Abstract
Climate warming is a major cause of the global decline of coral reefs. Active reef restoration, although still in its infancy, is one of several possible ways to help restore coral cover and reef ecosystem function. The deployment of mature coral larvae onto depauperate reef substratum has been shown to significantly increase larval recruitment, providing a novel option for the delivery of ex situ bred coral stock to the reef for restoration purposes. The success of such reef restoration approaches may be improved by the use of coral larval stock augmented for climate resilience. Here we explore whether coral climate resilience can be enhanced via interspecific hybridization through hybrid vigour. Firstly, we assessed cross-fertility of four pairs of Acropora species from the Great Barrier Reef. Temporal isolation in gamete release between the Acropora species was limited, but gametic incompatibility was present with varying strength between species pairs and depending on the direction of the hybrid crosses. We subsequently examined the fitness of hybrid and purebred larvae under heat stress by comparing their survival and settlement success throughout 10 days of exposure to 28 °C, 29.5 °C and 31 °C. Fitness of the majority of Acropora hybrid larvae was similar to that of the purebred larvae of both parental species, and in some instances it was higher than that of the purebred larvae of one of the parental species. Lower hybrid fertilization success did not affect larval fitness. These findings indicate that high hybrid fitness can be achieved after overcoming partial prezygotic barriers, and that interspecific hybridization may be a tool to enhance coral recruitment and climate resilience.
Similar content being viewed by others
Introduction
Elevated seawater temperatures, especially when above an organism’s thermal optimum, have well-documented adverse effects on marine organisms. Since 1985, coral reefs worldwide have been warming at a rate distinctly higher than the ocean average, at approximately 0.2 °C per decade1. Many corals live near their upper thermal tolerance limit2, and ocean warming is therefore detrimental to them. As for coral larvae, elevated seawater temperature is known to negatively affect their development, survival and settlement3,4,5, and larval thermal tolerance can cause a bottleneck to reef recruitment3,6,7,8. For coral recruits and adults, elevated seawater temperature can cause coral bleaching, where the symbiotic relationship between the coral host and its dinoflagellate endosymbionts (Symbiodiniaceae) is disrupted, often resulting in coral mortality9. In the last three decades, higher-than-usual seawater temperatures caused by global warming have resulted in multiple mass beaching events on coral reefs worldwide, including in 1998, 2010 and 2014–20171,10. On the Great Barrier Reef (GBR), 30% coral mortality was recorded after the 2016 mass bleaching event, and a further 20% mortality was recorded following the 2017 mass bleaching event11. Recent estimates suggest more than 50% of the world’s coral reefs have been lost since the 1980s, and areas such as the Caribbean, Kiritimati, and certain parts of Japan have lost more than 80% of their coral10,12. This loss of corals directly threatens the extraordinary diversity of marine life dependent on reefs, as well as the goods and services reefs provide and that support millions of people13,14.
Active restoration is one possible way to restore coral cover, ecosystem function and socio-economical values of degraded coral reefs. Although current restoration attempts have not yet succeeded at a scale that can reverse global coral loss, several promising advances have been made15,16,17,18,19,20. For example, dela Cruz and Harrison20 have shown that the deployment of mature Acropora larvae into large scale mesh enclosures attached to the reef substratum can re-establish a breeding population of Acropora tenuis in three years’ time. Both larval recruitment rates and the number of surviving Acropora colonies two years after larval deployment were significantly higher at the reseeded sites compared to the control sites20. The process of coral recruitment involves the supply of larvae, the survival and settlement of these larvae, as well as post-settlement survival of the recruits8,21. The success of interventions such as those by Heyward et al.15 and dela Cruz and Harrison20 may be further improved through the use of climate resilient coral stock.
Climate resilient coral stock can potentially be produced via hybrid vigour generated from interspecific hybridization22,23. The benefits of hybridization have been documented extensively in commercial crops for traits of economic interest, such as yield, and disease and drought tolerance24,25. Hybridization creates new gene combinations and increases genetic diversity, which enhances the adaptive potential of species and their prospects of survival under environmental changes22,26,27,28,29, facilitates their expansion into new environments27,30,31,32 and breaks genetic correlations that constrain the evolvability of parental species33. For example, hybridization has led to variation in beak morphology necessary to survive environmental change in Darwin’s finches34, altered chemical defense systems in brassicaceae plants and assisted their survival through the Last Glacial Maximum27, and facilitated large scale adaptive radiation in haplochromine cichlid fishes33. Fitness of the first generation (F1) hybrid relative to its parental species depends on whether the gene effect is dominant (i.e., hybrid fitness is equivalent to the dominant parent, who can either be the more fit or less fit parent), additive (i.e., hybrid fitness is higher than one parent but lower than the other), over-dominant (i.e., hybrid fitness is higher than that of both parents), and under-dominant (i.e., hybrid fitness is lower than that of both parents)25,35,36. The scenarios where hybrids are more fit than both parents, or at least more fit than one parent are relevant for coral reef restoration.
Although the use of hybridization in conservation is limited, existing examples have demonstrated that it can rescue small, inbred populations from extinction (i.e., genetic rescue)28,37. These examples include the highly threatened species of Florida panther38, the Norfolk Island boobook owl39 and the Mt. Buller mountain pygmy-possum40. Several examples have demonstrated that interspecific hybrid corals likely represent useful stock for use in reef restoration23,30,31. Acropora prolifera, for example, the natural interspecific hybrid of A. cervicornis and A. palmata in the Caribbean, has been shown to have equivalent or higher fitness in multiple life history stages and phenotypic traits compared to the parental purebred species41. Similar observations were found in experimentally produced hybrids between Acropora species. Chan et al.42 showed that certain hybrid offspring survived better and grew faster compared to purebred offspring under ambient and elevated temperature and pCO2 conditions. Willis et al.30 reported that hybrid offspring grew faster than purebred offspring in the reef-flat environment. These examples suggest that hybrid colonies of Acropora are often more resilient than purebred colonies, and may represent a superior stock for reseeding of damaged reefs.
The aim of this study was to investigate whether the high hybrid fitness of Acropora recruits and juvenile colonies is also observed in the larval stages. To achieve this aim, we examined four experimentally crossed pairs of Acropora spp. from the GBR using seven parental species, and asked whether hybrid Acropora larvae have enhanced survival and settlement success compared to purebred larvae under ambient and elevated temperatures. As a secondary aim, we examined the extent of temporal reproductive isolation and gametic incompatibility in the four interspecific crosses of Acropora species.
