Abstract
Motor symptoms are defining traits in the diagnosis of Parkinson’s disease (PD). A crucial component in motor function is the integration of afferent proprioceptive sensory feedback. Previous studies have indicated abnormal movement-related cortical oscillatory activity in PD, but the role of the proprioceptive afference on abnormal oscillatory activity in PD has not been elucidated. We examine the cortical oscillations in the mu/beta-band (8–30 Hz) in the processing of proprioceptive stimulation in PD patients, ON/OFF levodopa medication, as compared to that of healthy controls (HC). We used a proprioceptive stimulator that generated precisely controlled passive movements of the index finger and measured the induced cortical oscillatory responses following the proprioceptive stimulation using magnetoencephalography. Both PD patients and HC showed a typical beta-band desynchronization during the passive movement. However, the subsequent beta rebound after the passive movement that was almost absent in PD patients compared to HC. Furthermore, we found no difference in the degree of beta rebound attenuation between patients ON and OFF levodopa medication. The results demonstrate a disease-related deterioration in cortical processing of proprioceptive afference in PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disease. The diagnosis of PD is based on the presence of bradykinesia, tremor, and rigidity. The motor symptoms are mainly caused by deficient dopamine neurotransmission and are counteracted by dopamine replacement therapies1. The pathology of PD is characterized by the presence of alpha-synuclein-enriched protein aggregates called Lewy- bodies1,2. Lewy body pathology spreads progressively from the olfactory bulb and brainstem to larger parts of the brain giving rise to motor symptoms but also non-motor symptoms such as hyposmia, depression, sleep disorders, pain, and cognitive impairment1,3.
One of the core symptoms of PD is disturbances in proprioception crucial for successful control of movements and for maintaining balance and posture4,5. Proprioceptive signals are afferent neural signals from the peripheral nervous system (PNS) to the central nervous system (CNS). The proprioceptive signals primarily originate in the muscle spindles, Golgi tendon organ, and joint receptors, and project through the spinal cord to the dorsal spinocerebellar tract and from there to the thalamus; and from thalamus to cerebellum, basal ganglia, and further to the sensory-motor areas in the cortex6. Carrying out voluntary movements requires efferent signals from cortex through basal ganglia through the brainstem to the PNS, and afferent proprioceptive signals going back, transmitting information about the prior state of the locomotor system and its changes, e.g., in limb position.
Disturbances in proprioception in PD is associated with motor symptoms in PD4,5. PD patients are, for instance, worse compared to healthy controls (HC) at detecting passive movements of their limbs which is dependent on proprioceptive afferents7,8,9. The apparent deterioration in the utilization of proprioceptive information in PD does not appear to be caused by disturbances in the PNS. Recordings of muscle spindle responses by microneurography show no differences in afferent signals between HC and PD patients10. The early cortical processing of proprioceptive signals—measured with electroencephalography (EEG) as the event-related potentials (ERPs) following passive movements of the index fingers—does not differ between PD patients and HC11.
Behavioral studies indicate that loss of proprioceptive function arises due to errors in central processing and sensory integration. For example, illusions of movement induced by vibrating muscles in the limbs are reduced in PD patients compared to HC12,13. PD patients also show increased dependency on visual cues over proprioceptive feedback during active movements14,15, and postural control is more difficult for PD patients without visual feedback compared to HC16,17. The disturbances in proprioception in PD thus appear to arise in the higher levels of sensorimotor integration. Impaired utilization of proprioceptive information in PD patients also shows when switching from visually guided to the proprioceptive guided control of balance18. Errors in integrating proprioceptive signals are seen in grasping tasks where PD patients show increased grip force when grasping objects compared to HC, suggesting that proprioceptive feedback and active motor commands are not adequately integrated to facilitate optimal grasping15,19. Impaired proprioception degrades sensorimotor integration, which is compensated with feedback from other sensory domains5,20.
Disturbances in the proprioceptive processing in PD appears to be due to errors in the integration of proprioceptive afferents, but the actual mechanisms of the disturbances in the processing of proprioceptive signals are unknown. If loss of proprioception in PD is due to disturbed communication between the basal ganglia, thalamus, and cortical motor areas, due to a faulty integration of proprioceptive signals at a later processing stage, or outside the dopamine-dependent pathways has not yet been adequately explained12. Isolating the relative contribution from efferent and afferent signals on movement control to answer how afferents are affected in PD is difficult, as both are necessary for successfully carrying out the movements and depends on functional and anatomical overlapping neural processes21. In the present study, we investigate how proprioceptive afferents are processed in PD in induced passive movements.
PD is associated with changes in neural oscillatory behavior demonstrated both at local and global levels. Local field potentials from the subthalamic nucleus (STN) in PD patients show an increase in beta-band (~14–30 Hz) oscillations22. The abnormal beta oscillations are decreased by administration of dopaminergic medication, indicating a functional link between dopamine levels and background beta oscillations22,23. The decrease of beta-band oscillations in STN due to dopaminergic medication have been correlated to an overall reduction in motor symptoms in PD24. Although PD is associated with an increase of beta-oscillations in sub-cortical areas, cortical beta oscillations are decreased in PD patients compared to HC25,26. Though, mouse model recording of intracranial beta activity have shown increased focal beta oscillations during spontaneous movements due to dopamine depeltion27,28. The increase in beta oscillations in mice were associated with increased global synchronization in the beta band seen as increased spectral coherence across the basal ganglia-cortical loop. Several studies have reported a similar increase in the functional connectivity in the beta-band within the sensory-motor cortex in humans with PD26,29,30,31. The level of synchronicity of cortical beta activity is related to rigidity and action tremor in PD32.
