Abstract
Accurate evaluation of individual risk of intravenous immunoglobin (IVIG)-resistance is critical for adopting regimens for the first treatment and prevention of coronary artery lesions (CALs) in patients with Kawasaki disease (KD). Methods: The KD patients hospitalized in Chongqing Children’s Hospital, in west China, from October 2007 to December 2017 were retrospectively reviewed. Data were collected and compared between IVIG-resistant group and IVIG-responsive group. The independent risk factors were determined using multivariate regression analysis. A new prediction model was built and compared with the previous models. Results: A total of 5277 subjects were studied and eight independent risk factors were identified including higher red blood cell distribution width (RDW), lower platelet count (PLT), lower percentage of lymphocyte (P-LYM), higher total bile acid (TBA), lower albumin, lower serum sodium level, higher degree of CALs (D-CALs) and younger age. The new predictive model showed an AUC of 0.74, sensitivity of 76% and specificity of 59%. For individual’s risk probability of IVIG-resistance, an equation was given. Conclusions: IVIG-resistance could be predicted by RDW, PLT, P-LYM, TBA, albumin, serum sodium level, D-CALs and age. The new model appeared to be superior to those previous models for KD population in Chongqing city.
Similar content being viewed by others
Introduction
Kawasaki disease (KD) is an acute autoimmune systemic vasculitis disease, mainly affecting young children and characterized by bilateral conjunctival inflammation, atypical rash, etc. The most serious consequence of KD is coronary artery lesions (CALs), which is associated with the prognosis of KD1. Prompt treatment with high-dose (2 g/kg) intravenous immunoglobulin (IVIG) could significantly reduce manifestations of KD and CALs. However, 10–20% of the KD patients are resistant to IVIG2,3. Thus, after initial IVIG administration, recrudescent or persistent fever may occur and further treatment is required at 48 hours after the initial use of IVIG, such as the second administration of IVIG, corticosteroids, etc4. The incidence of CALs in IVIG-resistant KD group was significantly higher than that in the IVIG-sensitive KD group (71% versus 5%, p < 0.0001)5. Moreover, studies have suggested that IVIG-resistance is an independent risk factor for giant coronary aneurysms6,7. Therefore, to early detect the IVIG-resistant KD patients and improve prognosis, it is important to identify the risk possibility of IVIG-resistance and take appropriate regimens early.
The etiology and underlying biology of KD have not been completely elucidated. It is still a challenge for pediatricians to quickly diagnose KD, especially when diagnosing the children with atypical or incomplete KD. Many studies have tried to explore the methods to identify the disease more effectively and accurately. Previous studies reported that C-reactive protein, neutrophils, serum sodium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, erythrocyte sedimentation rate (ESR), age, etc. are the risk factors of IVIG-resistance5,8,9,10,11,12. Based on those risk factors, some prediction models for IVIG-resistant KD were established, including Fukunishi3, Egami8, Kobayashi9 and Sano5 scoring system from Japan and Yang10 et al., Wang11 et al. and Tang12 et al. models from China. Those prediction methods, however, have limitations considering they are specific for Japan, North China and East China. As acknowledged, the risk factors of IVIG-resistance are likely to be different in different regions and populations13,14,15. The prediction models developed from the Japanese population may not prove to be accurate and sensitive enough when applied in the Chinese population. For instance, Kobayashi prediction model, which was developed based on a sample of 546 Japanese patients, showed rather unsatisfactory results when applied in Chinese population. The sensitivity and specificity of Kobayashi model were reported as 86.0% and 67.0% respectively when applied in the Japanese population while as 48.8% and 71.6% when applied in 1177 Chinese KD patients15. Although there are prediction models based on KD population in east China and north China, we still lack a prediction model specific for the population in Chongqing city, one of the biggest cities in western China, considering the very large area of China.