Results
Spawning date and time
There were differences in the spawning date and time of the seven Acropora spp. from the central GBR that were used in this study (Fig. 1, Supplementary Table S1). A. tenuis, A. loripes and A. sarmentosa spawned on the earlier days after full moon (i.e., 3–8 days) and also spawned earlier in time (i.e., 19:10–21:40), whereas A. florida, A. hyacinthus, A. nobilis and A. cytherea spawned on the later days after full moon (i.e., 8–11 days) and also later in the evening (i.e., 21:35–22:15). As previously reported in the literature23, A. tenuis spawned at a distinctly earlier time (i.e., ~19:30) compared to the other species (i.e., ~20:30–22:15). Colonies of A. loripes, A. florida, A. hyacinthus, A. nobilis and A. cytherea all spawned within a narrow 45-minute window. Most species spawned for several consecutive days and overlapped with other species, except for A. nobilis where all colonies spawned on the 9th day after the full moon. Most species spawned within 0.5–1.5 h since setting was observed, with the exception of A. loripes, which spawned between 2 and 2.5 h since setting was observed.
Fertilization rates
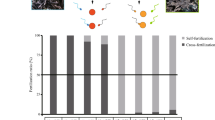
Fertilization rates, measured 2.5 h after the mixing of sperm and eggs, were high for purebreds (i.e., 75–100%), and low to moderate for hybrids (i.e., 0–68%) (Fig. 2). Interspecific hybridization was successful in three out of the four Acropora crosses, namely (1) the A. tenuis x A. loripes cross (Fig. 2a), (2) the A. florida x A. nobilis cross (Fig. 2b), and (3) the A. hyacinthus x A cytherea cross (Fig. 2c). For these successful crosses, hybrid fertilization was only observed in one direction (i.e., eggs from parent 1 were cross-fertile with sperm from parent 2, but the reciprocal cross was unsuccessful). These included TL (65–68%), FN (9–12% and HC 24–31%) (Fig. 2). Hybrid crosses in the other direction (i.e., LT, NF and CH) showed no fertilization (i.e., 0–0.3%). For the A. tenuis x A. sarmentosa cross, hybrid crosses failed in both directions (Fig. 2d) and this cross was thus excluded from the temperature stress experiment.
Fertilization rates for the four species pairs. (a) the A. tenuis (T) x A. loripes (L) cross, (b) the A. florida (F) x A. nobilis (N) cross, (c) the A. hyacinthus (H) x A. cytherea (C) cross, and (d) the A. tenuis (T) x A. sarmentosa cross (S). The first letter in the designation of the offspring groups represents its maternal parent species and the second letter its paternal parent species. Values are mean and error bars represent 95% CI calculated using the angular transformed data back-transformed into percentages.
Larval survival of the offspring groups from the A. tenuis (T) x A. loripes (L) cross, the A. florida (F) x A. nobilis (N) cross, and the A. hyacinthus (H) x A. cytherea (C) cross at (a) 28 °C, (b) 29.5 °C and (c) 31 °C. The first letter in the designation of the offspring groups represents its maternal parent species and the second letter its paternal parent species. Values are mean and error bars represent 95% CI calculated using the angular transformed data back-transformed into percentages. *Indicates significantly higher survival (i.e., p < 0.05) of this offspring group compared to the offspring group(s) indicated, or the same offspring group under the temperature treatment indicated.
Larval survival
Survival of hybrid larvae, measured at day seven since treatment commenced, was equivalent to or higher than that of at least one parental purebred species in most cases. Out of the nine species and temperature combinations, hybrid survival was equivalent to both parents in three cases, the same as the more fit parent in four cases, higher than both parents in one case and same as the less fit parent in one case (Fig. 3, Table 1). There was no instance where hybrid survival was lower than both parents (Fig. 3, Table 1). Offspring group (i.e., the specific hybrid or purebred offspring resulting from a cross, see caption of Fig. 3) had a substantial effect on larval survival, but treatment had very limited effects. For the A. tenuis x A. loripes cross (Fig. 3, Supplementary Table S2), neither offspring group nor treatment affected larval survival. For the A. florida x A. nobilis cross (Fig. 3, Supplementary Table S3), purebred FF had higher survival than NN and hybrid FN at 28 °C (p = 0.030 for both). At 29.5 °C and 31 °C, however, survival of both the hybrid FN and purebred offspring FF was higher than that of NN (29.5 °C: p = 0.002, <0.001 respectively; 31 °C: p < 0.001 for both). For FF and NN, survival within an offspring group was not different between treatments. However, survival of hybrid FN under 29.5 °C was higher than under 28 °C (p = 0.01). For the A. hyacinthus x A cytherea cross (Fig. 3, Supplementary Table S4), survival of hybrid HC and purebred HH was higher than that of purebred CC at all three temperatures (p < 0.001 for all pairs). At 28 °C, hybrid HC also had higher survival than purebred HH (p = 0.028). For HH and CC, survival within an offspring group was unaffected by treatment. However, survival of hybrid HC at 28 °C and 29.5 °C was higher than at 31 °C (p = 0.028, 0.047 respectively). The results of an overall comparison of hybrid vs. purebred larval survival are shown in Supplementary Table S8.
Larval settlement
The larval settlement results, assessed two days after the introduction of the settlement cue, were consistent with the survival results. The majority of the hybrid larvae had settlement rates either similar to those of purebred larvae of both parental species or higher than those of purebred larvae of one parental species. Out of the nine species and temperature combinations, hybrid settlement was the same as that of purebred larvae of both parental species in five cases, more fit than purebred larvae of one parental species in three cases, and the same as that of the less fit purebred larvae of one of the parental species in one case (Fig. 4, Table 1). In the cases of the FN cross at 29.5 °C and 31 °C, settlement of hybrid FN was higher than the less fit purebred NN larvae, but lower than the more fit purebred FF larvae (i.e. additive gene effect) (Fig. 4, Table 1). In none of the cases, hybrid settlement success was lower than both parents (Fig. 4, Table 1). Offspring group (i.e., the specific hybrid or purebred offspring resulting from a cross, see caption of Fig. 4) had a substantial effect on settlement, yet treatment had very limited effect. For the A. tenuis x A. loripes cross (Fig. 4, Supplementary Table S5), larval settlement was not affected by offspring group or treatment. For the A. florida x A. nobilis cross (Fig. 4, Supplementary Table S6), the hybrid FN had a higher proportion of settled larvae than the purebred NN at 29.5 °C and 31 °C (p = 0.005, 0.008 respectively). Purebred FF also had higher settlement rates than NN, as well as FN, at all temperatures (p < 0.001 for all pairs). Treatment did not affect settlement of FN and FF, however, settlement of NN at 31 °C was significantly lower than at 28 °C (p = 0.043). For the A. hyacinthus x A cytherea cross (Fig. 4, Supplementary Table S7), the settlement rate of the hybrid HC was higher than that of the purebred CC at 31 °C (p = 0.004). For all other comparisons in this cross, settlement did not differ between offspring groups or temperatures. Abnormal settlement behavior (i.e., metamorphosis without settlement cue and without attachment to a substrate) was frequently observed in the purebred CC at 29.5 °C and 31 °C. Such behavior was not observed in the hybrid HC or the other purebred FF. The results of an overall comparison of hybrid vs. purebred larval settlement rates are shown in Supplementary Table S8.