Cortical beta-band oscillations are actively involved in sensorimotor processing. Beta-band oscillations exhibit well-known event-related desynchronization (ERD) and event-related synchronization (ERS) during active states of the sensorimotor system (Fig. 1). Beta oscillations attenuate in the second before movement onset, known as the movement-related ERD, and is prevalent during the duration of the movement33,34,35,36. Once the movement stops, the beta oscillations temporarily show a relative increase, known as the post-movement ERS or beta rebound, before going settling back at the baseline level33,35,36,37,38. The origin of both the movement-related beta ERD and the beta rebound during voluntary movements is the primary somatosensory cortex39,40 with the cortical source of the movement-related being more posterior than the source of subsequent beta rebound34.
Typical movement-related beta-band activity. Typical event-related synchronization (ERS) and desynchronization (ERD) in the beta-band during movements measured from the cortex with EEG/MEG. Relative-change here means an increase or decrease in beta-band amplitude/spectral-power relative to the baseline (the units are arbitrary in the figure). When initiating a movement beta activity start to desynchronize and prevails as a persistent ERD during the movement execution phase. Once the movement ends, it is followed by an ERS referred to as the beta rebound.
The movement-related ERD and beta rebound seen during voluntary movements is attenuated in PD compared to HC. PD patients show less beta ERD before and during active movements and a smaller rebound after active movements41,42,43. It is currently unclear if the attenuated dynamics in the beta-band in PD is driven by deficits in efferent processes, processes of afferents, or at a higher level in the integration of afferent and efferent signals. Both efferent signals, afferent signals, and the integration of the two are needed for carrying out voluntary movements. The movement-related ERD is taken to reflect the active state of the motor system, receiving the afferent signals, and the cortical processes responsible for integrating the efferent and afferent signals44.
The beta rebound is linked to proprioceptive afferents. The beta rebound is present for passive induced movements in healthy subjects45,46 meaning the beta rebound is related to the processing of proprioceptive signals independent of the efferent motor commands from cortex to the periphery. The beta rebound is also diminished when applying a temporary ischemic nerve block to disrupt the proprioceptive feedback from the muscles in healthy subjects47. We hypothesize that the attenuated beta ERD and rebound during voluntary movement in PD to some extent related to disturbances in the cortical processing of proprioceptive signals—even though the relative contribution of the movement specific efferent signals, afferent signals, and the integration is unclear.
In the present study, we investigate the differences in cortical processing of proprioceptive information between PD patients compared to HC regarding the. In contrast to earlier studies that have used voluntary movements to study beta-oscillations in PD, we isolate the processing of afferent from that of movement specific efferent signals by using a computer-controlled proprioceptive stimulator that generates precise passive movements of the index finger. With this method, we examine the processing of proprioceptive signals in isolation, without the confounding effect of the efferent motor signals associated with self-initiated voluntary movements. If the attenuation of movement-related ERD and rebound during active movements is due to a defect in the processing of proprioceptive afferents in PD, we expect the movement-related ERD and beta rebound to be less salient for PD patients than for HC. Conversely, if the difference between PD and HC primarily depends upon deficits in efferent signaling processing, then we expect there to be no differences between PD and HC during passive movements. We furthermore investigated how Levodopa influences processing of proprioceptive information in PD patients, by examining PD patients both in ON and OFF Levodopa states. While Levodopa improves motor symptoms in PD, it is unclear to what extent it affects proprioceptive processing, as results from behavioral studies have been inconclusive with regards to the improvement of proprioceptive function due to medication5.
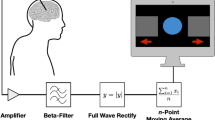
PD patients and healthy controls were subject to proprioceptive stimulation consisting of passive movements of the index finger (Fig. 2). The passive finger movements were evoked by a custom-made MEG compatible pneumatic movement actuator utilizing a pneumatic artificial muscle (PAM)48. The proprioceptive stimulation was administered to the index finger on the hand contralateral to PD dominant side for PD patients and on the dominant hand for HC. The cortical responses to the proprioceptive stimulation were measured with MEG. We tested PD patients in OFF (at least 12 hours after last levodopa intake) and ON (one hour after levodopa intake) medication status in two consecutive sessions on the same day. We extracted the time-frequency response from frequencies within the “mu” spectrum from 8 to 30 Hz, which encompasses the beta (14–30 Hz) and alpha (8–13 Hz) sensory motor rhythms. The time-frequency representations where then compared between PD patients and HC, and between PD patients ON/OFF medication.
Results
Subject variables
Table 1 shows a summary of demographic variables (age, sex), clinical scores (disease duration, Levodopa equivalent daily dose (LEDD)49, MDS-UPDRS-III scores in ON and OFF medication status, the Montreal Cognitive Assessment battery (MoCA), Hospital Anxiety and Depression Scale (HADS). The right column gives the Bayes Factor (BF) for the hypothesis that there is a difference between groups. PD patients and HC did not differ in the male/female ratio (BF = 0.42), nor in cognitive ability measured by MoCA (BF = 0.30), and anxiety score on HADS (BF = 0.52). There was a trend in favor of a difference on HADS depression score (BF = 1.23), with PD patients scoring higher than HC, and that HC on average was older than PD patients (BF = 2.02). None of these trends are sufficient to conclude clear differences between PD patients and HC50.
PD patients showed an improvement of motor symptoms after taking medication reflected by the difference in MDS-UPDRS-III score between ON and OFF states (BF = 4.30*104). The median score of the sum of the tremor-related items in MDS-UPDRS-III (items 15–18) was 5 (out of 40; range 0–20) in the OFF state and 2 (range 0–12) in the ON state.
