Abstract
Systemic lupus erythematosus (SLE) is mediated by a chronic and dysregulated inflammatory response. Interleukin (IL)-17, a proinflammatory cytokine, and T helper (Th)17 cells are associated with chronic autoimmune diseases. We hypothesized that inhibition of IL-17 would decrease the numbers of T cell subsets that function as B-cell helpers, as well as B-cell differentiation into plasma cells and autoantibody expression. The IL-17 level was increased markedly in Roquinsan/san mice. Loss of IL-17 in Roquinsan/san mice improved nephritis by downregulating immunoglobulin (Ig)G, IgG1, and IgG2a production. Formation of germinal centers (GCs), and follicular B- and T-cell differentiation was reduced, whereas the number of regulatory T (Treg) cells and immature B cells was increased, by IL-17 deficiency in Roquinsan/san mice. These results suggest that IL-17 inhibition can ameliorate SLE by inhibiting B-cell differentiation into GCs. Therefore, IL-17–producing Th17 cells show promise as a target for development of novel therapeutics for SLE.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease mediated by a chronic and excessive inflammatory response1. Damage to multiple organs results from the dysregulated immune inflammatory response mediated by autoantibodies (autoAbs) and immune complexes in SLE. For example, nephritis occurs in approximately 50% of SLE patients and induces premature death2,3,4,5.
Proinflammatory cytokines contribute to the pathogenesis of SLE. Indeed, serum levels of proinflammatory cytokines, such as interleukin (IL-1β, IL-6, and tumor necrosis factor (TNF)-α are correlated with SLE activity6. IL-17 is a proinflammatory cytokine involved in the development of several autoimmune diseases, including SLE. The serum level of IL-17A and numbers of IL-17–producing T cells were increased both in patients with SLE and in a mouse model of lupus7,8. In addition, the number of IL-17–producing T cells in peripheral blood is increased in SLE patients9,10, and an elevated serum IL-17 level is correlated with SLE progression10.
In SLE patients, the population of T follicular helper (Tfh) cells, which play a key role in B-cell differentiation into plasma cells in the germinal centers (GCs), as well as autoAb production, are increased11,12. IL-17 is associated with Tfh, GCs, and autoAbs. Indeed, autoAb overproduction is reportedly caused by IL-17 stimulation of peripheral blood mononuclear cells from patients with lupus nephritis13. Moreover, IL-17 activates B cells and promotes formation of GCs14, as do IL-17–producing Tfh cells15.
Roquin was identified as a CCCH-type zinc finger protein and diminish abnormal inducible T cell co-stimulator (ICOS) expression on T cells16,17. It has been demonsrated that Roquin deficiency leads to autoimmunity in Roquinsan/san mice that are homozygous for a point mutation in Rc3h1, the gene that encodes Roquin17,18. Indeed, Roquinsan/san mice showed dysregulation of immune response and used as a murine model of SLE16,19.
Thus, we hypothesized that IL-17 depletion would ameliorate the lupus-like characteristics of Roquinsan/san mice. Thus, we investigated the effect of loss of IL-17 on Tfh cells, GC formation, autoAb production, numbers of IL-17–producing T and B cells, and nephritis in Roquinsan/san and Roquinsan/san/IL-17−/− mice.
Results
AutoAb production and numbers of IL-17–producing T and B cells are increased in Roquinsan/san mice
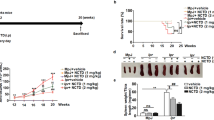
To investigate the potential function of Roquin on immune response, we used mouse genetics to mutant the Rc3h gene (Supplementary Fig. 1). We observed that IgG, IgG1, and IgG3 levels were increased significantly in Roquinsan/san mice compared to C57BL/6 mice (Fig. 1A). Moreover, the expression and production of IL-17 were upregulated significantly in Roquinsan/san mice compared to C57BL/6 mice (Fig. 1B). The numbers of IL-17–producing CD4+ T cells and CD19+ B cells were increased in Roquinsan/san mice (Fig. 1C,D). The frequency of IL-17–producing CD4+ T cells and CD19+ B cells in Roquinsan/san mice was increased by LPS treatment (Fig. 1E). These results suggest that the immune response in Roquinsan/san mice was enhanced by upregulation of IL-17 expression in T and B cells.