Larval settlement of the offspring groups from the A. tenuis (T) x A. loripes (L) cross, the A. florida (F) x A. nobilis (N) cross, the A. hyacinthus (H) x A. cytherea (C) cross at (a) 28 °C, (b) 29.5 °C and (c) 31 °C. The first letter in the designation of the offspring groups represents the origin of egg and the second letter the origin of sperm. Values are mean and error bars represent 95% CI calculated using the angular transformed data back-transformed into percentages. *Indicates significantly higher survival (i.e. p < 0.05) of this offspring group compared to the offspring group (s) indicated, or the same offspring group under the temperature treatment indicated.
Seawater chemistry
Experimental conditions of the treatment are summarized in Table 2. Treatment temperatures were maintained at 28.1 °C ± 0.2, 29.5 °C ± 0.1 and 31.0 °C ± 0.2. O2 levels of the seawater removed from the wells ranged from 95.8 to 96.6%, indicating that the seawater remained well oxygenated throughout the experiment.
Discussion
For sympatric broadcast spawning corals, temporal isolation and gametic incompatibility are two possible mechanisms that preclude interspecific hybridization in the wild43,44. Since considerable overlap in spawning date and time was observed for all but one species pairs in this study, temporal isolation is unlikely an effective prezygotic barrier. Similar observations have been reported for other Acropora spp.44,45,46 and Platygyra spp.47 from the GBR. However, one well-documented temporal isolation in Acropora spp. is that between the ‘early spawners’ and the ‘late spawners’ which are separated by about 1.5–3 h in the timing of gamete release48,49,50. The ‘early spawners’ are represented by only three species49,50. Relative to the 120–140 extant Acropora species, the existence of temporal isolation in this small group is not representative of the whole genus. Similar to the observation in Chan et al.42, the ‘early spawner’ A. tenuis spawned at a distinctly earlier time than all other species yet its gametes were compatible with a ‘late spawner’, A. loripes. Similarly, the Caribbean corals Orbicella franksi and Orbicella annularis have 2 h separation in spawning time but their gametes are compatible51. In both cases, a prezygotic barrier in the form of gametic incompatibility may not have evolved as the gametes are unlikely to encounter one another in nature.
Although temporal isolation was limited, gametic incompatibility was observed and its strength varied between species pairs and the direction of the hybrid cross. Fertilization rates were low to moderate in hybrid offspring groups and hybridization was only possible in one direction (i.e., asymmetric gametic incompatibility). Species-specific gametic incompatibility has previously been reported in experimental crossing of Acropora spp. Among 38 species pairs of Acropora from central GBR, eight pairs yielded high interspecific fertilization (50–80%), seven pairs had moderate fertilization (10–50%), three pairs had low fertilization (3–10%), and the remaining pairs were not cross-fertile52,53. Note that the fertilization rates within a species pair cross were highly variably with SDs ranging from 0 to 50%52,53. Experimental crosses of five Acropora species pairs from Okinawa (Japan) resulted in low interspecific fertilization (i.e., <2%) in all crosses, except the A. formosa x A. nasuta cross (i.e., 95%)54. Chan et al.42 reported high fertilization success (i.e., averaged 93%) in hybrids of both directions from A. tenuis x A. loripes and A. sarmentosa x A. florida crosses. Asymmetric gametic incompatibility as observed in this study is, however, not uncommon in Acropora spp. and has been reported in Hatta et al. (i.e., 40% vs. 95% in A. formosa x A nasuta cross)54, Fogarty et al. (i.e., 5–12% vs. 55–70% in A. palmata x A cervicornis cross)44 and Isomura et al. (i.e., 34% vs. 64% in A. florida x A. nobilis cross)55. Note that A. intermedia mentioned in Isomura et al.55 is the same species as A. nobilis. Many other taxa such as sea urchins56, mosquitoes57, tuna58, oak59 and walnut tree60 are also known to show asymmetric gametic incompatibility.
One possible explanation for the observed difference in gametic incompatibility is interspecific differences in gamete-recognition proteins, receptors and molecules. Gamete-recognition proteins can affect fertilization success within species61,62,63,64, as well as the extent of reproductive isolation between species43,65. Sperm proteins, such as bindin in sea urchin and sea star, and lysin in abalone, provide species-specific binding of sperm to egg and play an important role in reproductive isolation between species43,54,66,67. Bindin, for example, is a sperm protein in sea urchins that coats the acrosome of the sperm, binds sperm to the vitelline envelope of the egg, and facilitates the fusion of sperm and egg membranes68,69. Interspecific differences in bindin can result in failure of one or all of these processes, preventing fertilization from occurring70. In sea urchins, divergence in bindin amino acid sequence can predict gamete compatibility between species, and species with less than 1% difference in sequence are fully compatible43.
The complementary receptor on the egg surface (e.g., VERL in mollusk and EBR1 in echinoderms) mediates species-specific sperm adhesion and also plays a role in reproductive isolation71,72. The receptor, however, has been much less studied due to its relatively large size compared to the sperm protein (e.g., ~4595 amino acids in the bindin receptor EBR1 compared to 200–300 amino acids in bindin)66. Other than gamete-recognition proteins and receptors, species-specific diffusible molecules from the egg can also affect compatibility between species73. Eggs of marine invertebrates are known to produce diffusible chemo-attractants (e.g. ‘sperm-activating peptides’) that activate and attract sperm to swim toward the egg73,74,75,76. Abalone sperm, for example, has been shown to only respond to chemo-attractants from conspecific eggs77. To date, however, little is known about gamete recognition proteins and chemo-attractants in coral.
Gametic incompatibility can also vary between colonies of the same species and between locations. Hatta et al.54 and Isomura et al.55 reported interspecific fertilization rates ranged from 4–76% and 3–99% respectively between different Acropora colonies of the same species. Colonies of the A. tenuis x A. loripes cross in this study are from the same reef location as those in Chan et al.42 and crossed using similar methods. However, Chan et al.42 reported a hybridization rate of 79–95% in contrast to the 0–67% observed here. Experimental crossing of A. florida x A. nobilis yielded a fertilization rate of 34–64% in colonies from Okinawa Japan55, but the same cross in this study had a fertilization rate of only 0.3–10%. Further, hybridization between M. franksi and M. annularis was possible in both directions in Panama but was only possible in one direction in Bahamas51. We speculate that gametic incompatibilities can vary between genotypes of the same species, that minor differences in gamete-recognition proteins, receptors, and diffusible molecules associated with the gametes can exist between colonies of the same species, and that these are responsible for the variation in interspecific fertilization observed in these and our studies. For example, sperm from different individuals of the same sea urchin species has been shown to vary in chemotaxis (i.e., the ability to navigate toward the egg using chemical signals), which was demonstrated to influence individual fertilization success78.