Time-frequency responses in beta/mu band
The aim was to compare the induced responses to proprioceptive stimulation in the beta-band between PD patients and HC, and between PD patients ON/OFF Levodopa medication. The first effect we tested was for a general change in the beta-band response in PD compared to HC. We compared the OFF-state for PD patients to the first session for HC to get between-group comparison without additional variation introduced by medication.
The time-frequency responses to the proprioceptive stimulation in the beta and mu band showed a significant difference between PD patients OFF medication and HC. The significant difference was defined by a single cluster located 1.0 s after stimulation onset, lasting 0.5 s, covering a frequency range from 14 Hz to 25 Hz (p = 0.017). The cluster corresponds to the post-movement beta rebound, which was considerably attenuated, almost absent, in PD patients compared to HC (Fig. 3).
8–30 Hz oscillatory response to proprioceptive stimulation. Time-frequency responses (TFR) of the cortical response to proprioceptive stimulation (movement onset at time = 0) for HC in session one (A) and session 2 (B), and PD patients OFF medication (C) and ON medication (D). The relative change in spectral is expressed in gradient represents the log-ratio relative to the baseline. Below each TFR are traces showing the z-score of the absolute acceleration of the movements and muscle activation measured with EMG. (E) The temporal evolution of the band of the cluster that informed the significant difference between groups (14–25 Hz) for both sessions and both groups. Thick lines are averages per group (PD = blue, HC = red) and session (first session/OFF = solid lines, second session/ON = dotted lines) and thin lines are the responses per subject (also shown as seperate figures in the supplementary material). The shaded area indicates the time of the cluster that showed a significant difference between PD patients and HC.
The interaction between-groups and between-sessions comparisons of the effect of levodopa on the beta/mu response to the proprioceptive stimulation yielded no significant difference (p = 0.45). Comparing the sessions for PD patients alone, without including the interaction between groups to correct for repetition effects, did not reveal any difference (p = 0.55).
Peripheral muscle activation
We compared the EMG activation between the different groups and sessions to ensure that subjects did not voluntarily or unknowingly move their fingers during the proprioceptive stimulation. The absence of peripheral muscle activation was taken to indicate the absence of movement-related signals in the flexor carpi radialis muscle responsible finger movements. None of the subjects showed time-locked muscle activation to the passive finger movements.
To quantify whether there was any difference in peripheral muscle activation, we tested for differences in the measured EMG signals during the movement compared to a baseline consisting of EMG data from half a second before the movement with cluster-based permutation tests51. The tests did not show significant differences for neither PD patients (first session: p = 0.31, second session: p = 0.31) nor for HC (first session: p = 0.26, second session: p = 0.31). In addition, we compared the EMG time-course between groups and session in the time-window of the time-frequency analysis. Cluster-based permutation tests on the EMG time-series did not show significant differences between groups (first session: p = 0.80, second session: p = 0.84) or between sessions (p = 0.12 for PD patients and p = 0.68 for HC).
Relation between beta rebound and baseline beta power
The amount of movement-related beta-band ERS and ERD in the beta-band may depend on the spectral power in the beta-band within the baseline period leading up to the movements52,53. We tested for a relationship between the absolute power spectral density in the time-window from 1.25 s to 0.2 s before the passive movements were initiated and the relative change within clusters that showed significant differences in the primary analysis. We explored whether there was a relationship between baseline beta and the beta rebound by comparing regression models with baseline power as a predictor (H1) and a model without baseline power (H0).
The model comparison did not support the model containing baseline power over the model without it (BFH1/H0 = 0.83). Including interactions between baseline power, session, and group in the model (H2) and comparing it to the model without baseline power gave substantial evidence in favor of the model without baseline power (BFH2/H0 = 0.16).
Discussion
In the present study, we aimed at elucidating the processing of afferent proprioceptive information in PD by combining two different approaches. First, we used a computer-controlled proprioceptive stimulator that generates precisely controlled passive proprioceptive stimulation, with the aim of separating the processing of afferent proprioceptive information from that of efferent motor information. The controlled proprioceptive stimulation made sure the movements were identical for PD patients and HC in both sessions. Second, we studied PD patients ON and OFF Levodopa medication as compared to HC, with the aim of separating disease-related from medication-related effects. Our results show that when passive movements are used to generate proprioceptive stimulation, there is a definite difference in the cortical processing of afferent proprioceptive signals between PD patients and HC, manifested as a significant reduction—almost absence—of the beta rebound in PD patients as compared to HC (see Fig. 3). We did not find that the beta rebound attenuation was modulated by medication in PD, despite an evident effect from medication on overt motor symptoms, as assessed with MDS-UPDRS-III. The beta band attenuation hence emerges as a disease-related rather than medication-related change in the processing of afferent proprioceptive information in PD.
The different stages in the cortical beta response to movements reflect different aspects of the processing of motor commands and proprioceptive feedback36. The beta ERD, observed before and during movements (see Fig. 1), is taken to reflect a state of heightened sensitivity to efferent and afferent information within the motor system54. This notion has been supported by studies showing that reaction times to stimuli is negatively correlated with beta-band power, indicating that motor commands are executed more readily during an ERD when beta-band power is decreased52,53. Such heightened sensitivity facilitates events such as motor commands being carried out efficiently as well as the integration of proprioceptive feedback while carrying out movements. The role of the beta rebound has been suggested to function an effective inhibition of motor responses38,55. As such the increase of beta oscillation during the beta rebound might reflect a “resetting” of the sensory-motor system, in terms of integrating the proprioceptive feedback from the action into the body schemata, thereby constructing an updated model of the position of the body and the limbs44. The successful update of the body schemata is crucial for the calibration and execution of future actions as they will be dependent on the state of the body schemata to generate future motor commands and efferent signals.
