Abstract
Low bone mineral density (BMD) prevails among patients with schizophrenia. Antipsychotics use plays an important role in BMD. Previous cross-section study suggests that clozapine treatment may benefit BMD of women with schizophrenia. However, the effect of long-term clozapine therapy on BMD remains unknown. This prospective study compared clozapine and non-clozapine antipsychotics in long-term effects on BMD among both men and women with schizophrenia. Patients with schizophrenia and age-matched healthy individuals were enrolled from two centers. All patients, including clozapine receivers and non-clozapine antipsychotics recipients, kept clinically stable with unchanged antipsychotics and doses for at least 6 months at enrollment and during the follow-up period. BMD was examined by dual-energy X-ray absorptiometer upon enrollment and at 1- or 3-year follow-up. Thorough clinical and laboratory variables were measured too. The mean BMD of patients receiving clozapine was higher than that of the non-clozapine patients at both enrollment and follow-up. Overall, the patients in the clozapine group gained BMD, while those in the non-clozapine group lost BMD after 1–3 years (p = 0.015). There was no significant difference of BMD change between clozapine-treated patients and healthy controls. Factors associated with BMD change in the clozapine group included calcium level (B = −0.607, p = 0.021) and T3 level (B = −0.077, p = 0.007). This longitudinal study suggests that long-term clozapine treatment may protect BMD compared to prolactin-raising and non-clozapine prolactin-sparing antipsychotics among patients with schizophrenia. Future prospective studies are warranted to testify whether switching from non-clozapine antipsychotics to clozapine can rescue BMD.
Similar content being viewed by others
Introduction
Decreased bone mineral density (BMD), partially due to medication use, is widespread among both male and female patients with schizophrenia1,2. Low BMD, associated with osteoporosis and fracture, contributes to high morbidity and mortality3,4. Moreover, patients with schizophrenia, while receiving antipsychotic treatment, are prone to fall, further worsening their life quality5.
The long-term impacts of various antipsychotics on BMD have been gaining more attention6,7. However, previous studies showed inconsistent findings. Abraham et al. did not find a significant BMD change in a 12-month prospective study but noticed higher rates of bone formation and resorption in patients with high prolactin levels8. Among antipsychotics, prolactin-raising (PR) antipsychotics, are more likely to influence BMD of patients, due to antipsychotic-induced hyperprolactinemia and/or secondary hypogonadism9,10. In accordance, Meaney and O’Keane found BMD loss in patients receiving PR antipsychotics but BMD gain in the prolactin-sparing (PS) recipients over one year11. While the effect of hyperprolactinemia stays inconclusive8,12,13, a longer observational study, with average follow-up at 3.4 ± 1.6 (SD) years, revealed a negative influence of PR antipsychotics on BMD14. Different PS antipsychotics generate diverse short-term impacts on BMD15. Of note, the long-term effects of different PS antipsychotics on BMD remain unknown.
Our previous study found that women with schizophrenia receiving clozapine had better BMD than those taking PR antipsychotic treatment; however, there was no correlation between prolactin level and BMD12. Cui et al. also found that schizophrenia patients receiving clozapine had higher BMD than those receiving non-clozapine16. Patients with refractory schizophrenia who receive clozapine therapy usually need long duration of treatment, because it is the last-line therapy for schizophrenia even while novel compounds are under development17,18. Therefore, long-term clozapine effects on BMD require elucidation. Animal study suggests that clozapine can interact with the glycine site of the N-methyl-D-aspartic acid receptor (NMDAR)19 and chronic clozapine administration can up-regulate NMDARs20,21. NMDARs are expressed in osteoblasts and osteoclasts22,23, and down-regulation of NMDARs may result in the decrease of osteogenesis24. It has not yet been clear whether clozapine exerts different effects on BMD compared to other antipsychotics, particularly non-clozapine PS antipsychotics that do not cause hyperprolactinemia. This study prospectively followed up BMD for one to three years in both male and female patients with schizophrenia under stable antipsychotics treatment and in healthy men and women, aiming to investigate the long-term effect of clozapine vs. non-clozapine antipsychotics (including PR and non-clozapine PS antipsychotics) on BMD.