In this study, we retrospectively reviewed 5277 KD patients from Chongqing, trying to identify risk factors and establish a new prediction model for IVIG-resistance in Chongqing city. The predictive ability, sensibility and specificity of our new model were further compared with the previously established models including Egami8, Kobayashi9 and Sano5 scoring system from Japan and the model established by Yang10 et al. from China
Materials and Methods
Patients
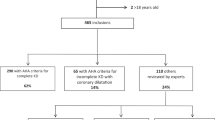
The KD patients who were hospitalized in Chongqing Children’s Hospital from October 2007 to December 2017 with discharge diagnosis of KD were enrolled into the study. According to the diagnostic guidelines of Kawasaki Disease Version 516, the diagnosis criteria were at least 5 days of fever accompanied by 4 or 5 items of the following clinical manifestations: bilateral conjunctival injection, changes in the lips and oral cavity, non-purulent cervical lymphadenopathy, polymorphous exanthema and changes in the extremities. The presence of three or fewer of the above manifestations was defined as incomplete KD. The inclusion criterion was KD as main diagnosis when the patients were first discharged. The exclusion criteria were incomplete KD and other diseases which are easily confused with KD, such as toddler’s idiopathic arthritis; those patients were also excluded who had been given IVIG treatment in other medical institutions before admission and who didn’t receive IVIG treatment during hospitalization.
Definition and data collection
IVIG-resistant KD was defined as the KD patients with a persistent or recurrence of fever >37.3 °C at any time during 48 hours to two weeks after initial IVIG treatment, and accompanied by one or more of the main symptoms15. The presence of coronary artery lesion was defined as coronary artery diameter ≥2.5 mm in patients aged 0–3 years old, ≥3.0 mm in patients aged 3–9 years old and ≥3.5 mm in patients older than 9 years old17. As for the degree of CALs (D-CALs), localized dilatation with internal diameter ≤4 mm, the dilatation with the internal diameter between 4 mm and 8 mm, and the dilatation with the internal diameter ≥8 mm were defined as slight CALs, moderate CALs and severe CALs respectively18. The patients were also classified according to age, that was age ≤6 months and age >6 months8,12.
All demographic characteristics, imaging data and the laboratory data prior to the initial use of IVIG were collected. The demographic characteristics included age (month), sex, total cost and in-hospital time; imaging data prior to the initial use of IVIG included presence of coronary artery lesions and degree of CALs. The laboratory data included red blood cell(RBC), absolute value of red blood cell distribution (RDWa), red blood cell distribution width (RDW), packed cell volume, erythrocyte morphology, mean platelet volume (MPV), platelet distribution width (PDW), thrombocytocrit, platelet count (PLT), white blood cell (WBC), leucocyte morphology, mean corpuscular hemoglobin (MCH), lymphocyte count, percentage of lymphocyte (P-LYM), neutrophil count, percentage of neutrophil, monocyte, platelet-large-cell ratio (P-LCR), hemoglobin (HB), lymphocyte/neutrophil(LNR), urinary bile proto, leucocyte morphology, hematuria, urine specific gravity (low/normal/high), phagocyte, urine protein, white blood cell (stool), urobilirubin, gamma-glutamyl transpeptidase(GGT), alanine transaminase (ALT), aspartate aminotransferase (AST), lactic dehydrogenase(LDH), alkaline phosphatase (ALP), AST/ALT, total bile acid (TBA), direct bilirubin (DBIL), total bilirubin (TBIL), total protein (TP), albumin, prealbumin, globulin, creatinine, blood urea nitrogen (BUN), ketone body (KET), uric acid (UA), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum inorganic phosphorus, serum sodium, serum potassium, serum magnesium, serum chlorine and serum calcium. If there were more than two laboratory reports concerning CRP, blood-routine, kidney function, urine-routine, electrolytes and liver function, we used the reports with the greatest value of CRP, neutrophils ratio, urea nitrogen, urinary protein and lowest albumin and sodium ion concentration. All those laboratory variables were routinely obtained in our clinical practice.