The reduced beta rebound response in PD following proprioceptive stimulation might be understood as a deterioration in the processing and integration of movement-related proprioceptive signals, where errors in integrating proprioceptive signals lead to errors in the internal representation of body-state, and more imprecise efferent motor commands. The reduction of the beta rebound in PD appears not just to be an after-effect of reduced beta ERS during active movements but related to the distinct processing of proprioceptive afferents.
The time of the difference in the beta-band between PD patients and HC suggest that disturbances in processing proprioceptive signals in PD arise at a late stage. How this relates to motor symptoms during active movements is unclear. We show that processing of proprioceptive afferents is disturbed in PD even without muscle activation of the flexor carpi radialis required for self-initiated movement of the index finger. We cannot rule out that efferent signals not specific to movement might influence the cortical beta band responses. We only measured muscle activity from the flexor carpi radialis to ensure that muscle activity related to moving the index finger were not present during the passive movements. It is a possibility that efferent (or afferent) signals to and from other parts of the body, e.g. the axial muscles, lower limb muscles, or other parts of the body do not influence cortical responses in a non-trivial manner. For example, efferent signals related to tremor or rigidity might influence the processing of proprioceptive signals in PD patients. One explanation of the reduced beta rebound is that ongoing efferent and afferent signals due to tremor or rigidity introduce noise in the later stages of the sensory-motor processing thus leading to less pronounced post-movement processing seen as an attenuated beta-rebound. The results did, however, not suggest that the beta rebound was less attenuated as motor symptoms improved due to medication in the ON state. However, due to the relatively small sample size in the present study, we are unable to further qualify the relation between specific motor symptoms and cortical beta-band responses.
Although the overt motor symptoms in PD patients changed upon Levodopa medication as compared to an initial OFF state (as rated by MDS-UPDRS-III), the attenuation of the beta rebound in PD patients did not change between Levodopa medication states. The effect of dopaminergic medication on restoring proprioceptive function in PD is mixed. Behavioral studies have shown that the error in detecting proprioceptive feedback for PD patients is not affected by Levodopa medication17. Other studies have even shown that Levodopa might worsen detection of proprioceptive feedback56,57. However, others have also reported that the Levodopa equivalent dose of medication has a small effect in restoring proprioceptive function in a haptic discrimination task58. The combination of dopaminergic medication and deep brain stimulation have been shown to improve haptic discrimination in PD patients59. The seemingly conflicting results might be due to the diverse and complex processing of proprioceptive signals from the PNS. Processing of proprioceptive is done in parallel pathways between thalamus, cerebellum, basal ganglia, and cerebral cortex, in which the signals are integrated into the wider sensory-motor system6. It is possible that dopaminergic medication has a more substantial effect on certain stages in the processing of proprioceptive signals, e.g., the stages where dopaminergic neurons are involved. It has been proposed that processing of proprioceptive information in PD might rely less on the dopamine-dependent loop between basal ganglia, thalamus, and cortex, and instead involve pathways from the thalamus, through cerebellum to cortical areas60. Since MEG primarily detects activity from synchronous populations of pyramidal neurons in the cortex, we can, however, only speculate about the sub-cortical pathways responsible for propagating proprioceptive signals.
We acknowledge that it is possible that there might be effects of the medication in our study that we do not have sufficient statistical power to pick up due to the sample size. As the lack of effect of medication is a null-result, we cannot conclude that there is no effect of medication: it is possible that dopaminergic medication has a small effect on the cortical processing of proprioceptive feedback that we did not detect. If there is a missed effect of Levodopa on the cortical processing of proprioceptive signals, these effects appear to be smaller than the difference in the beta-rebound response observed between PD patients and HC. Hence, even before we elucidate the post-movement beta rebound and its underlying mechanisms, this finding offers a potential marker for assessing the loss of proprioceptive function PD and disease progression in PD. Due to the relatively small sample size of PD patients in our current study, we cannot make a decisive conclusion about specific motor symptoms in PD. More studies with larger sample sizes are needed to better understand the role of the beta rebound—both in the general processing of sensory-motor signals and why it is attenuated in PD—and how the attenuation of the beta rebound is related to motor symptoms in PD.
Nevertheless, we can conclude is that at the cortical level, there appears to be a deficit in the processing of proprioceptive signals at the later cortical stage of processing and appears to be little affected by Levodopa medication.
Materials and Methods
Participants
Thirteen PD patients (age 41–75; three female) and seventeen HC (age 54–76; five female) participated in the study. One patient had to abort the session due to severe tremor in the OFF-medication state and subsequently had to cancel the participation in the study. One HC was excluded from analysis due to insufficient quality of the MEG recording.
The inclusion criteria for the PD group were: a clinical diagnosis of PD according to the United Kingdom Parkinson’s Disease Society Brain Bank Diagnostic Criteria with Hoehn and Yahr stage 1 to 361, and treatment with Levodopa, COMT inhibitors, MAO-B inhibitors, or dopamine receptor agonists. Additional inclusion criteria were: age between 40 to 80 years, healthy according to a physical examination (excluding parkinsonism), adequate cognitive status in terms of well-functioning and being able to give written consent after being informed about the procedure and purpose of the study. Exclusion criteria were: diagnosis of major depression dementia, history or presence of schizophrenia, bipolar disorder, epilepsy, or history of alcoholism or drug addiction according to Diagnostic and Statistical Manual of Mental Disorders, fifth edition62. Table 1 summarize motor symptoms measured by MDS-UPDRS-III in the ON- and OFF state, Levodopa equivalent daily dose (LEDD), scores on MoCA, scores on HADS, and demographical variables of PD patients and HC.