Although prezygotic barriers in the form of gametic incompatibility were observed, the majority of the hybrid offspring groups were either as fit as or more fit than one of the parental purebred offspring groups, which had higher fertilization rates. This is a common phenomenon in Acropora species. Hybrid larvae from an A. florida x A. nobilis cross showed higher survival than purebred larvae at 5–8 days after fertilization, despite their low fertilization rate55. This is a critical time as Acropora larvae become competent for settlement and metamorphosis at about 5 days of age. High larval survival during the first week in life will thus result in a larger number of larvae that may settle. Similarly, survival of hybrid larvae and 6-week old hybrid recruits from an A. palmata x A. cervicornis cross was equivalent to that of purebreds despite lower hybrid fertilization rate41. The hybrids also had similar settlement rates compared to purebred larvae41.
We observed abnormal settlement behavior in the purebred offspring group CC under elevated temperatures, but not in the corresponding hybrid offspring group HC. Hybrid offspring that can settle normally under elevated temperatures is likely to have an advantage over some of the purebred offspring under climate change scenarios. Overall, existing evidence indicates that gametic incompatibility does not negatively affect hybrid fitness in Acropora corals, and the more resilient hybrid offspring may provide superior coral stock for coral reef restoration. Long term field and aquarium studies have shown higher survival and growth rate in some Acropora hybrids compared to purebreds23,30, suggesting that high hybrid fitness is not limited to the larvae but may also manifest in the later life stages.
Elevated seawater temperatures have well-documented negative effects on coral larvae in terms of larval development and motility (i.e., ciliary activity)4, survival3,4,5, settlement4, metamorphosis69, ability to establish symbiosis5,79, post-settlement mortality80,81, photosynthesis3, as well as respiration and rubisco protein expression74 (Table 3). Although we used treatment temperatures similar to those in the studies cited above, treatment had a limited effect on larval survival and settlement. Studies with short exposure times (i.e., 1 to 48 h) have also reported that elevated temperatures did not have a negatively impact on survival6,81, motility3, settlement, metamorphosis, photosynthesis and respiratory demand81, post-settlement mortality80, and positive effects on settlement of coral larvae was reported in some instances80,82 (Table 3). Most studies with longer exposure times (i.e., over 48 h), however, observed negative effects of elevated temperatures on coral larvae (Table 3). Randall and Szmant6 for example, showed that elevated temperatures did not affect larval survival after 48 h of exposure, but had a negative impact after 7 days of exposure. This is not the case in the present study where we used ten days of exposure time.
A possible explanation for the observed discrepancy may be the lower sensitivity of aposymbiotic larvae (i.e., without Symbiodiniaceae) to elevated temperatures compared to symbiotic larvae. The Acropora spp. used in the present study are broadcast spawners and their larvae are aposymbiotic. The majority of the relevant larval studies in the literature are from brooding species that release larvae already harbouring Symbiodiniaceae (Table 3). Symbiotic larvae are potentially more sensitive to elevated temperatures as they are exposed to reactive oxygen species (ROS) produced as by-products of photosynthesis3,83. Aposymbiotic larvae have been shown to have higher survival than their symbiotic counterparts of the same species under elevated temperatures83, possibly explaining the limited effect of temperature observed in our experiment.
Elevated temperatures may also have a delayed negative effect in later life stages that were not examined in this study. Latent negative responses to environmental stress have been documented in a variety of marine invertebrate larvae84. Nozawa and Harrison80 and Ross et al.81 showed that elevated temperatures had no or a positive effect on coral larvae initially, but were followed by high post-settlement mortality. In another coral species examined in the same experiment, however, post-settlement mortality was unaffected80. This hypothesis also does not hold for purebreds and hybrids of A. tenuis x A. loripes examined here, as Chan et al.42 showed that high hybrid fitness was consistently observed under seven months of exposure to elevated temperature and pCO2 conditions and no delayed negative effect was reported. Alternatively, pre-exposure to a stressor may result in preconditioning and enhance an organism’s tolerance to subsequent stress events22,85,86,87. Pre-exposure to elevated temperatures of the larvae from the present study may increase their tolerance to coral bleaching during subsequent temperature stress and possibly to a different extent in hybrid and purebred juveniles. Future longer-term studies investigating the impact of exposure of hybrid and purebred corals to sub-lethal stress on tolerance to a subsequent stress event will be invaluable.
Our findings on coral larvae show that high hybrid fitness can still be achieved after overcoming partial prezygotic barriers, and that interspecific hybridization has the potential to enhance coral recruitment and climate resilience. Although interspecific fertilization is lower than conspecific fertilization, mass-spawning corals are highly fecund and the number of larvae resulting from low or medium fertilization is still enormous. Experimental crossings of A. palmata and A. cervicornis showed low fertilization (i.e., 5–12%) in one hybrid direction44. Nonetheless, naturally produced hybrids of both directions are present on the reef88. The next important questions to investigate are whether these hybrid corals can persist in nature and continue to maintain high fitness in later generations. In the most ideal scenario, F1 hybrids are able to reproduce sexually via hybridization with other F1 hybrids and/or backcrossing with parental species. This process generates novel genotypes that are climate resilient, and high fitness may be maintained in advanced generation hybrids and backcrosses. In this case, the introduction of hybrids can bring large spatial and temporal scale benefits to the reef they are out-planted to and beyond.
Although knowledge on the reproductive potential of hybrid corals is currently limited, Isomura et al.32 have demonstrated that experimentally produced F1 hybrids of A. intermedia × A. florida were fertile and able to produce an F2 generation with high fertilization success (i.e. >80%). These hybrids were also able to backcross with either the maternal parental species only or with both parental species. Given the vast volume and great surface area of the ocean compared to laboratory conditions, the fertilization rates for F2 hybrids and backcrosses may be lower in the wild due to lower sperm concentrations44. Despite this, evidence of unidirectional gene flow from A. palmata into A. cervicornis in the Caribbean indicates that their hybrid A. prolifera is fertile and can successfully backcross with at least one parental species88,89.
In the case where hybrids have limited success in sexual reproduction, it is possible for hybrids to persist asexually. Fragmentation is a common way of asexual reproduction of mass spawning corals90,91. In the Caribbean, the hybrid A. prolifera is known to persist and spread across large reef areas through asexual reproduction91. The conservation benefits of this scenario is less than the former as the hybrids are not able to promote introgression of genes across the parental species or continue to generate novel genotypes. Nevertheless, Acropora corals are long-lived (up to 13–24 years for some species92) and F1 hybrid corals with high climate resilience may maintain ecosystem function and buy time for the reef while global warming is being addressed. In the least favorable scenario, hybrids are able to hybridize with other F1 hybrids and backcross with parental species, but hybrid breakdown (i.e., outbreeding depression) occurs in later generations. The occurrence of hybrid breakdown has been documented in certain species, although it is more commonly associated with the crossing of geographically or phenologically distant species37,93. If hybrid breakdown occurs, natural selection will likely remove the unfit genotypes29,94,95 and therefore prevent them from propagating further.
The development of novel interventions is becoming increasingly important to reef systems worldwide which are rapidly losing coral, genetic diversity and ecosystem function following multiple high mortality bleaching events. The efficacy of hybridization as a tool to produce coral stock for restoration purposes is supported by our earlier work, which demonstrated hybrid corals survived equally or better compared to purebreds and grew faster over a seven months period of exposure to ambient and elevated temperature and pCO2 conditions23. The next step towards safe implementation of this reef restoration intervention will be to assess F1 hybrid reproductive potential, and the fitness of F1 and advanced generation hybrids in controlled field trials.