For using the previous models, we kept the predictors in those models and re-estimate the coefficients with the current data set to build the models. The predictive ability of the new model was compared with the previous models including Kobayashi9, Egami8 and Sano5 scoring systems from Japan and Yang prediction model10 from China.
Statistical analysis
All data were presented as count with percentage for categorical variables and mean ± standard deviation (SD) for continuous variables. For the variables with miss rate <25%, multiple imputation was used. The Mann-Whitney U test was used for the comparison of the intergroup continuous variables; the Chi-square test was used for the comparison of categorical variables between the two groups. P < 0.05 was considered statistically significant. The selected variables significantly different between groups entered into the multivariate analyses. For building the prediction model of IVIG-resistant KD, 70% of the patients were randomly selected from the whole sample, including the IVIG–resistant KD and IVIG-responders, by generating random list of number; the other 30% of the patients’ data were used for testing the new model. To determine independent predictors of IVIG resistance, multivariate logistic regression analysis with least absolute shrinkage and selection operator (LASSO) was performed using the indicators with significant difference derived from the univariate analysis; the OR and 95% CI were calculated. The OR value was used to determine the score of an independent risk factor and build the new prediction model. Hosmer-Lemeshow goodness of fit (GOF) test was used to test the model, and p > 0.05 indicated that the prediction model fit the sample data. Receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to determine the predictive ability, sensitivity and specificity of the prediction model. To identify personal risk probability of IVIG-resistance that could be used in the nomogram, an equation was given.
Data analysis was conducted using R Project for Statistical Computing (R version 3.4.1).
Ethic statement
The present study protocol was reviewed and approved by the Ethics Committee of the Children’s Hospital Affiliated to Chongqing Medical University, and with the its approval, this study required no conformed consent. All methods were performed in accordance with Declaration of Helsinki and the relevant guidelines.
Results
Sample collection
A total of 5277 subjects met the inclusion criteria and were enrolled into the study, including 348 cases of IVIG resistance (348/5277, 6.59%) and 4929 cases of IVIG responder (4929/5277, 93.41%). Fifty-seven variables were collected, including 4 demographic variables, 1 imaging variable and 52 laboratory variables. The variable, unconjugated bilirubin, was excluded due to its missing rate of 58%.
Comparison between IVIG-resistant KD and IVIG-responsive KD by univariate analysis
According to univariate analysis (Table 1), 24 variables were significantly higher in the IVIG-resistant group than in the IVIG-responsive group, including RDWa, RDW, erythrocyte morphology, MPV, PDW, Neutrophil count, Percentage of neutrophil, P-LCR, GGT, ALT, AST, lactic dehydrogenase, TBA, DBIL, TBIL, creatinine, BUN, UA, urine protein (positive), leucocyte morphology (urine, positive), urobilirubin (positive), white blood cell (stool, positive), CRP and D-CALs; 18 items were significantly lower in the IVIG-resistant group including RBC, PCV, thrombocytocrit, PLT, lymphocyte, HB, P-LYM, LNR, AST/ALT, TP, albumin, PALB, serum inorganic phosphorus, serum sodium, serum potassium, serum magnesium, serum calcium and age. Besides, the total cost and in-hospital time were significantly higher in IVIG-resistant group, indicating higher burden in IVIG-resistant KD patients.
Analysis of independent risk factors and establishment of predicting model
For multiple logistic regression analysis, the variables with statistical significance derived from univariate analysis were further selected by LASSO constraints in order to find the optimal value of lambda by balancing accuracy and simplicity. The result suggested that the log of the optimal value of lambda was eleven. Among the eleven variables, eight indicators presented statistical significance and were used for multivariate logistic regression analysis (Table 2). The independent risk factors for IVIG-resistant KD were higher RDW, lower platelet count, lower P-LYM, higher TBA, lower albumin, lower serum sodium level, higher degree of CALs and younger age. The OR values (95%Cl) of those risk factors were listed in Table 2.