HC were recruited from a pool of healthy participants who previously had participated in other studies within the preceding year, or amongst eligible spouses of PD patients. Exclusion criteria for HC were: Diagnosis of PD or any other movement disorder, depression, dementia, the presence of- or history of schizophrenia, bipolar disorder, epilepsy, or history of alcoholism or drug addiction.
The local ethics committee in Stockholm approved the study (Etikprövningsnämden, 2016/911–31/1). We obtained informed consent from participants prior to their participation following the Declaration of Helsinki. The patients were recruited from the Parkinson’s Outpatient Clinic, Department of Neurology, Karolinska Huddinge University Hospital (Stockholm, Sweden).
Proprioceptive stimulation
The proprioceptive stimulation consisted of passive movements of the index finger on the hand contralateral to PD dominant side for PD patients and on the dominant hand for HC. The passive finger movements were evoked by a custom-made MEG compatible pneumatic movement actuator48. The movement actuator contained a pneumatic artificial muscle which contract when filled with pressurized air and expand when air-pressure is released, resulting in movement along a single axis. The flow of pressurized air to the device was controlled from outside the magnetically shielded room using Presentation software (v. 18.3; www.neurobs.com). Each session had a total of 90 induced movements. Subjects watched a movie with sound throughout the proprioceptive stimulation. All subjects confirmed that they felt the movements in the index finger before we started the recording.
The induced movements consisted of one contraction and extension of the pneumatic artificial muscle with a 200 ms interval followed by a silent period between 3.5 s to 4.0 s until the next induced movement. The movements had a total distance of 0.5 cm resulting in movements in the distal interphalangeal joint of approximately 15° and the proximal interphalangeal joint of approximately 10° in the index finger (Fig. 2B). Extending and contracting the pneumatic artificial muscle took around 50 ms giving a movement velocity of 0.1 m/s. The type of movement actuator we used has previously been shown to induce proprioceptive signals coherent with MEG signals measured from primary sensorimotor cortex48. Since the movements are induced through contact with the distal phalanx, activation of mechanoreceptors in the skin will contribute to the afferent signals generated by the stimulation in addition to the signals from the proprioceptors in the finger.
Procedure
MEG recordings in the MSR were acquired twice: first in OFF medication state and then after an hour’s break in ON. The motor subscale of the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale (MDS-UPDRS part III)63 was assessed in both ON and OFF, while MoCA and the Hospital Anxiety and Depression Scale (HADS) were assessed in ON. A neurologist certified in the use of MDS-UPDRS did all assessments.
HC was tested twice to accommodate the effect of time between repeated sessions64. The experiment was repeated in HC with the same hour distance break as PD subjects but did not include any PD medication for HC. By repeating the assessment on both PD patients and HC, we were able to isolate the effect of the medication this being the only difference between the two recordings among our groups.
MEG data acquisition
MEG data were recorded with an Elekta Neuromag TRIUX 306-channel MEG system, with 102 magnetometers and 204 planar gradiometers. The MEG scanner was located inside a two-layer magnetically shielded room. Data were sampled at 1000 Hz with an online 0.1 Hz high-pass filter and 330 Hz low-pass filter. Internal active shielding was active to suppress electromagnetic artifacts from the surrounding environment. The subjects’ head position and head movements inside the MEG helmet were sampled with MEG data using head-position indicator coils (HPI). The HPI was attached to subjects’ heads, and the location of the HPI location was digitalized with a Polhemus Fastrak motion tracker.
Horizontal and vertical electrooculogram (EOG) and electrocardiogram (ECG) were recorded simultaneously with bipolar Ag/AgC electrodes located above/below the left eye (vertical EOG) and on each side of the eyes (horizontal EOG). Electromyography (EMG) was measured on the forearms above the flexor carpi radialis with bipolar Ag/AgC electrodes located 7–8 cm apart. The position of the EMG electrodes was determined by asking subjects to tap their index finger and then locate muscle movements. An accelerometer attached to the nail of the index finger measured the acceleration of the finger movements along three orthogonal axes. The continuous time-course of the accelerometer were sampled together with the MEG data.
Data processing
Pre-processing
MEG data were processed off-line first by applying temporal signal space separation (tSSS) to suppress artifacts from outside the scanner helmet and correct for head movement during the recordings65,66. The tSSS had a buffer length of 10 s and a cut-off correlation coefficient of 0.98. Head origin was shifted to a position based on the average initial position from the first and second scan for each subject.
The continuous data from both sessions were concatenated for each subject, and independent component analysis (ICA) was then performed on the combined data using the fastica algorithm67 implemented in MNE-Python68. Components related to eye-blinks and heartbeats were identified by selecting components correlating with peaks of the measured EOG and ECG and removed from the raw MEG data. The ICA cleaned continuous MEG data was chopped into epochs from 1.5 s before movement onset to 3.5 s after movement. We rejected trials with extreme jump-artifacts based on min-to-max peak range exceeding 10 pT for the magnetometer and exceeded 2000 fT/cm for gradiometers.
The accelerometer data was filtered with a band-pass filter between 1–195 Hz, before averaging the three orthogonal channels by calculating the Euclidian norm and z-score normalized. The accelerometer provided a high accuracy estimate of the movements of the finger that is not influenced by high-frequency noise of biological or non-biological origin. The accelerometer was used to monitor that induced movement occurred in all trials and to ensure that subjects did not accidentally move their finger during the trials. Trials in which subjects made movements were rejected from the analysis. Accidental movements were defined as movement measured by the accelerometer outside the time window from 0–0.5 s relative to stimulus onset.