Immunoglobulin (Ig) production and the numbers of interleukin (IL)-17-producing T and B cells are increased in splenocytes and serum from Roquinsan/san mice compared to C57BL/6 and Roquinsan/san/IL-17−/− mice. (A,B) Serum IgG, IgG1, IgG3, and IL-17 levels were measured by enzyme-linked immunosorbent assay (ELISA) (each group n = 9). (B) IL-17 expression in splenocytes was determined by real-time PCR. (C,D) Confocal micrographs (n = 3). The numbers of CD4+ IL-17+ T cells and CD19+ IL-17+ B cells were determined by confocal microscopy and flow cytometry. (E) Splenocytes were simulated with lipopolysaccharide (LPS) (100 ng/mL) for 3 days and IL-17+ CD4 T cells and CD19 B cells were enumerated by flow cytometry. The numbers of CD4+ IL-17+ T cells and CD19+IL-17+ B cells were significantly increased in Roquinsan/san mice. Original magnification × 400. *p < 0.05.
Ig production and nephritis in Roquinsan/san/IL-17−/− mice
Since we observed the upregulation of IL-17 level in Roquinsan/san mice, we hypothesized that IL-17 deficiency can reduce immune inflammatory response and SLE development. We used mouse genetics to mutant the Rc3h gene and IL-17a gene deficiency (Supplementary Fig. 1). Compared to Roquinsan/san mice, serum IgG, IgG1, and IgG3 levels were reduced significantly in Roquinsan/san/IL-17−/− mice (Fig. 2A). Nephritis was attenuated by IL-17 deficiency in Roquinsan/san mice (Fig. 2B). Therefore, IL-17 likely plays an important role in dysregulated humoral immunity in SLE.
Ig production and nephritis in Roquinsan/san/IL-17−/− mice. (A) Serum IgG, IgG1, IgG3, and IL-17 levels in Roquinsan/san and Roquinsan/san/IL-17−/− mice (n = 9) were measured by ELISA. (B) Hematoxylin and eosin (H&E)-stained kidney sections from 15-week-old mice. The severity of the kidney pathology score was graded (n = 3). Original magnification × 200 and × 400. **p < 0.01, ***p < 0.001.
Treg cell differentiation is promoted by IL-17 deficiency in Roquinsan/san mice
The number of Treg cells was increased significantly in Roquinsan/san/IL-17−/− mice compared to Roquinsan/san mice. Moreover, Th2 cell differentiation was increased, but Th17 cell differentiation was reduced significantly, in Roquinsan/san/IL-17−/− mice compared to Roquinsan/san mice (Fig. 3A). The frequency of Treg cells was confirmed by flow cytometry (Fig. 3B). Foxp3 expression in Tfh cells was also increased by IL-17 deficiency in Roquin mice (Fig. 3C). These findings suggest that loss of IL-17 results in an increased Treg population in Roquinsan/san mice.
Effect of IL-17 deficiency on T-cell subsets in Roquinsan/san mice (n = 3). (A,B) The numbers of Foxp3+ regulatory T (Treg) cells, interferon (IFN)-γ+ Th1 cells, and IL-4+ Th2 cells were analyzed by confocal microscopy; representative images and a bar chart (right) are shown (Original magnification × 200). (C) The numbers of T helper (Th) cells and Tregs among splenocytes were determined by confocal microscopy (Original magnification × 400). **p < 0.01, ***p < 0.001.