Based on the above result, a nomogram was derived for personal risk probability of IVIG-resistance (Fig. 1). The underlying logistic model is given by the following equation:
The nomogram for personal risk probability of intravenous immunoglobin-resistant Kawasaki disease. As for age, the patients were classified as age ≤6 months and age >6 months. The risk score represents probability of intravenous immunoglobin-resistance. RDW, RBC; PLT, platelet count; P-LYM, percentage of lymphocyte; TBA, total bile acid; ALB, albumin; Na, serum sodium; D-CALs, degree of coronary artery lesions.
Thus, individual risk probability of IVIG-resistance could be identified. The coefficients indicate the contribution of the variables. Take RDW for an example, when the other variables are fixed, the odds ratio of having IVIG resistance increases by 18.9% (exp(0.173) − 1 = 1.189 − 1 = 0.189) with one unit increase in RDW. The increase unit in PLT, P-LYM, Na, albumin and age with negative coefficients, would decrease the odds ratio of IVIG-resistant KD; the increase of RDW, TBA, GLB and D-CALs with positive coefficients would increase the likelihood of having IVIG-resistance. The McFadden’s R squared was 0.1223 for this model.
For model testing, 30% of the total cohort were applied to the new prediction model. GOF test indicated that the prediction model fit the sample data (χ2 = 2.3227, p = 0.508). The new predictive model for IVIG-resistance showed an AUC of 0.74 (Fig. 2), sensitivity of 76% and specificity of 59%. Multiple testing was performed to further evaluate the validity of the new prediction model, and the AUCs were shown in Fig. 3. The AUC on average was 0.72 (range 0.65–0.80), indicating the value of AUC was valid.
ROC and AUC of the prediction models for IVIG-resistance. The new predictive model for IVIG-resistance showed an AUC of 0.74, Compared with previous IVIG-resistant scoring systems, the new model presented a higher AUC value than the Kobayashi (AUC = 0.68), Egami (AUC = 0.65), Sano (AUC = 0.55) and Yang (AUC = 0.67) methods. ROC, receiver-operator characteristic curves; AUC, area under the curve.
Comparison between the new-established model and the previous scoring systems
Compared with previous IVIG-resistant scoring systems, the new model (AUC = 0.74) presented a higher AUC value than the Kobayashi (AUC = 0.68), Egami (AUC = 0.65), Sano (AUC = 0.55) and Yang (AUC = 0.67) methods. Those previous scoring systems were applied to the cohort in this study, and the result showed that sensitivity and specificity of the new prediction model were better than those previous scoring systems (Table 3).
Discussion
Currently, the treatment of KD mainly depends on high dose of IVIG, however, IVIG-resistant KD is not sensitive to IVIG and additional treatment cannot quickly and effectively reduce vascular inflammation after the initial use of IVIG or after the diagnosis of IVIG-resistance19,20,21. Thus, it results in increased incidence of CALs, which is harmful to the KD prognosis. It would possibly reduce the CALs incidence in the IVIG-resistant KD patients if additional treatment is adopted early before the initial use of IVIG5. Therefore, there is an urgent need to build a prediction model for IVIG-resistant KD with high predictive ability for specific populations in different areas. Here, we reviewed 5277 KD patients from Chongqing city, in West China, and built a new prediction model which appeared to be superior to those previous models when applied in this KD population.
In this study, the percentage of IVIG-resistance was 6.25%, which was far below the percentage of 10%-20% in the studies of Fukunishi3 et al. and Sleeper2 et al. and was close to the percentage of 5.1% in Tang’s study12 and 5.0% in Qian’s22. This might be attributed to the different study populations, the bigger sample size of this study, and the definition of IVIG-resistance. In Sano’s study, IVIG-resistance was defined as persistent fever >24 hours after the completion of initial IVIG infusion while in our study it’s defined as persistent fever >48 hours and duration of initial IVIG use ≥5 days. The initial incidence of CALs judged by absolute diameter was 68% in IVIG-resistant group and 47% in IVIG-responsive group in this study. The initial CALs incidence in IVIG-resistant group was close to Han’s study23 and was much higher than Chantasiriwan’s study24, but the incidences in IVIG-responsive groups were similar to theirs.