The number of trials used in the analysis of cortical responses to the proprioceptive stimulation after data cleaning ranged between 66–90 trials with a median of 87 trials. Comparison of the number of useful trials after data cleaning in a “Bayesian ANOVA”69 yielded support for no differences between session (BFH1/H0 = 0.33), between groups (BFH1/H0 = 0.45) or in the interactions between session and group (BFH1/H0 = 0.062). The remaining epochs were averaged per session for each subject. The averaged response was subtracted from every single trial for each subject, to enhance sensitivity to non-phase locked responses70,71.
The EMG data from the forearms were cut into epochs corresponding to the time-windows of interest for the MEG data (after applying a discrete Fourier transform filter to suppress 50 Hz line noise), and the signals were rectified. The rectified EMG epochs were averaged for each session per subject. For further comparison, we calculated the power spectral density of the non-rectified EMG signals by fast Fourier transform after applying a Hann window.
Time-frequency analysis of MEG
For the time-frequency analysis and the subsequent statistical analysis, we used the FieldTrip toolbox72 in Matlab R2016a (MathWorks Inc., Natick, MA).
We focused the statistical comparison on the combined gradiometer-pair that showed the highest amplitude in the time interval from 50 ms to 110 ms after stimulation onset. We averaged all trials across conditions per subject, after applying a 90 Hz low-pass filter and combine each orthogonal gradiometer pairs by taking the root of the squared values. The combined gradiometer-pair that showed the highest mean value across the specified time interval in the phase-locked domain was taken to represent the sensory-motor response to the optimal proprioceptive stimulation. The peak channel was used for the statistical analysis of the induced time-frequency beta-band responses to proprioceptive stimulation.
We obtained the event-related induced responses in the beta, alpha/mu, and theta bands by time-frequency decomposition using wavelets with a width of five cycles in the time window starting 1.25 s before movement onset and ending 2.5 seconds after on all frequencies from 2–40 Hz in steps of one Hz. After time-frequency decomposition, we combined the orthogonal gradiometer pairs to get a single time-frequency representation for each gradiometer-pair location.
We extracted the time-frequency response from the selected channel in the frequencies within the “mu spectrum” from 8 to 30 Hz, which encompasses the beta (14–30 Hz) and alpha (8–13 Hz) sensory motor rhythms73. Separating the two motor rhythms is difficult due to the harmonic component of the alpha-component of the mu rhythm leaking into the beta-band range36,73. Our comparisons of the “beta-band” response to proprioceptive stimulation, therefore, included the spectrum from 8–30 Hz. This is also in accordance with other definition of the beta-band as the entire 8–30 Hz spectrum, often used in intracranial recordings74.
The time-frequency representation of the data was log transformed and the average log-transformed power in a baseline-window from 1.25 s to 0.2 s before stimulation onset was subtracted per frequency bin from the log-transformed time-frequency data.
Statistical analysis
Beta/mu band response
The baseline corrected log-transformed time-frequency representation was compared between groups using a cluster-based permutations test51. This method for inferential statistics identifies clusters of adjacent time-frequency points along either the time or frequency domains that differ in a point-wise two-tailed t-test comparison (df = 26 for between-group comparisons, df = 11 for comparison within the PD group, and df = 15 for comparison within the HC group). The t-values of all points in each cluster were summed, and the sum was compared to a distribution of summed cluster values drawn from the same test in which data had been randomly sampled assigned groups using Monte Carlo simulation (n = 1000). The p-values reported in the results is not the analytical p-values from the t-tests, but the proportion of random clusters giving a larger cluster-statistic than the observed statistics. Clusters which sum greater than 95% of the sum of the permutated clusters are considered a significant difference between groups. The cluster-based permutation test accommodates the multiple comparison problems by giving a single test-statistic for the entire time-frequency window of interest, rather than test-statistics for each time-frequency bin51. The cluster-based permutation test is a non-paramedic that does not assume the probability distribution of the data to be normal.
The second effect we investigated was the effect of Levodopa medication on the beta-band response to the proprioceptive stimulation. To accommodate the effect of repetition upon the effect of medication, we tested the effect of medication in PD patients with a pseudo-two-by-two design. The test-retest effect would be present for both the patient group and the HC group, but any medication specific effect would only be present in the patient group since they were the only group who did take any medication. The interaction effect between group and session would regress out the retest effect and represent the effect of the medication.
The comparison was made by subtracting the log-transformed time-frequency responses from the first and second session for each subject. The time-frequency difference was then baseline corrected by subtracting the average of the difference in a baseline from 1.25 s to 0.2 s before stimulation onset. Finally, we tested for differences between the groups with a cluster-based permutation test with 1000 random permutations, where clusters beyond the critical alpha (alpha = 0.05, two-tailed) of the permutation distribution were considered significant.
Peripheral muscle activation
We tested for differences in peripheral muscle activation during the induced movements by comparing the EMG signals during the movement to EMG from a baseline period. The comparison was done with cluster-based permutation tests51 between EMG from 0 s to 0.5 s from stimulus onset, encompassing the movement, compared to EMG from 0.5 to 0 seconds before stimulus onset within the same session. The tests were done by comparing each time point of the EMG time-series with a two-tailed t-test, and by summing the t-value of neighboring time-points with a p-value < 0.05 (two-tailed). The sum of the clusters of t-values was compared to a distribution cluster values calculated from random permutations of data assigned to the groups using Monte Carlo simulation (n = 1000). Clusters in which the total sum of t-values was on the edges of the permutation distribution beyond the critical alpha (alpha = 0.05, two-tailed) were considered a significant difference. EMG activation during movement and baseline was compared for each group and session: PD patients ON/OFF (df = 11) and test/re-test for HC (df = 15).