Results
Subjects
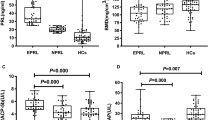
A total of 155 participants (111 schizophrenia patients and 44 healthy controls) were recruited. Among 111 patients with schizophrenia, 22 patients (19.8%) were from the outpatient clinics and the other 89 (80.2%) were from the inpatient units. Patients with schizophrenia were classified into two groups accordingly to the antipsychotics they had been taking: clozapine group and non-clozapine antipsychotics group. Overall, there were three groups, including two groups of schizophrenia patients and one group of healthy individuals.
The mean age was similar among the three groups. The percentage of women in healthy individuals was higher than those in schizophrenia groups (p = 0.004, χ2 test). The healthy individuals had higher education level, lower body weight and BMI, smaller waist and hip circumstances than the schizophrenia patients (all p values < 0.001, Table 1). Between the two groups of schizophrenia patients, patients in the clozapine group had lower antipsychotic dose and severity of psychotic symptoms (mainly in the negative and general-subscales of PANSS) (Table 1). The global functioning, duration of disease, duration of antipsychotic treatment, and concomitant mood stabilizers use were similar between the two patient groups (all p values > 0.05, Table 1). Healthy individuals had lower alkaline phosphatase level, higher estradiol level and lower testosterone level than patients with schizophrenia. Patients in the clozapine group had higher TSH level than non-clozapine group (p = 0.011, Mann-Whitney U test). Patients in the non-clozapine group had higher prolactin level and higher percentage of hyperprolactinemia than the clozapine group and healthy controls (both p values < 0.001). Patients in the clozapine group had higher baseline BMD (determined by DEXA T score and DEXA Z score) than patients in the non-clozapine group (p = 0.007 and 0.017, respectively, post-hoc test using Bonferroni method). Patients in the non-clozapine group had higher percentage of baseline LBMDZ than patients in the clozapine group and healthy controls (p = 0.017 and < 0.001, respectively, Fisher’s Exact test). The three groups had similar calcium, T3 and cortisol levels (all p values > 0.05).
Patients taking clozapine had more bone density gain than patients taking other antipsychotics
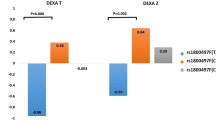
For overall subjects as whole (both subjects with 1-year follow-up and subjects with 3-year follow-up), healthy individuals gained more BMD than patients with schizophrenia (both clozapine and non-clozapine patients) after 1–3 years (DEXA Z scores differences 0.16 ± 0.47, −0.10 ± 0.66, respectively, p = 0.013, Mann-Whitney U test). Noteworthy, clozapine-treated patients and healthy controls gained similar BMD (p = 0.31, Mann-Whitney U test); on the contrary, non-clozapine antipsychotics patients significantly lost BMD, compared to healthy controls after 1–3 years (p = 0.003, Mann-Whitney U test) (Table 2).
At 1-year follow-up, there was no significant difference of BMD change between clozapine and non-clozapine groups (p = 0.81, Mann-Whitney U test) (Table 2).
At 3-year follow-up, patients taking non-clozapine antipsychotics had more BMD loss than those in the clozapine group and healthy controls (p = 0.032 and 0.033, respectively, Mann-Whitney U test). There was no significant difference of BMD change between clozapine group and healthy controls (p = 0.33, Mann-Whitney U test) (Table 2).
For further examining the effects of PR and non-clozapine PS antipsychotics on BMD, we also classified patients into three groups based on their antipsychotics used: PR, non-clozapine PS, and clozapine. Overall, patients in PR and non-clozapine PS groups significantly lost BMD compared to clozapine-treated patients and healthy controls after 1–3 years (p = 0.037, Kruskal-Wallis test) (Supplementary Table 1).
Predictive factors of bone density change in patients with schizophrenia
Backward multiple linear regressions were applied to identify the factors associated with BMD change (DEXA Z score difference after 1–3 years) in patients with schizophrenia (Supplementary Table 2). Potential factors included gender, education duration, age at onset, baseline BMD, duration of schizophrenia, duration of antipsychotic treatment, body weight, height, BMI, waist and hip circumstances, clozapine or non-clozapine antipsychotics use, PR or PS antipsychotics dose, concomitant mood stabilizers, PANSS scores, GAF score, calcium level, alkaline phosphatase level, TSH level, T3 level, cortisol level, estradiol level, testosterone level, prolactin level, and hyperprolactinemia.
Simple linear regressions separately examined all potentially variables aforementioned prior to application of multiple linear regressions. The variables with significant influence on the DEXA Z score change in single linear regressions were then selected to be variables for the multiple linear regression models.