There were several prediction models for IVIG-resistant KD. The risk factors in those models include age of month <6; IVIG treatment within 4 days of illness; abnormal first echocardiographic results; higher levels of CRP, ALT, AST, PCT, neutrophil ratio, percentage of band cell, TBIL and LDH; and lower levels of PLT, serum sodium, hemoglobin and pericardial effusion, etc9,11,12,15,25,26,27. However, those models could not present high predictive ability in populations from different regions. The independent risk factors reported in the previous prediction model, such as CRP, AST, ALT, TBL, NEU%, ALB, GGT, LDH and LNR were significantly different between IVIG-responsive and IVIG-resistant group in our study, but they failed to enter in the final logistic regression model3,5,8,9,10,11. Besides, the results of univariate analysis may be different in different populations between IVIG-responsive and IVIG-resistant group. For instance, GGT level was significantly different between the two groups in our and Wang’s study, while wasn’t in Yang’s and Kobayashi’s study. Serum chlorine level was significantly different between the IVIG-responders and the IVIG-resistant in Kobayashi’s study, while was not in our and Wang’s study. AST was an independent risk factor in Kobayashi’s and Sano’s study and ALT was an independent risk factor in Egami’s study, but those two factors didn’t show statistical difference in the univariate analysis in Yang’s study5,8,9,10,11. It might be attributed to that KD pathology is related with genetic polymorphisms, and the reported genetic determinants of KD were different in various populations28. The genetic polymorphisms and unknown etiology might make the risk factors of IVIG-resistant different in different populations.
We expected to identify new risk factors of IVIG-resistant KD and establish a more accurate prediction model for Chongqing city. Therefore, this study collected demographic, imaging and laboratory information from 5277 KD patients as completely as possible. The sample size and the variables included in our study were much larger than previous IVIG-resistant KD prediction models. In the present study, a total of 57 variables were successfully collected and included in the univariate analysis, of which 42 factors showed significant difference between the two groups. Eight independent risk factors were identified, among which RDW, P-LYM and D-CALs were not identified as predictive indicators for IVIG-resistance in previous studies. We also found that some new factors were significantly different between the two groups, including PCV, MPV, PDW, thrombocytocrit, P-LCR, lymphocyte/neutrophil, urine protein, urobilirubin, AST/ALT, PALB, serum inorganic phosphorus, serum magnesium and serum calcium. But those were not independent risk factors. In this study, we didn’t include the patients who received initial IVIG treatment within 4 days of illness because the diagnosis criteria of Kawasaki disease required fever duration ≥5 days.
Several studies reported that the platelet counts decreased in IVIG-resistant KD patients9,11,29. In our study, we found platelet changed in morphology in addition to the decreased counts in IVIG-resistance group. The reduction of platelets might be associated with CAA-induced platelet consumption, which was followed by a compensatory change in platelet morphology, with an increased volume of platelet and a larger PDW. Urinary protein was higher in IVIG-resistance group than in IVIG-responsive group, which might imply a more severe glomerular vasculitis and increased glomerular vascular permeability in IVIG-resistant KD patients. Besides the increase of ALT, AST, TBIL and LDH, we also observed higher urobilirubin and lower PALB in IVIG-resistant KD group, which suggested that the patients with IVIG-resistance might have more severe systemic inflammation and vasculitis in liver5.