In addition, we compared EMG activation within- and between groups on the entire time-window of the average rectified EMG data. The comparison was done with cluster-based permutation51 tests as described above, but using the same time-window as in the TFR analysis. The EMG time-series were compared with two-tailed t-tests session-wise between group (df = 26), between sessions within the PD group (df = 11), and between sessions within the HC group (df = 15). Clusters in which the total sum of t-values was on the edges of the permutation distribution beyond the critical alpha = 0.05 were considered a significant difference.
Baseline beta oscillations
We tested for a relationship between baseline power and relative power change due to proprioceptive stimulation by fitting a linear regression model that explained the mean spectra power change within the cluster as a function of baseline power and regressors indicating the session, group, and individual intercepts per subject. The model was compared by Bayesian model comparison69 to a model containing only regressors indicating the session, group, and individual intercepts, but not baseline power.
Subject variables and clinical scores
We tested for group differences in scores of MoCA, HADS, and age between PD patients and healthy controls through “Bayesian t-tests”75. The test gives the ratio of evidence for the hypothesis that there is a group difference versus the hypothesis of no difference between groups. The same test was used to test differences in UPDRS-III scores. To test for difference in the male-female ratio between groups, we used a Bayesian test for unequal multinomial distributions76.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Kalia, L. V. & Lang, A. E. Parkinson’s disease. The Lancet 386, 896–912 (2015).
Rodriguez-Oroz, M. C. et al. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 8, 1128–1139 (2009).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Dietz, V. Proprioception and locomotor disorders. Nat. Rev. Neurosci. 3, 781–790 (2002).
Konczak, J. et al. Proprioception and Motor Control in Parkinson’s Disease. J. Mot. Behav. 41, 543–552 (2009).
Proske, U. & Gandevia, S. C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 92, 1651–1697 (2012).
Konczak, J., Krawczewski, K., Tuite, P. & Maschke, M. The perception of passive motion in Parkinson’s disease. J. Neurol. 254, 655–663 (2007).
Maschke, M., Gomez, C. M., Tuite, P. J. & Konczak, J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126, 2312–2322 (2003).
Zia, S., Cody, F. & O’Boyle, D. Joint position sense is impaired by Parkinson’s disease. Ann. Neurol. 47, 218–228 (2000).
Mano, T., Yamazaki, Y. & Mitarai, G. Muscle spindle activity in human rigidity. Neurosci. Lett. 11, 22 (1979).
Seiss, E., Praamstra, P., Hesse, C. & Rickards, H. Proprioceptive sensory function in Parkinson’s disease and Huntington’s disease: evidence from proprioception-related EEG potentials. Exp. Brain Res. 148, 308–319 (2003).
Rabin, E., Muratori, L., Svokos, K. & Gordon, A. Tactile/proprioceptive integration during arm localization is intact in individuals with Parkinson’s disease. Neurosci. Lett. 470, 38–42 (2010).
Rickards, C. & Cody, F. W. Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain 120, 977–990 (1997).
Demirci, M., Grill, S., McShane, L. & Hallett, M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann. Neurol. 41, 781–788 (1997).
Nowak, D. A. & Hermsdörfer, J. Predictive and reactive control of grasping forces: on the role of the basal ganglia and sensory feedback. Exp. Brain Res. 173, 650–660 (2006).
Adamovich, S. V., Berkinblit, M. B., Hening, W., Sage, J. & Poizner, H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 104, 1027–1041 (2001).
Jacobs, J. V. & Horak, F. B. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with parkinson’s disease. Neuroscience 141, 999–1009 (2006).
Bronstein, A. M., Hood, J. D., Gresty, M. A. & Panagi, C. Visual Control of Blanace in Cerebellar and Parkinsonian Syndromes. Brain 113, 767–779 (1990).
Fellows, S., Noth, J. & Schwarz, M. Precision grip and Parkinson’s disease. Brain 121, 1771–1784 (1998).
Abbruzzese, G. & Berardelli, A. Sensorimotor integration in movement disorders. Mov. Disord. 18, 231–240 (2003).
Prud’homme, M. J. & Kalaska, J. F. Proprioceptive activity in primate primary somatosensory cortex during active arm reaching movements. J. Neurophysiol. 72, 2280–2301 (1994).
Alonso-Frech, F. et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 129, 1748–1757 (2006).
Neumann, W.-J. et al. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s disease. Clin. Neurophysiol. 128, 2286–2291 (2017).
Kühn, A. A., Kupsch, A., Schneider, G.-H. & Brown, P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease: STN activity and motor improvement. Eur. J. Neurosci. 23, 1956–1960 (2006).
Bosboom, J. L. W. et al. Resting state oscillatory brain dynamics in Parkinson’s disease: An MEG study. Clin. Neurophysiol. 117, 2521–2531 (2006).
Heinrichs-Graham, E. et al. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson’s disease: a pharmaco-magnetoencephalography study. J. Neurophysiol. 112, 1739–1747 (2014).
Sharott, A. et al. Dopamine depletion increases the power and coherence of β-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 21, 1413–1422 (2005).
Mallet, N. et al. Disrupted Dopamine Transmission and the Emergence of Exaggerated Beta Oscillations in Subthalamic Nucleus and Cerebral Cortex. J. Neurosci. 28, 4795–4806 (2008).
Bosboom, J. L. W., Stoffers, D., Wolters, E. C., Stam, C. J. & Berendse, H. W. MEG resting state functional connectivity in Parkinson’s disease related dementia. J. Neural Transm. 116, 193–202 (2009).
Pollok, B. et al. Increased SMA–M1 coherence in Parkinson’s disease — Pathophysiology or compensation? Exp. Neurol. 247, 178–181 (2013).
Silberstein, P. et al. Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain 128, 1277–1291 (2005).
Airaksinen, K. et al. Somatomotor mu rhythm amplitude correlates with rigidity during deep brain stimulation in Parkinsonian patients. Clin. Neurophysiol. 123, 2010–2017 (2012).