For all patients with schizophrenia (including 1-year and 3-year follow-up patients), gender (B = −0.269, p = 0.033), age at onset (B = −0.017, p = 0.026), baseline DEXA Z score (B = −0.170, p < 0.001), serum calcium level (B = −0.378, p = 0.031) and T3 level (B = −0.005, p = 0.021) were associated with DEXA Z score change in simple linear regressions. In multiple linear regressions, only baseline DEXA Z score (B = −0.152, p = 0.001) and serum calcium level (B = −0.396, p = 0.019) were associated with DEXA Z score change (adjusted R square = 0.157, Supplementary Table 2). For schizophrenic patients with 3-year follow-up (N = 35), clozapine or non-clozapine antipsychotics use (B = 0.468, p = 0.080) were marginally associated with DEXA Z score change in simple linear regression.
For patients taking clozapine, serum calcium level (B = −0.713, p = 0.012) and T3 level (B = −0.008, p = 0.004) were associated with DEXA Z score change in simple linear regressions. In multiple linear regressions, serum calcium level (B = −0.607, p = 0.021) and T3 level (B = −0.077, p = 0.007) were also associated with DEXA Z score change (adjusted R square = 0.258, Supplementary Table 3).
Discussion
It is important to monitor BMD change for schizophrenia patients with long-term, usually lifelong antipsychotics use. To our knowledge, the current study is the first one to follow up BMD after long-term use of clozapine versus non-clozapine (including PR and non-clozapine PS) antipsychotics in patients with chronic schizophrenia. The 3-year BMD change of healthy individuals was also measured for comparison. The findings suggest that patients receiving clozapine gained BMD, while those receiving non-clozapine (including PR and non-clozapine PS) antipsychotics lost BMD after 1–3 years. In consistent to our previous study that prolactin level itself was not correlated with BMD12, there was no significant association between prolactin level/hyperprolactinemia and BMD changes in this study. This longitudinal study conducted in a new cohort echoes the finding of our previous cross-sectional study in another cohort, suggesting that clozapine may have protective effect on BMD that may be unrelated to its prolactin-sparing effect12.
In the 1-year follow-up site, there was no significant difference of BMD change between clozapine and non-clozapine group. However, the baseline and endpoint BMD of the clozapine group were significantly higher than those of the non-clozapine group in the 1-year follow-up site. In the 3-year follow-up site, patients in the clozapine group had increased BMD, while patients in the non-clozapine group had decreased BMD after 3 years (Table 2). The insignificant difference of BMD change between clozapine and non-clozapine groups in the 1-year follow-up site may have been partially due to the relatively shorter duration of follow-up. Overall, our findings suggest that clozapine may be beneficial in protecting BMD among patients with schizophrenia after longer duration of follow-up.
Although the multiple linear regressions analysis did not show that clozapine or non-clozapine antipsychotics use was a significant predictive factor for BMD change in schizophrenic patients, it is interesting to elucidate clozapine’s effects on BMD.
The multiple linear regression models showed that serum calcium levels and T3 levels were negatively associated with BMD changes in patients receiving clozapine (Supplementary Table 3). A rat study found that clozapine can up-regulate calcium sensors, such as visinin-like protein 1 and neurocalcin δ25. An in vitro study also revealed that clozapine exerted activity by altering cell excitability and firing via actions on T-type calcium channels26. Thyroid function may be important for BMD in clozapine recipients. Studies have suggested the association between TSH level and BMD or fracture in both women and men27,28,29. Our previous study also found that TSH level was associated with BMD in women receiving clozapine12. However, studies that examine the interactions between clozapine and calcium or T3 are scanty. The role of calcium and T3 in BMD of clozapine recipients needs further investigation.
As aforementioned, clozapine may be able to protect BMD by activating NMDARs; however, other mechanisms deserve attention too. A recent animal study showed that clozapine had protective effect on bones possibly via causing sex-specific increase in pro-inflammatory cytokines30. It will be interesting to explore the effect of clozapine on bone mass and other related parameters.