IL-17 deficiency inhibits Tfh cell differentiation in Roquinsan/san mice
Tfh cells regulate the differentiation of B cells into plasma cells, which are involved in the pathogenesis of SLE. Therefore, we investigated the effect of IL-17 deficiency in Roquinsan/san mice on the numbers of cytokine-producing Tfh cells. The number of CD4+ICOS+ CXCR5+PD1+Tfh cells within the GC area was decreased in Roquinsan/san/IL-17−/− mice (Fig. 4A). The numbers of IL-17-, IFN-γ-, and IL-4-producing Tfh cells were determined by confocal microscopy and flow cytometry (Fig. 4B,C). These findings suggest that loss of IL-17 reduces the numbers of cytokine-producing Tfh cells in Roquinsan/san mice.
Effect of IL-17 deficiency on cytokine-producing Tfh cells in Roquinsan/san mice (n = 3). The numbers of CD4+ICOS+ CXCR5+PD1+ T follicular helper (Tfh) (A) cells and IL-17-, IFN-γ-, and IL-4-producing Tfh cells were analyzed by confocal microscopy. (B) Representative confocal micrographs of each mouse (n = 3). (C) IL-17+ Tfh, IFN-γ+ Tfh, and IL-4+ Tfh cells among splenocytes were analyzed by flow cytometry. Original magnification × 200. *p < 0.05.
IL-17 deficiency suppresses B-cell differentiation and GC formation in Roquinsan/san mice
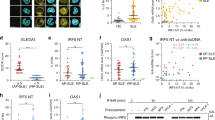
GC formation and plasma cell activation are hallmarks of SLE. Therefore, we investigated the effect of IL-17 deficiency on B cell differentiation and GC formation in spleen tissue from Roquinsan/san mice. GC formation, CD138+ plasma cell, and CD19+IgD+ mature B cell differentiation were reduced in Roquinsan/san/IL-17−/− mice, while formation of CD19+IgM+ immature B cells was increased (Fig. 5A). The numbers of B cells, GC, plasma cells, immature B cells, and mature B cells were determined by flow cytometry (Fig. 5B). These results suggest that IL-17 deficiency in Roquinsan/san mice influences B-cell populations and inhibits the differentiation of pathogenic plasma cells and mature B cells.
Effect of IL-17 deficiency on B-cell differentiation and germinal center (GC) formation in the spleen of Roquinsan/san mice (n = 3). (A) The numbers of B220-CD138+ plasma cells, B220+IgM+ immature B cells, and B220+IgD+ mature B cells were analyzed by confocal microscopy (Original magnification × 200 and × 400). (B) The numbers of plasma cells, immature B cells, mature B cells, CD21highCD23low MZB cells, and CD21midCD23high FOB cells were determined by flow cytometry. IL-17 deficiency resulted in a significantly increased number of immature B cells in Roquinsan/san mice. *p < 0.05.
Effect of IL-17 deficiency on regulatory B cells in the spleen of Roquinsan/san mice
The absence of regulatory B cells (Bregs) exacerbates pathologic inflammatory responses in autoimmune diseases. Therefore, we investigated the numbers of Bregs in IL-17-deficient Roquinsan/san mice. The numbers of CD19+IL-10+ Breg cells and CD19+CD1d+CD5+ Breg cells were increased in IL-17–deficient Roquinsan/san mice (Fig. 6A,B). We have shown the existence of IL-17–producing B cells in Roquinsan/san mice. These results suggest that IL-17+ B cells and Breg cells exert opposite effects on the autoimmune response in SLE.
Effect of IL-17 deficiency on the numbers of IL-10-producing B cells and CD19+CD1d+CD5+ regulatory B cells (Bregs) in the spleen of Roquinsan/san mice (n = 3). (A) IL-10-producing B cells and Foxp3+ Bregs were analyzed by confocal microscopy. The number of IL-10-producing B cells was significantly increased in Roquinsan/san/IL-17−/− mice. (B) The number of CD19+ CD1d+ CD5+ regulatory Bregs as determined by flow cytometry. Original magnification × 400. *p < 0.05.
