript; P.Q., S.L., D.L. and F.L. analyzed and interpreted the data; P.Q., S.L., D.L., F.L., S.D. and L.Z. collected and cleared the data. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qu, P., Li, S., Liu, D. et al. A propensity-matched study of the association between optimal folic acid supplementation and birth defects in Shaanxi province, Northwestern China. Sci Rep 9, 5271 (2019). https://doi.org/10.1038/s41598-019-41584-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41584-5
This article is cited by
-
Environmental and parental risk factors for congenital solitary functioning kidney — a case–control study
Pediatric Nephrology (2023)
-
Maternal periconceptional folic acid supplementation reduced risks of non-syndromic oral clefts in offspring
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.