This study has several limitations. First, the sample size was modest. Among the 35 schizophrenic patients with 3-year follow-up, only 13 patients were in the clozapine group. The insignificant finding for clozapine or non-clozapine antipsychotics use in the linear regression analysis may be partly due to the limited sample size and the relatively short follow-up duration (1 year) for some patients. Second, the follow-up durations and the exercise programs for the participants were different between the two hospitals. Third, it was difficult to restrict diet and exercise rigorously for patients and healthy controls at outpatient clinics although adequate education had been provided for them. Fourth, the amount of exercise was not measured quantitatively. Daily habits such as dietary calcium consumption31,32, exercise9,33 and sun exposure9 affect bone density. These factors should be included as variables in future study. Fifth, the bone metabolism related parameters were not measured completely due to the limited amount of blood sample. Sixth, measurements on prolactin, T3, calcium levels and several factors that may be associated with BMD at the endpoint were lacking, making it difficult to link baseline prolactin, T3, and calcium levels with BMD after 1- or 3-years follow up. Measurements on these factors should be included in future study. Lastly, the findings of the current study may not be unable to be extrapolated to all patients with schizophrenia because the population of the present study was in single race (Han Chinese) and had relatively long duration of disease and chronically ill symptoms.
In summary, this multicenter, longitudinal follow up study suggests that long-term clozapine treatment may be protective for BMD compared to non-clozapine antipsychotics in patients with chronic schizophrenia. The underlying mechanisms of clozapine’s effects on bone metabolism warrant further elucidation. Patients receiving long-term non-clozapine antipsychotic treatment are at higher risks for osteoporosis, fall and fracture. If the finding of this study can be replicated and confirmed in future studies, clozapine may be a potential choice for patients with schizophrenia who have high risk of osteoporosis. It is also interesting to demonstrate in prospective study whether switching from non-clozapine antipsychotics to clozapine helps to rescue BMD loss.
Methods
Setting
Patients with schizophrenia were recruited from outpatient clinics and chronic inpatient units of two major psychiatric centers in Taiwan (Changhua Hospital in central Taiwan [site 1] and Kaohsiung Chang Gung Memorial Hospital in southern Taiwan [site 2]). Healthy individuals including caregivers of patients were recruited from Kaohsiung Chang Gung Memorial Hospital. The study was approved by the institutional review boards of both hospitals (Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital and Institutional Review Board of Changhua Hospital). All participants gave written informed consent in accordance with the Declaration of Helsinki after complete description of the study.
Both hospitals provided balanced diets which contained calcium about 600 mg and 2,000 calories per day for inpatients. Kaohsiung Chang Gung Memorial Hospital also provided regular daily exercise program (walking for one hour per day) for chronic inpatients.
Participants
Patients with chronic schizophrenia were diagnosed and assessed by research psychiatrists using the DSM-IV criteria34. All patients had been under stable clinical condition with unchanged antipsychotics and doses for at least 6 months prior to the enrollment35,36. Patients recruited from Changhua Hospital were followed up for one year, while patients and healthy individuals recruited from Kaohsiung Chang Gung Memorial Hospital were followed up for three years. The antipsychotics and doses of patients from both sites had been kept unchanged during the follow-up period.
The patients were classified into two groups: clozapine group (clozapine was the only antipsychotic drug for the patients) and non-clozapine antipsychotics group. The non-clozapine antipsychotics included both PR (risperidone, amisulpride, paliperidone, ziprasidone or first-generation antipsychotics) and PS (olanzapine, quetiapine or aripiprazole) antipsychotics except for clozapine.
Healthy individuals were free from any axis I psychiatric disorder. Patients and healthy individuals with the following mental or physical conditions that might affect the BMD were excluded: substance abuse or dependence (including smoking and alcohol drinking that were forbidden at public places and hospitals in Taiwan), eating disorder, pregnancy or lactation, bone metabolism diseases, electrolyte imbalance, renal function impairment, pituitary tumor, thyroid or parathyroid diseases, and co-medications known to affect BMD (e.g. drugs for osteoporosis such as alendronate37, parathyroid hormone38, estrogens, selective estrogen receptor modulators39, bisphosphonates, and calcitonin40, heparin41, and glucocorticoids35) except antidepressants and benzodiazepines that did not consistently influence BMD42.
Assessment
The BMD was measured by dual-energy X-ray absorptiometer (DEXA)43,44,45 at L2-L4 lumbar spine in a supine position at baseline and after 1-year (for site 1) or 3-year (for site 2) follow-up. The DEXA data was reviewed by experienced radiologists who were unaware of the clinical characteristics of the participants. Osteopenia was defined by an absolute DEXA T score between –2.5 and –146, while osteoporosis was defined by a DEXA T score of –2.5 or lower47. T score ≤ –1 was defined as low BMD (LBMDT, including osteoporosis and osteopenia)46. T scores were obtained from the comparison with 30-year-old population. Therefore, we also measured DEXA Z score to help determine whether the bone density loss resulted from aging48. Z score ≤ –1 (LBMDZ) was considered as bone density loss due to causes other than age itself48.