Materials and Methods
Parental colony collection and in vitro fertilization
Parental colonies (5–11 for each species: Acropora tenuis, Acropora loripes, Acropora florida, Acropora nobilis, Acropora hyacinthus, Acropora cytherea and Acropora sarmentosa) were collected from Trunk Reef (18°35′S, 146°80′E), central GBR. Colonies were collected prior to the full moon on 14th Nov and held in flow-through aquaria of the National Sea Simulator (SeaSim) at the Australian Institute of Marine Science (AIMS) in Townsville, Australia. When signs of imminent spawning were observed (i.e., ‘setting’, where the egg-sperm bundles of a colony are pushed to the mouth of its polyps), colonies were isolated in individual aquaria to avoid unintentional mixing of gametes prior to experimental crossing. Egg-sperm bundles from the four or five most profusely spawning colonies of a species were collected and separated using a 100 µm filter. Eggs were washed three times with filtered seawater to remove any residual sperm and placed in an individual 3 L bowl until the egg-sperm separation step was completed for all targeted colonies (within 3 h).
Similar quantities of sperm (i.e., 107 sperm mL−1) were pooled from colonies of the same species to create a mixed sperm solution. For making the hybrid offspring, 300 mL of the pooled interspecific sperm solution was added to the eggs of each colony of the receiving species to achieve a final volume of 3 L and a sperm concentration of 106 sperm mL−1. There were four to five replicates for each direction of the hybrid crosses, and each replicate was a different colony. Fertilization was conducted separately for each colony to avoid unintended fertilization by sperm from other conspecific colonies that was not washed away (if any). Note that self-fertilization is uncommon in Acropora corals. For making the purebred offspring, eggs of the conspecific colonies were pooled and 1.1 L of the pooled conspecific sperm solution was added to achieve a final volume of 11 L and a sperm concentration of 106 sperm mL−1. There were two replicates of each purebred cross. We considered two replicates sufficient as each replicate received the same mixed eggs and sperm solution and the containers themselves were unlikely to have an effect on fertilization success. Fertilization was conducted under ambient conditions and fertilization rates were assessed at 2.5 h after introduction of the sperm.
Four species pair crosses were carried out: (1) the A. tenuis x A. loripes cross, (2) the A. florida x A. nobilis cross, (3) the A. hyacinthus x A cytherea cross, and (4) the A. tenuis x A. sarmentosa cross. Four offspring groups were produced from each cross (i.e., two hybrid offspring groups and two purebred offspring groups, Fig. 5a). The four species pairs were selected to represent two phylogenetically divergent crosses (i.e., A. tenuis x A. loripes and A. tenuis x A. sarmentosa), and two phylogenetically closely related crosses (i.e., A. florida x A. nobilis and A. hyacinthus x A cytherea). The phylogeny of Acropora spp. consists of two distinct groups: the ‘early spawners’ and the ‘late spawners’, where the latter group spawns approximately 1.5–3 h earlier than the ‘early spawners’48,49,50. A. tenuis (early spawner) is phylogenetically divergent from loripes (late spawner) and A. sarmentosa (late spawner), while A. florida and A. nobilis, as well as A. hyacinthus and A cytherea are all late spawners and are closely related to their targeted breeding partner48,49,50. For the fertilization rate assessment, three samples of approximately 100 eggs of each offspring group taken at 2.5 h since the introduction of sperm were placed into 12-well plates and imaged using a high-resolution camera (Nikon D810). The numbers of fertilized/unfertilized embryos were visually counted. Three samples of approximately 100 eggs were also collected as self-fertilization and “no-sperm” controls in each cross conducted.
Illustrations showing the experimental setup. (a) The three successful Acropora spp. crosses (i.e., the A. tenuis (T) x A. loripes (L) cross, (2) the A. florida (F) x A. nobilis (N) cross, (3) the A. hyacinthus (H) x A. cytherea (C) cross, and the three resultant offspring groups of each cross used in the experiment, (b) a set of 6-well plates in each experimental tank with 3 × 10 larvae from each offspring group, and (c) the three temperature treatments (i.e., 28, 29.5, and 31 °C) with four replicate tanks each. The abbreviation of the offspring groups throughout this paper is that the first letter represents the origin of the eggs and the second letter the origin of sperm (e.g., TL is a hybrid crossing A. tenuis eggs with A. loripes sperm).
Little information is available from the literature about the relative resilience of these four parental species, but this has limited relevance for this study as our purpose was to increase genetic diversity (and thus adaptive potential) via hybridization, and not to conduct targeted breeding with species of known relative bleaching tolerance.
Temperature stress experiment
Coral larvae were reared under ambient conditions for five days until they reached the planula stage. They remained aposymbiotic (i.e., without Symbiodiniaceae) throughout the experiment. The A. tenuis x A. sarmentosa interspecific cross was unsuccessful (i.e., no fertilization occurred) and thus this species pair was excluded from the experiment. For the remaining three crosses, three offspring groups (i.e., one hybrid group and two purebred groups) of each cross were used for the heat stress experiment (Fig. 5a). Hybrids in one direction of each cross were excluded due to their low fertilization success (and thus low larval yields). Using a glass pipette, planula larvae of each offspring group were carefully transferred into 6-well plates and reared under 28 °C (i.e., mean annual temperature at Davies Reef over the period 1991–2016, proximal to Trunk Reef), 29.5 °C (i.e., mean summer maximum at Davies Reef) and 31 °C (i.e., elevated temperature) (Fig. 5b). Temperatures followed the diurnal variation of 0.6 °C that typically occurs on Davies Reef and were ramped to the targeted temperatures at a rate of 0.5 °C per day. A total of 360 larvae of each of the nine offspring groups were loaded into 36 wells (i.e., 10 larvae per well) and randomly distributed among the 12 experimental tanks (Fig. 5b,c). In other words, each treatment had 120 larvae per offspring group that were distributed among 12 wells and among its four replicate tanks (Fig. 5b,c). The experimental tanks served as a water bath to maintain seawater temperatures inside the 6-well plates, and were also a seawater source for daily water change of the wells. Each treatment had four replicate tanks and five 6-well plates were placed in each tank (Fig. 5c). Positions of the tanks were randomized in the experimental room and positions of the 6-well plates were randomized within a tank (Fig. 5c).
After the larvae were transferred to the wells, the plates were covered with a lid to avoid evaporation, and floated in the treatment tanks to maintain their temperatures. Each day, 80% of the seawater of a well was exchanged using a transfer pipette. Dead or decomposing larvae that were observed during the water change were removed to maintain quality of the seawater inside the wells. Light was provided at 120 µE m−2 s−1 using Aquaillumination Hydra following the natural summer light/dark cycle.