As stated in the 5-minute Pediatric Consult (Second Edition)30, the concentration of serum inorganic phosphorus and serum sodium decreased in patients with KD and low concentration serum potassium was related with CAA31. The significantly lower concentration of serum inorganic phosphorus, serum potassium, serum magnesium, serum calcium and serum sodium and higher concentration of BUN were observed in IVIG-resistant group in our study, which indicated that kidney vasculitis might exert negative effect on renal function and tubular reabsorption.
The final risk factors selected to undergo multivariate analysis for predicting IVIG-resistance, including RDW, platelet count, P-LYM, TBA, albumin, serum sodium level, D-CALs and age. Among those variables, TBA, serum sodium, albumin, platelet count and age of month have been reported in previous studies. The increased RDW was related to anemia, which was consistent with Durongpisitkul’s study. The reduced P-LYM represented higher percentage of neutrophil in blood and more severe inflammation, which was also reported in the studies of Durongpisitkul et al. and Wang et al.11,32. The new model for IVIG-resistant KD prediction was generated based on those risk factors, with the AUC of 0.74, sensitivity of 76% and specificity of 59%. The AUC value, sensitivity and specificity in this study showed better accuracy compared with the previous models when applied in the population from Chongqing city.
This study has considered more medical information besides the factors included in the previous prediction models, in order to find the potential hint of the disease. The factors including RDW, D-CALs and P-LYM were rarely mentioned in previous prediction models. In the future, we will conduct a prospective study to further evaluate the effectiveness of this new model. Still, this study has some limitations. Firstly, it’s a retrospective study in a single center. Secondly, multiple clinical teams participated in the care and measurement of the patients. Thirdly, due to the lack of patients’ height data, no mean body-surface-area (BSA) adjusted Z-score was available; we will take it into consideration in our future study. Last, some data items were missing, which might result in bias in statistical analysis; for the variables with miss rate <25%, multiple imputation was done to decrease bias in this study. With the large sample size, we thought we could still draw a relatively valid conclusion.
Conclusion
The IVIG-resistance could be predicted using the values of RDW, PLT, P-LYM, TBA, ALB, serum sodium level, D-CALs and age. The new model of predicting IVIG-resistant KD appeared to be superior to those previous prediction models for the KD population in Chongqing city. Further study is necessary to validate the utility of this new model.
References
Tsuda, E. et al. A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J 167, 249–258 (2014).
Sleeper, L. A. et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr 158, 831–835.e3 (2011).
Fukunishi, M. et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr 137, 172–176 (2000).
Brogan, P. A. et al. Kawasaki disease: an evidence based approach to diagnosis, treatment, and proposals for future research. Arch Dis Child 86, 286–290 (2002).
Sano, T. et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 166, 131–137 (2007).
Burns, J. C., Capparelli, E. V., Brown, J. A., Newburger, J. W. & Glode, M. P. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J 17, 1144–1148 (1998).
Tremoulet, A. H. et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr 153, 117–121 (2008).
Egami, K. et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 149, 237–240 (2006).
Kobayashi, T. et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 113, 2606–2612 (2006).
Yang, S., Song, R., Zhang, J., Li, X. & Li, C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child (2018).
Hua, W. et al. A new model to predict intravenous immunoglobin-resistant Kawasaki disease. Oncotarget 8, 80722–80729 (2017).
Tang, Y. et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an East China population. Clin Rheumatol 35, 2771–2776 (2016).
Berdej-Szczot, E. et al. Risk factors of immunoglobulin resistance and coronary complications in children with Kawasaki disease. Kardiol Pol 75, 261–266 (2017).
Sánchez-Manubens, J. et al. Role of the Egami score to predict immunoglobulin resistance in Kawasaki disease among a Western Mediterranean population. Rheumatol Int 36, 905–910 (2016).
Fu, P. P., Du, Z. D. & Pan, Y. S. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J 32, e319–323 (2013).
Ayusawa, M. et al. Revision of diagnostic guidelines for Kawasaki disease (the5th revised edition). Pediatr Int 47, 232–234 (2005).