Blood samples were collected at 8 AM. Bone-remodeling related factors were measured, including serum calcium, alkaline phosphatase, TSH (thyroid-stimulating hormone), T3, cortisol, estradiol, testosterone and prolactin. Complete blood and platelet count, BUN (blood urea nitrogen), creatinine, GOT (glutamic oxaloacetic transaminase) and GPT (glutamic pyruvic transaminase) were also measured to exclude major physical problems. Hyperprolactinemia is defined as an elevation of prolactin level above the upper limit of the reference laboratory, 25 ng/ml in women49,50 and 17 ng/ml in men51. Physical examinations including height, weight, body mass index (BMI), waist and hip circumstances were also determined.
For patients with schizophrenia, clinical data including psychiatric and physical illness history, medication kind and duration of use were collected by research psychiatrists by structured clinical interview and chart review. Positive and Negative Syndrome Scale (PANSS)52,53 was used to assess the severity of psychopathological symptoms. Global Assessment of Functioning (GAF) (axis V of DSM-IV) was also used to evaluate the overall functioning. The intra-class correlation coefficient (ICC) among the raters was 0.9.
Statistical Analysis
Chi square tests or Fisher’s exact test was applied for the comparisons of categorical data between groups. Shapiro-Wilk W test was used to examine the normality. Continuous data were analyzed by t-test and ANOVA test, or the corresponding non-parametric method, Mann-Whitney U test and Kruskal-Wallis test, for variables with non-normal distributions. The multiple regression models were applied to explore the contributing factors related to DEXA Z score change after 1–3 years. All tests were two-tailed, and significance of tests was defined as p-value < 0.05. Data were analyzed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Role of the Sponsor
The sponsors were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
Renn, J. H. et al. Bone mass in schizophrenia and normal populations across different decades of life. BMC musculoskeletal disorders 10, 1 (2009).
Lin, C. H. et al. Sex-specific factors for bone density in patients with schizophrenia. International clinical psychopharmacology 30, 96–102, https://doi.org/10.1097/YIC.0000000000000062 (2015).
Stubbs, B. et al. Schizophrenia and the risk of fractures: a systematic review and comparative meta-analysis. General hospital psychiatry 37, 126–133, https://doi.org/10.1016/j.genhosppsych.2015.01.004 (2015).
Kishimoto, T., De Hert, M., Carlson, H. E., Manu, P. & Correll, C. U. Osteoporosis and fracture risk in people with schizophrenia. Current opinion in psychiatry 25, 415–429, https://doi.org/10.1097/YCO.0b013e328355e1ac (2012).
Stubbs, B. et al. Predictors of falls and fractures leading to hospitalization in people with schizophrenia spectrum disorder: A large representative cohort study. Schizophrenia research, https://doi.org/10.1016/j.schres.2018.05.010 (2018).
Crews, M. P. & Howes, O. D. Is antipsychotic treatment linked to low bone mineral density and osteoporosis? A review of the evidence and the clinical implications. Human psychopharmacology 27, 15–23, https://doi.org/10.1002/hup.1265 (2012).
Chen, C. Y., Lane, H. Y. & Lin, C. H. Effects of Antipsychotics on Bone Mineral Density in Patients with Schizophrenia: Gender Differences. Clinical psychopharmacology and neuroscience: the official scientific journal of the Korean College of Neuropsychopharmacology 14, 238–249, https://doi.org/10.9758/cpn.2016.14.3.238 (2016).
Abraham, G. et al. Effects of elevated serum prolactin on bone mineral density and bone metabolism in female patients with schizophrenia: a prospective study. The American journal of psychiatry 160, 1618–1620 (2003).
Halbreich, U. & Palter, S. Accelerated osteoporosis in psychiatric patients: possible pathophysiological processes. Schizophrenia bulletin 22, 447–454 (1996).
Meaney, A. M. et al. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry 184, 503–508 (2004).
Meaney, A. M. & O’Keane, V. Bone mineral density changes over a year in young females with schizophrenia: relationship to medication and endocrine variables. Schizophrenia research 93, 136–143 (2007).