Larval survival and settlement
Survival and settlement of the larvae were used as proxies for fitness. Larval survival was assessed under a dissecting microscope at day seven after treatment commenced. After the survival assessment, a crustose coralline algae (CCA) chip was introduced into each well to induce larval settlement. These CCA were collected from the same reef as the parental coral colonies and maintained in flow-through aquaria at the SeaSim. On the day of larval settlement, the CCA were cut into similarly sized chips (i.e., approximately 4 mm2) using a bone cutter. Larval settlement rates were assessed under a dissecting microscope two days after the CCA chips were introduced. A larva was counted as ‘settled’ when it was (1) attached to a substrate (i.e., either on the surface of the well or the CCA chip) and (2) was fully metamorphosed.
Statistical analysis
Statistical analyses were conducted separately for the A. tenuis x A. loripes cross, the A. florida x A. nobilis cross, and the A. hyacinthus x A cytherea cross using the raw data (n = 12 wells per offspring group per treatment). The response (i.e., larval survival or settlement) was treated as a binomial variable (i.e., survived/dead, settled/not settled) in the analyses. Generalized linear mixed models (GLMM)96 for binomial data with logistic link functions were used to test the effects of treatment and offspring group on larval survival and settlement. A random tank effect was incorporated into the models to account for possible tank effects. Models assumptions were checked visually, and models were assessed for overdispersion using a Chi-square test and goodness of fit using Akaike Information Criteria, and all of which were satisfactory. Tukey’s pairwise comparisons were then used to test for differences between treatment and offspring group and p-values of the pairwise comparisons were corrected using the Benjamini-Hochberg method. An overall comparison of hybrids vs. purebreds was also conducted using a GLMM. Statistical analyses were completed using R97 with packages lme4 and multcomp. For illustration purpose, mean values are shown in the figures with the error bars representing 95% CI calculated using the angular transformed data that were back-transformed into percentages.
Seawater chemistry
Automated controls of seawater temperatures were provided by SeaSim via the SCADA (Supervisory Control and Data Acquisition) system. Seawater temperature of each tank was monitored hourly using resistance temperature detector (RTD). To confirm the treatment conditions inside the 6-well plates (i.e., where the larvae were located), seawater that was removed from the wells during water change was collected for measurement of O2 level, salinity, temperature and pH every day at 12:00 using the HACH HQ40D Portable Multi Meter. Salinity measurements were calibrated with IAPSO Standard Seawater. Seawater from several wells of the same tank was combined for measurement due to depth requirement of the measurement probes. Total alkalinity (AT) was measured twice during the 10-day experiment using VINDTA calibrated to Dickson’s Certified Reference Material. Ωarag (aragonite saturation state) and DIC (dissolved inorganic carbon) were calculated using the measured values of seawater AT, pH, temperature and salinity, with the program CO2SYS98 as implemented in Microsoft Excel by Pierrot et al.99.
Data Availability
The datasets generated during the present study are publicly available via the Australian Institute of Marine Science data at: https://apps.aims.gov.au/metadata/view/69f17afe-378b-41a2-8c90-5fff3318898c.
References
Heron, S. F., Maynard, J. A., van Hooidonk, R. & Eakin, C. M. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci Rep 6 (2016).
Berkelmans, R. & Willis, B. L. Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228 (1999).
Edmunds, P., Gates, R. & Gleason, D. The biology of larvae from the reef coral Poritesastreoides, and their response to temperature disturbances. Marine Biology 139, 981–989 (2001).
Bassim, K. & Sammarco, P. Effects of temperature and ammonium on larval development and survivorship in a scleractinian coral (Diploriastrigosa). Marine Biology 142, 241–252 (2003).
Schnitzler, C. E., Hollingsworth, L. L., Krupp, D. A. & Weis, V. M. Elevated temperature impairs onset of symbiosis and reduces survivorship in larvae of the Hawaiian coral, Fungia scutaria. Mar Biol 159, 633–642 (2012).
Randall, C. J. & Szmant, A. M. Elevated Temperature Affects Development, Survivorship, and Settlement of the Elkhorn Coral, Acropora palmata (Lamarck 1816). Biol Bull 217, 269–282 (2009).
Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Marine Environmental Research 76, 3–15 (2012).
Putnam, H. M., Mayfield, A. B., Fan, T. Y., Chen, C. S. & Gates, R. D. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Mar Biol 160, 2157–2173 (2013).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshwater Res. 50, 839–866 (1999).
Eakin, C. et al. Global coral bleaching 2014–2017: status and an appeal for observations. Reef Encounter 20–26 (2016).
Ward, S. How the 2016 bleaching altered the shape of the northern Great Barrier Reef. The Conversation Available at, http://theconversation.com/how-the-2016-bleaching-altered-the-shape-of-the-northern-great-barrier-reef-95142 (Accessed: 18th July 2018).
50 Reefs. 50 Reefs Available at, https://50reefs.org/ (Accessed: 19th October 2017).
Burke, L., Reytar, K., Spalding, M. & Perry, A. Reefs at risk revisited (2011).
de Groot, R. et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosystem Services 1, 50–61 (2012).
Heyward, A. J., Smith, L. D., Rees, M. & Field, S. N. Enhancement of coral recruitment by in situ mass culture of coral larvae. Marine Ecology Progress Series 230, 113–118 (2002).
Omori, M. Degradation and restoration of coral reefs: Experience in Okinawa, Japan. Marine Biology Research 7, 3–12 (2011).
Nakamura, R. et al. Corals mass-cultured from eggs and transplanted as juveniles to their native, remote coral reef. Marine Ecology Progress Series 436, 161–168 (2011).
Villanueva, R. D., Baria, M. V. B. & Cruz, D. W. dela. Growth and survivorship of juvenile corals outplanted to degraded reef areas in Bolinao-Anda Reef Complex, Philippines. Marine Biology Research 8, 877–884 (2012).
Guest, J. R., Baria, M. V., Gomez, E. D., Heyward, A. J. & Edwards, A. J. Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs 33, 45–55 (2014).
dela Cruz, D. W. & Harrison, P. L. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Scientific Reports 7, 13985 (2017).
Ritson-Williams, R. et al. New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithsonian Contributions to the Marine Sciences (2009).
van Oppen, M. J. H., Oliver, J. K., Putnam, H. M. & Gates, R. D. Building coral reef resilience through assisted evolution. PNAS 112, 2307–2313 (2015).
Chan, W. Y., Peplow, L. M., Menéndez, P., Hoffmann, A. A. & Van Oppen, M. J. H. Interspecific Hybridization May Provide Novel Opportunities for Coral Reef Restoration. Frontiers in Marine Science in press (2017).
Fu, D. et al. Utilization of crop heterosis: a review. Euphytica 197, 161–173 (2014).
Lippman, Z. B. & Zamir, D. Heterosis: revisiting the magic. Trends in Genetics 23, 60–66 (2007).
Hoffmann, A. A. & Sgrò, C. M. Climate change and evolutionary adaptation. Nature 470, 479–485 (2011).