Discussion
Although IL-17 is associated with the pathogenesis of SLE, and Roquinsan/san is related to the SLE phenotype, the relationship between IL-17 and Roquinsan/san is unclear. SLE is characterized by systemic inflammation and overproduction of proinflammatory cytokines, including IL-176,7,8. However, little information is recognized about the interaction of IL-17 and Roquinsan/san in T and B cells. Our results suggest that IL-17 deficiency in Roquinsan/san mice results in an increased number of IL-17-producing T and B cells, improvement of nephritis, and amelioration of the inflammatory response. Moreover, Roquinsan/san induced IL-17 expression in T and B cells. To our knowledge, this is the first report of increased IL-17 expression in T and B cells from Roquinsan/san mice. On the other hand, loss of IL-17 in Roquinsan/san mice ameliorated nephritis that is characteristic of SLE. Notably, IL-17 deficiency reduced the severity of inflammation in Roquinsan/san mice. This observation can elucidate IL-17 function related with inappropriate immune inflammation in SLE.
Dysregulation of IL-17 and Tfh cells is related to the pathogenesis of SLE. Differentiation of IL-17-producing Tfh cells was enhanced in BXD2 mice, which may be related to the development of SLE20. Moreover, Roquinsan/san mice exhibit an accumulation of Tfh cells and develop SLE21,22. Although suppression of IFN-γ production by Th1 cells reduces the severity of SLE in Roquinsan/san mice23, IL-17 expression in Tfh cells was not investigated. In this study, IL-17 deficiency in Roquinsan/san mice resulted in reduced numbers of IL-17-producing Tfh cells, leading to improvement of nephritis. These results suggest that IL-17 inhibition could be a therapeutic strategy in SLE.
AutoAb production and B-cell activation are related to the pathogenesis of SLE. B-cell activation leads to autoAb secretion24. Indeed, SLE is characterized by B-cell differentiation to plasma cells25. Moreover, the serum autoAb level is elevated in SLE patients26. B cell-targeted therapy involving inhibition of B-cell activation has been proposed27. In this study, IL-17 deficiency reduced B-cell differentiation and GC formation in Roquinsan/san mice. Serum IgG, IgG1, and IgG3 levels were decreased by IL-17 deficiency in Roquinsan/san mice. These results suggest that IL-17 has promise as a target for the development of novel therapeutics in SLE.
Because SLE is an inflammatory autoimmune disease24, Tregs and IL-10 are important factors in its treatment. Indeed, the frequency of CD4+CD25highFoxP3+ Tregs is decreased in SLE patients28,29. IL-10 is produced as an effector molecule by Tregs, and IL-10 receptor expression was reduced in a mouse model of SLE30,31. In this study, IL-17 deficiency enhanced Treg differentiation and IL-10 production by effector T cells in Roquinsan/san mice. Furthermore, IL-17 deficiency reduced the severity of SLE by increasing the number of Treg cells and the production of IL-10.
The effect of Roquin mutation on IL-17 production has to date been unclear. Our results provide insight into the role of IL-17 in the pathogenesis of SLE: Roquin mutation increased the expression of IL-17 in T and B cells. Thus, IL-17 can be considered a therapeutic target for SLE.
Materials and Methods
Ethics statement
The Animal Care Committee of The Catholic University of Korea approved the experimental protocol. All experimental procedures were evaluated and carried out in accordance with the protocols approved by the Animal Research Ethics Committee at the Catholic University of Korea (ID number:CUMC-2014-0103-03). All procedures performed followed the ethical guidelines on animal use.
Animals
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA), Roquinsan/san mice (Jackson Laboratory), and Roquinsan/san/IL-17−/− mice aged 15–20 weeks were maintained in groups (n = 9 per group) in polycarbonate cages in a specific pathogen-free environment. IL-17 KO mice were obtained from Dr. Y. Iwakura (University of Tokyo, Tokyo, Japan). Roquinsan/san mice were backcrossed with IL-17 knockout mice over 10 generations, and the mice were selected by genotyping PCR. The mice were provided with ad libitum access to mouse chow (Ralston Purina, St. Louis, MO, USA) and water.