Lin, C. H. et al. Clozapine protects bone mineral density in female patients with schizophrenia. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 15, 897–906, https://doi.org/10.1017/S1461145711001507 (2012).
Lean, M. & De Smedt, G. Schizophrenia and osteoporosis. International clinical psychopharmacology 19, 31–35 (2004).
Takahashi, T. et al. The impact of prolactin-raising antipsychotics on bone mineral density in patients with schizophrenia: findings from a longitudinal observational cohort. Schizophrenia research 147, 383–386, https://doi.org/10.1016/j.schres.2013.04.015 (2013).
Halbreich, U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS drugs 21, 641–657 (2007).
Cui, J. et al. Prevalence, risk factors and clinical characteristics of osteoporosis in Chinese inpatients with schizophrenia. Schizophrenia research 195, 488–494, https://doi.org/10.1016/j.schres.2017.10.027 (2018).
Correll, C. U., Rummel-Kluge, C., Corves, C., Kane, J. M. & Leucht, S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophrenia bulletin 35, 443–457 (2009).
Lin, C. H. et al. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biological psychiatry 84, 422–432, https://doi.org/10.1016/j.biopsych.2017.12.006 (2018).
Schwieler, L., Linderholm, K. R., Nilsson-Todd, L. K., Erhardt, S. & Engberg, G. Clozapine interacts with the glycine site of the NMDA receptor: electrophysiological studies of dopamine neurons in the rat ventral tegmental area. Life sciences 83, 170–175 (2008).
Gray, L., van den Buuse, M., Scarr, E., Dean, B. & Hannan, A. J. Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 12, 45–60 (2009).
Lane, H. Y. et al. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biological psychiatry 60, 645–649 (2006).
Patton, A. J., Genever, P. G., Birch, M. A., Suva, L. J. & Skerry, T. M. Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 22, 645–649 (1998).
Chenu, C., Serre, C. M., Raynal, C., Burt-Pichat, B. & Delmas, P. D. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22, 295–299 (1998).
Ho, M. L. et al. Down-regulation of N-methyl D-aspartate receptor in rat-modeled disuse osteopenia. Osteoporos Int 16, 1780–1788 (2005).
Kedracka-Krok, S. et al. Clozapine influences cytoskeleton structure and calcium homeostasis in rat cerebral cortex and has a different proteomic profile than risperidone. Journal of neurochemistry 132, 657–676, https://doi.org/10.1111/jnc.13007 (2015).
Choi, K. H. & Rhim, H. Inhibition of recombinant Ca(v)3.1 (alpha(1G)) T-type calcium channels by the antipsychotic drug clozapine. European journal of pharmacology 626, 123–130, https://doi.org/10.1016/j.ejphar.2009.09.035 (2010).
Murphy, E. et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. The Journal of clinical endocrinology and metabolism 95, 3173–3181, https://doi.org/10.1210/jc.2009-2630 (2010).
Baqi, L. et al. The level of TSH appeared favourable in maintaining bone mineral density in postmenopausal women. Endocrine regulations 44, 9–15 (2010).
Kim, B. J. et al. The association between serum thyrotropin (TSH) levels and bone mineral density in healthy euthyroid men. Clinical endocrinology 73, 396–403, https://doi.org/10.1111/j.1365-2265.2010.03818.x (2010).
Nikolic, T. et al. Haloperidol affects bones while clozapine alters metabolic parameters - sex specific effects in rats perinatally treated with phencyclidine. BMC pharmacology & toxicology 18, 65, https://doi.org/10.1186/s40360-017-0171-4 (2017).
Wyszogrodzka-Kucharska, A. & Rabe-Jablonska, J. [Calcium balance and regulation in schizophrenic patients treated with second generation antipsychotics]. Psychiatria polska 39, 1157–1171 (2005).
Baastrup, P. C., Christiansen, C. & Transbol, I. Calcium metabolism in schizophrenic patients on long-term neuroleptic therapy. Neuropsychobiology 6, 56–59 (1980).
Kishimoto, T. et al. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. The Journal of clinical psychiatry 69, 385–391 (2008).
American Psychiatric Association. Structured Clinical Interview for DSM-IV. American Psychiatric Press, Washington DC (1994).
Suzuki, Y. & Sato, S. Secondary osteoporosis UPDATE. Clinical significance of glucocorticoid-induced osteoporosis. Clinical calcium 20, 645–653 (2010).