Wang, Y. et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum 65, 805–814 (2013).
Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease. Pediatr Int 47, 711-732 (2005).
Sundel, R. P., Burns, J. C., Baker, A., Beiser, A. S. & Newburger, J. W. Gamma globulin re-treatment in Kawasaki disease. J Pediatr 123, 657–659 (1993).
Harada, K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn 33, 805–810 (1991).
Wright, D. A., Newburger, J. W., Baker, A. & Sundel, R. P. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr 128, 146–149 (1996).
Qian, W., Tang, Y., Yan, W., Sun, L. & Lv, H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr 44, 33 (2018).
Han, R. K., Silverman, E. D., Newman, A. & McCrindle, B. W. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch Pediatr Adolesc Med 154, 694–699 (2000).
Chantasiriwan, N., Silvilairat, S., Makonkawkeyoon, K., Pongprot, Y. & Sittiwangkul, R. Predictors of intravenous immunoglobulin resistance and coronary artery aneurysm in patients with Kawasaki disease. Paediatr Int Child Health 38, 209–212 (2018).
Ashouri, N., Takahashi, M., Dorey, F. & Mason, W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr 153, 365–368 (2008).
Rigante, D. et al. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol Int 30, 841–846 (2010).
Sato, S., Kawashima, H., Kashiwagi, Y. & Hoshika, A. Inflammatory cytokines as predictors of resistance to intravenous immunoglobulin therapy in Kawasaki disease patients. Int J Rheum Dis 16, 168–172 (2013).
Del, P. D. et al. Pathogenetic determinants in Kawasaki disease: the haematological point of view. J Cell Mol Med 21, 632–639 (2017).
Seo, E. et al. Prediction of unresponsiveness to second intravenous immunoglobulin treatment in patients with Kawasaki disease refractory to initial treatment. Korean J Pediatr 59, 408–413 (2016).
Paston & Carrie. The 5 Minute Pediatric Consult: Second Edition. Pediatr Emerg Care 305–306 (2000).
Manlhiot, C., Millar, K., Golding, F. & Mccrindle, B. W. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol 31, 242–249 (2010).
Durongpisitkul, K. et al. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol 24, 145–148 (2003).
Acknowledgements
This work was financially supported by the Science and Technology Innovation of Social Undertakings and People’s Livelihood Security of Chongqing(NO.cstc2015shms-ztzx0031). We gratefully acknowledge the contribution of Yidu Cloud (Beijing) Technology Co., Ltd. for the statistical advice.
Author information
Authors and Affiliations
Contributions
Xu-Hai Tan designed the protocol, collected, analysed the data and wrote the manuscript, Xiao-Wei Zhang built the model and prepared all figures, Xiao-Yun Wang reviewed and edited the manuscript, Xiang-Qian He and Chu Fan collected he data, Tie-Wei Lyu and Jie Tian designed the protocol, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, XH., Zhang, XW., Wang, XY. et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep 9, 1722 (2019). https://doi.org/10.1038/s41598-019-39330-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39330-y
This article is cited by
-
Predictive value of albumin for intravenous immunoglobulin resistance in a large cohort of Kawasaki disease patients
Italian Journal of Pediatrics (2023)
-
Predicting immunoglobulin resistance in Kawasaki disease: an assessment of neutrophil to lymphocyte platelet ratio
Italian Journal of Pediatrics (2022)
-
Serum sodium level associated with coronary artery lesions in patients with Kawasaki disease
Clinical Rheumatology (2022)
-
Predictive value of Albumin-Bilirubin grade for intravenous immunoglobulin resistance in a large cohort of patients with Kawasaki disease: a prospective study
Pediatric Rheumatology (2021)
-
Prognostic value of pretreatment prognostic nutritional index in intravenous immunoglobulin-resistant Kawasaki disease
Heart and Vessels (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.