Measurement of immunoglobulin (Ig) and IL-17 concentrations
Serum concentrations of IgG, IgG1 and IgG3 were measured using mouse IgG, IgG1 and IgG3 enzyme-linked immunosorbent assay (ELISA) quantitation kits (Bethyl Laboratories, Montgomery, TX, USA). The serum IL-17 level was measured using an IL-17 DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA).
Flow cytometry analysis of T, B and TFH cell populations
Splenocytes were isolated from the spleens of 15–20-week-old C57BL/6 and Roquinsan/san mice. B- and T-cell populations were identified using specific antibodies (eBioscience; San Diego, CA, USA). The 5 × 105 T cells or B cells were stimulated for 4 h ex vivo with PMA (25 ng/ml, Sigma-Aldrich, St Louis, MO) and ionomycin (250 ng/ml, Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences, Sparks, MD). To examine B-cell populations, splenocytes were stained with anti-B220- allophycocyanin (APC), anti-CD21-fluorescein isothiocyanate (FITC), anti-CD23- phycoerythrin (PE), anti-CD138-PE, anti-IgD-FITC, anti-IgM-PE, anti-CD1D-PE, anti- CD19-peridinin chlorophyll protein complex (PerCP), and anti-IL-17-PE antibodies. To analyze T helper and regulatory T (Treg) populations, splenocytes were stained with anti-CD4-PerCP, anti-IL-17-PE, anti-IL-4-PE, anti-interferon (IFN)-γ-FITC, anti-CD25-APC, and anti-Foxp3-PE antibodies. To analyze T follicular helper (TFH) cell populations, splenocytes were stained with anti-CD4-eFluor450, anti-CXCR5-PerCP-eFluor 710, anti-PD1-FTIC, anti-ICOS-PE cyanine7, anti-BCL6-APC, anti-IFN-γ-PE, anti-IL-4-PE, anti-IL-17-PE and anti-Foxp3-PE antibodies (Thermo Fisher Scientific, Waltham, MA). Flow cytometry was performed using a CytoFLEX flow cytometer (Beckman Coulter, IN, USA). The expressions of cell population were analyzed by CytExpert 2.3 software.
Confocal microscopy
Spleen tissues were obtained from mice at 15–20 weeks after primary immunization, and the B- and T-cell populations of interest were identified using the following specific antibodies (eBioscience): anti-B220-APC, anti-GL7-FITC (or PerCP), anti-CXCR5-PerCP, anti-CD138-PE, anti-ICOS-PE (or APC), anti-IgD-FITC, anti-IgM-PE, anti-CD19-PE (or PerCP), anti-CD5-FITC, anti-IL-10-FITC, anti-Foxp3-PE, anti-CD4-PerCP (or FITC, PE), anti-CD25-APC, anti-Foxp3-FITC (or APC), anti-IL-17-PE, anti-IL-4-PE (or FITC), and anti-IFN-γ-FITC (or PE). Stained sections were visualized by confocal microscopy (LSM 510 Meta, Carl Zeiss, Oberkochen, Germany). The expression of GC or plasma B cells were estimated by comparing the mean fluorescence intensity using LSM 510 Meta, Carl Zeiss software.
Immunohistopathological analysis of kidney
Mouse kidney tissues were fixed in 4% paraformaldehyde, decalcified in ethylenediaminetetraacetic acid (EDTA) bone decalcifier, and embedded in paraffin. Tissues were sectioned at 7 μm thickness, dewaxed using xylene, dehydrated through a gradient of alcohol, and stained with hematoxylin and eosin (H&E). The severity of the kidney pathology score was graded on a 0–4 scale as follows32: 0 = normal; 1, a small increase of cells in the glomerular mesanguim; 2, a larger number of cells in the mesangium; 3, glomerular lobular formation and thickened basement membrane; 4 glomerular crescent formation, sclerosis, tubular atrophy and casts.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20 for Windows (IBM Corp., Armonk, NY, USA). The significance of differences among multiple groups was evaluated by one-way analysis of variance (ANOVA); if a significant difference was detected, the Bonferroni post hoc test was used to assess the significance of differences between individual groups. Comparisons of numerical data were performed using the nonparametric Mann–Whitney U test (two-tailed). Values of p < 0.05 were considered indicative of statistical significance. Data are presented as means ± standard deviation (SD).