Boling, E. P. Secondary osteoporosis: underlying disease and the risk for glucocorticoid-induced osteoporosis. Clinical therapeutics 26, 1–14 (2004).
Iwamoto, J., Sato, Y., Uzawa, M., Takeda, T. & Matsumoto, H. Seven years’ experience with alendronate in postmenopausal Japanese women with osteoporosis. Therapeutics and clinical risk management 6, 201–206 (2010).
Rosen, C. J. The role of parathyroid hormone in the management of osteoporosis. Hormone research 64(Suppl 2), 81–85 (2005).
Kulak Junior, J., Kulak, C. A. & Taylor, H. S. SERMs in the prevention and treatment of postmenopausal osteoporosis: an update. Arquivos brasileiros de endocrinologia e metabologia 54, 200–205 (2010).
Bakker, S. C. et al. The PIP5K2A and RGS4 genes are differentially associated with deficit and non-deficit schizophrenia. Genes, brain, and behavior 6, 113–119 (2007).
Wawrzynska, L., Tomkowski, W. Z., Przedlacki, J., Hajduk, B. & Torbicki, A. Changes in bone density during long-term administration of low-molecular-weight heparins or acenocoumarol for secondary prophylaxis of venous thromboembolism. Pathophysiology of haemostasis and thrombosis 33, 64–67 (2003).
Kinjo, M., Setoguchi, S., Schneeweiss, S. & Solomon, D. H. Bone mineral density in subjects using central nervous system-active medications. The American journal of medicine 118, 1414 (2005).
Placide, J. & Martens, M. G. Comparing screening methods for osteoporosis. Current women’s health reports 3, 207–210 (2003).
Theodorou, D. J., Theodorou, S. J. & Sartoris, D. J. Dual-energy X-ray absorptiometry in diagnosis of osteoporosis: basic principles, indications, and scan interpretation. Comprehensive therapy 28, 190–200 (2002).
Sturtridge, W., Lentle, B. & Hanley, D. A. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 2. The use of bone density measurement in the diagnosis and management of osteoporosis. Cmaj 155, 924–929 (1996).
Czerwinski, E. Radiologic diagnosis and densitometry of osteoporosis. Przeglad lekarski 54, 220–225 (1997).
Kanis, J. A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359, 1929–1936 (2002).
Swaminathan, K., Flynn, R., Garton, M., Paterson, C. & Leese, G. Search for secondary osteoporosis: are Z scores useful predictors? Postgraduate medical journal 85, 38–39 (2009).
Karasek, M., Pawlikowski, M. & Lewinski, A. [Hyperprolactinemia: causes, diagnosis, and treatment]. Endokrynologia Polska 57, 656–662 (2006).
Iwasa, T. et al. Comparison and problems of measured values of LH, FSH, and PRL among measurement systems. Endocrine journal 53, 101–109 (2006).
Perez-Iglesias, R. et al. Long-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosis. Journal of clinical psychopharmacology 32, 804–808, https://doi.org/10.1097/JCP.0b013e318272688b (2012).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin 13, 261–276 (1987).
Buchanan, R. W. et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophrenia bulletin 31, 5–19 (2005).
Acknowledgements
This study was funded by Chang Gung Memorial Hospital Research Grant, Taiwan (CMRPG891601, CMRPG8D0051) and Changhua Hospital Research Grant, Taiwan (101-15, 102-09).
Author information
Authors and Affiliations
Contributions
Chieh-Hsin Lin and Hsien-Yuan Lane designed the study, analyzed the data, and wrote the manuscript. Chieh-Hsin Lin, Chun-Yuan Lin and Hong-Song Wang recruited the subjects.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, CH., Lin, CY., Wang, HS. et al. Long-term Use of Clozapine is Protective for Bone Density in Patients with Schizophrenia. Sci Rep 9, 3895 (2019). https://doi.org/10.1038/s41598-019-40691-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40691-7
This article is cited by
-
Does clozapine really affect bone mineral density? An experimental study
Journal of Orthopaedic Surgery and Research (2021)
-
Altered levels of BMD, PRL, BAP and TRACP-5b in male chronic patients with schizophrenia
Scientific Reports (2020)
-
The use of clozapine is protective for low bone mineral density induced by prolactin-raising antipsychotics in inpatients with schizophrenia
Archives of Osteoporosis (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.