Becker, M. et al. Hybridization may facilitate in situ survival of endemic species through periods of climate change. Nature Clim. Change 3, 1039–1043 (2013).
Carlson, S. M., Cunningham, C. J. & Westley, P. A. H. Evolutionary rescue in a changing world. Trends in Ecology & Evolution 29, 521–530 (2014).
Hamilton, J. A. & Miller, J. M. Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conservation Biology 30, 33–41 (2016).
Willis, B. L., Oppen, M. J. H., van, Miller, D. J., Vollmer, S. V. & Ayre, D. J. The role of hybridization in the evolution of reef corals. Annual Review of Ecology, Evolution, and Systematics 37, 489–517 (2006).
Fogarty, N. Reproductive Isolation and Hybridization Dynamics in Threatened Caribbean Acroporid Corals. Oceanography Faculty Theses and Dissertations (2010).
Isomura, N., Iwao, K., Morita, M. & Fukami, H. Spawning and fertility of F1 hybrids of the coral genus Acropora in the Indo-Pacific. Coral Reefs 35, 851–855 (2016).
Meier, J. I. et al. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nature Communications 8, 14363 (2017).
Grant, P. R. & Grant, B. R. Conspecific versus heterospecific gene exchange between populations of Darwin’s finches. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365, 1065–1076 (2010).
Li, L. et al. Dominance, overdominance and epistasis condition the heterosis in two heterotic rice hybrids. Genetics 180, 1725–1742 (2008).
Chen, Z. J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet 14, 471–482 (2013).
Whiteley, A. R., Fitzpatrick, S. W., Funk, W. C. & Tallmon, D. A. Genetic rescue to the rescue. Trends in Ecology & Evolution 30, 42–49 (2015).
Johnson, W. E. et al. Genetic restoration of the Florida Panther. Science 329, 1641–1645 (2010).
Garnett, S. T., Olsen, P., Butchart, S. H. M. & Hoffmann, A. A. Did hybridization save the Norfolk Island boobook owl Ninox novaeseelandiae undulata? Oryx 45, 500–504 (2011).
Weeks, A. R. et al. Genetic rescue increases fitness and aids rapid recovery of an endangered marsupial population. Nature Communications 8, 1071 (2017).
Fogarty, N. D. Caribbean acroporid coral hybrids are viable across life history stages. Mar Ecol Prog Ser 446, 145–159 (2012).
Chan, W. Y., Peplow, L. M., Menéndez, P., Hoffmann, A. A. & Van Oppen, M. J. H. Interspecific Hybridization May Provide Novel Opportunities for Coral Reef Restoration. Front. Mar. Sci. 5 (2018).
Zigler, K. S., McCartney, M. A., Levitan, D. R., Lessios, H. A. & Harrison, R. Sea urchin bindin divergence predicts gamete compatibility. Evolution 59, 2399–2404 (2005).
Fogarty, N. D., Vollmer, S. V. & Levitan, D. R. Weak prezygotic isolating mechanisms in threatened Caribbean Acropora corals. PLoS ONE 7, e30486 (2012).
Harrison, P. L. et al. Mass Spawning in Tropical Reef Corals. Science 223, 1186–1189 (1984).
Babcock, R. C. et al. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394 (1986).
Miller, K. & Babcock, R. Conflicting Morphological and Reproductive Species Boundaries in the Coral Genus Platygyra. The Biological Bulletin 192, 98–110 (1997).
Fukami, H., Omori, M. & Hatta, M. Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zoological Science 17, 689–696 (2000).
van Oppen, M. J. H., McDonald, B. J., Willis, B. & Miller, D. J. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol 18, 1315–1329 (2001).
Márquez, L. M., Van Oppen, M. J. H., Willis, B. L., Reyes, A. & Miller, D. J. The highly cross-fertile coral species, Acropora hyacinthus and Acropora cytherea, constitute statistically distinguishable lineages. Molecular Ecology 11, 1339–1349 (2002).
Levitan, D. R. et al. Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the montastraea annularis species complex. Evolution 58, 308–323 (2004).
Willis, B. L., Babcock, R. C., Harrison, P. L. & Wallace, C. C. Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16, S53–S65 (1997).
Van Oppen, M. J. H., Willis, B. L., Van Rheede, T. & Miller, D. J. Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol. Ecol. 11, 1363–1376 (2002).
Hatta, M. et al. Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol Biol Evol 16, 1607–1613 (1999).
Isomura, N., Iwao, K. & Fukami, H. Possible natural hybridization of two morphologically distinct species of Acropora (Cnidaria, Scleractinia) in the Pacific: fertilization and larval survival rates. PLoS ONE 8, e56701 (2013).
Levitan, D. R. The relationship between conspecific fertilization success and reproductive isolation among three congeneric sea urchins. Evolution 56, 1599–1609 (2002).
Donnelly, M. J., Pinto, J., Girod, R., Besansky, N. J. & Lehmann, T. Revisiting the role of introgression vs shared ancestral polymorphisms as key processes shaping genetic diversity in the recently separated sibling species of the Anopheles gambiae complex. Heredity (Edinb) 92, 61–68 (2004).
Durand, J. D., Collet, A., Chow, S., Guinand, B. & Borsa, P. Nuclear and mitochondrial DNA markers indicate unidirectional gene flow of Indo-Pacific to Atlantic bigeye tuna (Thunnus obesus) populations, and their admixture off southern Africa. Marine Biology 147, 313–322 (2005).
Lepais, O. et al. Species relative abundance and direction of introgression in oaks. Molecular Ecology 18, 2228–2242 (2009).
Bai, W. N., Liao, W. J. & Zhang, D.-Y. Nuclear and chloroplast DNA phylogeography reveal two refuge areas with asymmetrical gene flow in a temperate walnut tree from East Asia. New Phytologist 188, 892–901 (2010).
Palumbi, S. R. All males are not created equal: Fertility differences depend on gamete recognition polymorphisms in sea urchins. PNAS 96, 12632–12637 (1999).
Levitan, D. R. & Ferrell, D. L. Selection on gamete recognition proteins depends on sex, density, and genotype frequency. Science 312, 267–269 (2006).
Levitan, D. R. & Stapper, A. P. Simultaneous Positive and Negative Frequency-Dependent Selection on Sperm Bindin, a Gamete Recognition Protein in the Sea Urchin Strongylocentrotus Purpuratus. Evolution 64, 785–797 (2010).
Levitan, D. R. Contemporary Evolution of Sea Urchin Gamete-Recognition Proteins: Experimental Evidence of Density-Dependent Gamete Performance Predicts Shifts in Allele Frequencies Over Time. Evolution 66, 1722–1736 (2012).
Zigler, K. S. & Lessios, H. A. 250 Million Years of Bindin Evolution. The Biological Bulletin 205, 8–15 (2003).
Knowlton, N. & Leray, M. Exploring Coral Reefs Using the Tools of Molecular Genetics. SpringerLink 117–132, https://doi.org/10.1007/978-94-017-7249-5_6 (2015).