References
Tsokos, G. C. Systemic lupus erythematosus. The New England journal of medicine 365, 2110–2121, https://doi.org/10.1056/NEJMra1100359 (2011).
Sada, K. E. & Makino, H. Usefulness of ISN/RPS classification of lupus nephritis. Journal of Korean medical science 24, S7–10, https://doi.org/10.3346/jkms.2009.24.S1.S7 (2009).
Alarcon, G. S. et al. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 11, 95–101, https://doi.org/10.1191/0961203302lu155oa (2002).
Cervera, R. et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 82, 299–308, https://doi.org/10.1097/01.md.0000091181.93122.55 (2003).
Krishnan, E. & Hubert, H. B. Ethnicity and mortality from systemic lupus erythematosus in the US. Annals of the rheumatic diseases 65, 1500–1505, https://doi.org/10.1136/ard.2005.040907 (2006).
Umare, V. et al. Effect of proinflammatory cytokines (IL-6, TNF-alpha, and IL-1beta) on clinical manifestations in Indian SLE patients. Mediators of inflammation 2014, 385297, https://doi.org/10.1155/2014/385297 (2014).
Ouyang, W., Kolls, J. K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467, https://doi.org/10.1016/j.immuni.2008.03.004 (2008).
Yang, J. et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis and rheumatism 60, 1472–1483, https://doi.org/10.1002/art.24499 (2009).
Crispin, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. Journal of immunology 181, 8761–8766 (2008).
Wong, C. K. et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clinical immunology 127, 385–393, https://doi.org/10.1016/j.clim.2008.01.019 (2008).
Malissen, B. Revisiting the follicular helper T cell paradigm. Nature immunology 10, 371–372, https://doi.org/10.1038/ni0409-371 (2009).
Dong, W., Zhu, P., Wang, Y. & Wang, Z. Follicular helper T cells in systemic lupus erythematosus: a potential therapeutic target. Autoimmunity reviews 10, 299–304, https://doi.org/10.1016/j.autrev.2010.11.004 (2011).
Dong, G. et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chinese medical journal 116, 543–548 (2003).
Subbarayal, B., Chauhan, S. K., Di Zazzo, A. & Dana, R. IL-17 Augments B Cell Activation in Ocular Surface Autoimmunity. Journal of immunology 197, 3464–3470, https://doi.org/10.4049/jimmunol.1502641 (2016).
Wichner, K. et al. Dysregulated development of IL-17- and IL-21-expressing follicular helper T cells and increased germinal center formation in the absence of RORgammat. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 30, 761–774, https://doi.org/10.1096/fj.15-274001 (2016).
Linterman, M. A. et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity 30, 228–241, https://doi.org/10.1016/j.immuni.2008.12.015 (2009).
Yu, D. et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303, https://doi.org/10.1038/nature06253 (2007).
Vinuesa, C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458, https://doi.org/10.1038/nature03555 (2005).
Lee, S. Y. et al. Metformin Suppresses Systemic Autoimmunity in Roquin(san/san) Mice through Inhibiting B Cell Differentiation into Plasma Cells via Regulation of AMPK/mTOR/STAT3. J Immunol 198, 2661–2670, https://doi.org/10.4049/jimmunol.1403088 (2017).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature immunology 9, 166–175, https://doi.org/10.1038/ni1552 (2008).
Athanasopoulos, V. et al. The ROQUIN family of proteins localizes to stress granules via the ROQ domain and binds target mRNAs. The FEBS journal 277, 2109–2127, https://doi.org/10.1111/j.1742-4658.2010.07628.x (2010).
Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. The Journal of experimental medicine 206, 561–576, https://doi.org/10.1084/jem.20081886 (2009).
Lee, S. K. et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 37, 880–892, https://doi.org/10.1016/j.immuni.2012.10.010 (2012).
Dorner, T., Giesecke, C. & Lipsky, P. E. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 13, 243, https://doi.org/10.1186/ar3433 (2011).
Grammer, A. C. & Lipsky, P. E. B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther 5(Suppl 4), S22–27, https://doi.org/10.1186/ar1009 (2003).
Han, S., Zhuang, H., Shumyak, S., Yang, L. & Reeves, W. H. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol 6, 228, https://doi.org/10.3389/fimmu.2015.00228 (2015).
Md Yusof, M. Y., Vital, E. M. & Emery, P. B-cell-targeted therapies in systemic lupus erythematosus and ANCA-associated vasculitis: current progress. Expert Rev Clin Immunol 9, 761–772, https://doi.org/10.1586/1744666X.2013.816479 (2013).
Sharabi, A. & Mozes, E. Harnessing regulatory T cells for the therapy of lupus and other autoimmune diseases. Immunotherapy 1, 385–401, https://doi.org/10.2217/imt.09.2 (2009).
Suen, J. L. & Chiang, B. L. CD4(+)FoxP3(+) regulatory T-cells in human systemic lupus erythematosus. J Formos Med Assoc 111, 465–470, https://doi.org/10.1016/j.jfma.2012.05.013 (2012).
Shevach, E. M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2, 389–400, https://doi.org/10.1038/nri821 (2002).
Wilhelm, A. J., Rhoads, J. P., Wade, N. S. & Major, A. S. Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr−/− mice. Annals of the rheumatic diseases 74, 778–785, https://doi.org/10.1136/annrheumdis-2013-203759 (2015).
Nicoletti, F. et al. Dichotomic effects of IFN-gamma on the development of systemic lupus erythematosus-like syndrome in MRL-lpr/lpr mice. European journal of immunology 30, 438–447, https://doi.org/10.1002/1521-4141 (2000).
Acknowledgements
We thank the Institutional Animal Care and Use Committee, School of Medicine, Catholic University of Korea, for help with animal care and study. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2B3007688), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A2A01051677).
Author information
Authors and Affiliations
Contributions
S.Y.L., S.H.P. and M.L.C. designed and performed most of the experiment and analyzed data; S.Y.L. and S.H.L. wrote the manuscript; H.B.S., J.G.R. and J.H.Y. performed mice in vivo experiments and analyzed data; K.A.J. and J.W.C. performed confocal and immunochemistry of mice tissue; J.Y.J., J.S.P., J.Y.K. and S.K.K. performed genotyping of Roquin mutant and IL-17 KO mice and breeding and care.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, Sy., Lee, S.H., Seo, HB. et al. Inhibition of IL-17 ameliorates systemic lupus erythematosus in Roquinsan/san mice through regulating the balance of TFH cells, GC B cells, Treg and Breg. Sci Rep 9, 5227 (2019). https://doi.org/10.1038/s41598-019-41534-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41534-1
This article is cited by
-
The star target in SLE: IL-17
Inflammation Research (2023)
-
Norcantharidin ameliorates the development of murine lupus via inhibiting the generation of IL-17 producing cells
Acta Pharmacologica Sinica (2022)
-
Dysregulation of immunity in COVID-19 and SLE
Inflammopharmacology (2022)
-
Molecular biochemical aspects of salt (sodium chloride) in inflammation and immune response with reference to hypertension and type 2 diabetes mellitus
Lipids in Health and Disease (2021)
-
Roquin1 inhibits the proliferation of breast cancer cells by inducing G1/S cell cycle arrest via selectively destabilizing the mRNAs of cell cycle–promoting genes
Journal of Experimental & Clinical Cancer Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.