Patiño, S. et al. Sperm Bindin Divergence under Sexual Selection and Concerted Evolution in Sea Stars. Mol Biol Evol 33, 1988–2001 (2016).
Vacquier, V. D. & Moy, G. W. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. PNAS 74, 2456–2460 (1977).
Ulrich, A. S., Otter, M., Glabe, C. G. & Hoekstra, D. Membrane Fusion Is Induced by a Distinct Peptide Sequence of the Sea Urchin Fertilization Protein Bindin. J. Biol. Chem. 273, 16748–16755 (1998).
Metz, E. C., Kane, R. E., Yanagimachi, H. & Palumbi, S. R. Fertilization Between Closely Related Sea Urchins Is Blocked by Incompatibilities During Sperm-Egg Attachment and Early Stages of Fusion. The Biological Bulletin 187, 23–34 (1994).
Kamei, N. & Glabe, C. G. The species-specific egg receptor for sea urchin sperm adhesion is EBR1,a novel ADAMTS protein. Genes Dev. 17, 2502–2507 (2003).
Hart, M. W. Structure and evolution of the sea star egg receptor for sperm bindin. Mol Ecol 22, 2143–2156 (2013).
Jagadeeshan, S., Coppard, S. E. & Lessios, H. A. Evolution of gamete attraction molecules: evidence for purifying selection in speract and its receptor, in the pantropical sea urchin Diadema. Evolution &. Development 17, 92–108 (2015).
Lillie, F. R. The Production of Sperm Iso-Agglutinins by Ova. Science 36, 527–530 (1912).
Miller, A. Sperm chemo-orientation in the metazoa. (Academic press, 1985).
Kaupp, Ub, Hildebrand, E. & Weyand, I. Sperm chemotaxis in marine invertebrates—molecules and mechanisms. J. Cell. Physiol. 208, 487–494 (2006).
Riffell, J. A., Krug, P. J. & Zimmer, R. K. The ecological and evolutionary consequences of sperm chemoattraction. Proc Natl Acad Sci USA 101, 4501–4506 (2004).
Hussain, Y. H., Sadilek, M., Salad, S., Zimmer, R. K. & Riffell, J. A. Individual female differences in chemoattractant production change the scale of sea urchin gamete interactions. Developmental Biology 422, 186–197 (2017).
Abrego, D., Willis, B. L. & Oppen, M. J. Hvan Impact of Light and Temperature on the Uptake of Algal Symbionts by Coral Juveniles. PLOS ONE 7, e50311 (2012).
Nozawa, Y. & Harrison, P. L. Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis. Mar Biol 152, 1181–1185 (2007).
Ross, C., Ritson-Williams, R., Olsen, K. & Paul, V. J. Short-term and latent post-settlement effects associated with elevated temperature and oxidative stress on larvae from the coral Porites astreoides. Coral Reefs 32, 71–79 (2013).
Nozawa, Y. & Harrison, P. Larval settlement patterns, dispersal potential, and the effect of temperature on settlement rates of larvae of the broadcast spawning reef coral, Platygyra daedalea, from the Great Barrier Reef. Proceedings 9th International Coral Reef Symposium 409–415 (2002).
Yakovleva, I. M. et al. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Marine Ecology Progress Series 378, 105–112 (2009).
Pechenik, J. A. Larval experience and latent effects–metamorphosis is not a new beginning. Integr. Comp. Biol. 46, 323–333 (2006).
Middlebrook, R., Hoegh-Guldberg, O. & Leggat, W. The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211, 1050–1056 (2008).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014).
Chan, W. Y. & Eggins, S. M. Calcification responses to diurnal variation in seawater carbonate chemistry by the coral Acropora formosa. Coral Reefs 1–10, https://doi.org/10.1007/s00338-017-1567-8 (2017).
Vollmer, S. V. & Palumbi, S. R. Hybridization and the Evolution of Reef Coral Diversity. Science 296, 2023–2025 (2002).
Vollmer, S. V. & Palumbi, S. R. Restricted gene flow in the caribbean staghorn coral Acropora cervicornis: implications for the recovery of endangered reefs. J Hered 98, 40–50 (2007).
Highsmith, R. C. Reproduction by fragmentation in corals. Marine Ecology Progress Series 7, 207–226 (1982).
Irwin, A. et al. Age and intraspecific diversity of resilient Acropora communities in Belize. Coral Reefs 36, 1–10 (2017).
Guzner, B., Novoplansky, A. & Chadwick, N. E. Population dynamics of the reef-building coral Acropora hemprichii as an indicator of reef condition. Marine Ecology Progress Series 333, 143–150 (2007).
Hwang, A. S., Northrup, S. L., Peterson, D. L., Kim, Y. & Edmands, S. Long-term experimental hybrid swarms between nearly incompatible Tigriopus californicus populations: persistent fitness problems and assimilation by the superior population. Conserv Genet 13, 567–579 (2012).
Jones, T. A. & Monaco, T. A. A role for assisted evolution in designing native plant materials for domesticated landscapes. Frontiers in Ecology and the Environment 7, 541–547 (2009).
Aitken, S. N. & Whitlock, M. C. Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics 44, 367–388 (2013).
McCulloch, C. E. & Neuhaus, J. M. Generalized linear mixed models. (John Wiley Sons, Ltd., 2013).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2016).
Lewis, E. & Wallace, D. W. R. CO2SYS program developed for the CO2 system calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee (1998).
Pierrot, D., Lewis, E. & Wallace, D. W. R. CO2SYS DOS program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee (2016).
Acknowledgements
This study was funded by the Paul G. Allen Family Foundation and the Australian Institute of Marine Science (AIMS). We thank the SeaSim staff at AIMS for their technical support and P. Menéndez for developing and sharing the statistical analysis code of the GLMM applied in this study. We are also grateful to B. Lenz, J. Davidson, K. Hughes, I. Huizingh and K. Damjanovic for help with coral spawning and experimental setup, and M. Shanahan for seawater alkalinity measurements. W.Y. Chan acknowledges receipt of the University of Melbourne International Research Scholarship and Fee Remission Scholarship.
Author information
Authors and Affiliations
Contributions
W.Y.C. and M.v.O. designed the experiment. W.Y.C. and L.M.P. conducted the experiment and collected the data. W.Y.C. undertook data analyses. W.Y.C. and M.v.O. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chan, W.Y., Peplow, L.M. & van Oppen, M.J.H. Interspecific gamete compatibility and hybrid larval fitness in reef-building corals: Implications for coral reef restoration. Sci Rep 9, 4757 (2019). https://doi.org/10.1038/s41598-019-41190-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41190-5
This article is cited by
-
Integrating cryptic diversity into coral evolution, symbiosis and conservation
Nature Ecology & Evolution (2024)
-
Genomic insights into hybridization of reef corals
Coral Reefs (2020)
-
Ocean acidification partially mitigates the negative effects of warming on the recruitment of the coral, Orbicella faveolata
Coral Reefs (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.