Cheyne, D. MEG studies of sensorimotor rhythms: A review. Exp. Neurol. 245, 27–39 (2013).
Jurkiewicz, M. T., Gaetz, W. C., Bostan, A. C. & Cheyne, D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. NeuroImage 32, 1281–1289 (2006).
Kilavik, B. E., Zaepffel, M., Brovelli, A., MacKay, W. A. & Riehle, A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 245, 15–26 (2013).
Salmelin, R. & Hari, R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60, 537–550 (1994).
Neuper, C. & Pfurtscheller, G. Post-movement synchronization of beta rhythms in the EEG over the cortical foot area in man. Neurosci. Lett. 216, 17–20 (1996).
Pfurtscheller, G., Stancák, A. & Neuper, C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr. Clin. Neurophysiol. 98, 281–293 (1996).
Druschky, K. et al. Somatosensory evoked magnetic fields following passive movement compared with tactile stimulation of the index finger. Exp. Brain Res. 148, 186–195 (2003).
Xiang, J. et al. Somatosensory evoked magnetic fields following passive finger movement. Cogn. Brain Res. 6, 73–82 (1997).
Devos, D. et al. Subthalamic stimulation influences postmovement cortical somatosensory processing in Parkinson’s disease. Eur. J. Neurosci. 18, 1884–1888 (2003).
Heinrichs-Graham, E. et al. Neuromagnetic Evidence of Abnormal Movement-Related Beta Desynchronization in Parkinson’s Disease. Cereb. Cortex 24, 2669–2678 (2014).
Pfurtscheller, G., Pichler-Zalaudek, K., Ortmayr, B., Diez, J. & Reisecker, F. Postmovement Beta Synchronization in Patients With Parkinson’s Disease. J. Clin. Neurophysiol. 15, 243–250 (1998).
Engel, A. K. & Fries, P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165 (2010).
Alegre, M. et al. Beta electroencephalograph changes during passive movements: sensory afferences contribute to beta event-related desynchronization in humans. Neurosci. Lett. 331, 29–32 (2002).
Parkkonen, E., Laaksonen, K., Piitulainen, H., Parkkonen, L. & Forss, N. Modulation of the ~20-Hz motor-cortex rhythm to passive movement and tactile stimulation. Brain Behav. 5 (2015).
Cassim, F. et al. Does post-movement beta synchronization reflect an idling motor cortex? Neuroreport 12, 3859–3863 (2001).
Piitulainen, H., Bourguignon, M., Hari, R. & Jousmäki, V. MEG-compatible pneumatic stimulator to elicit passive finger and toe movements. NeuroImage 112, 310–317 (2015).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease: Systematic Review of LED Reporting in PD. Mov. Disord. 25, 2649–2653 (2010).
Wetzels, R. et al. Statistical Evidence in Experimental Psychology An Empirical Comparison Using 855 t Tests. Perspect. Psychol. Sci. 6, 291–298 (2011).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Heinrichs-Graham, E. & Wilson, T. W. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage 134, 514–521 (2016).
Shin, H., Law, R., Tsutsui, S., Moore, C. I. & Jones, S. R. The rate of transient beta frequency events predicts behavior across tasks and species. eLife 6 (2017).
Jenkinson, N. & Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 34, 611–618 (2011).
Salmelin, R., Hämäläinen, M., Kajola, M. & Hari, R. Functional Segregation of Movement-Related Rhythmic Activity in the Human Brain. NeuroImage 2, 237–243 (1995).
Mongeon, D., Blanchet, P. & Messier, J. Impact of Parkinson’s disease and dopaminergic medication on proprioceptive processing. Neuroscience 158, 426–440 (2009).
O’Suilleabhain, P., Bullard, J. & Dewey, R. B. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J. Neurol. Neurosurg. Amp Psychiatry 71, 607 (2001).
Konczak, J., Li, K., Tuite, P. J. & Poizner, H. Haptic Perception of Object Curvature in Parkinson’s Disease. PLoS ONE 3, e2625 (2008).
Aman, J. E., Abosch, A., Bebler, M., Lu, C.-H. & Konczak, J. Subthalamic nucleus deep brain stimulation improves somatosensory function in Parkinson’s disease: STN-DBS Improves Haptic Perception in PD. Mov. Disord. 29, 221–228 (2014).
Wu, T. & Hallett, M. The cerebellum in Parkinson’s disease. Brain 136, 696–709 (2013).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression, and mortality. Neurology 17, 427–427 (1967).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (Fifth edition. Arlington, VA: American Psychiatric Publishing, [2013] ©2013, 2013).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47 (2007).
Wilson, T. W., Heinrichs-Graham, E. & Becker, K. M. Circadian modulation of motor-related beta oscillatory responses. NeuroImage 102, 531–539 (2014).
Taulu, S., Kajola, M. & Simola, J. Suppression of Interference and Artifacts by the Signal Space Separation Method. Brain Topogr. 16, 269–275 (2004).
Taulu, S. & Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51, 1759–1768 (2006).
Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 10, 626–634 (1999).
Gramfort, A. et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7 (2013).
Rouder, J. N., Morey, R. D., Speckman, P. L. & Province, J. M. Default Bayes factors for ANOVA designs. J. Math. Psychol. 56, 356–374 (2012).
Kalcher, J. & Pfurtscheller, G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalogr. Clin. Neurophysiol. 94, 381–384 (1995).
Pfurtscheller, G. & L da Silva, F. L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857 (1999).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive ElectrophysiologicalData. Comput. Intell. Neurosci. 2011, 1–9 (2011).
Hari, R. Action–perception connection and the cortical mu rhythm. in Progress in Brain Research 159, 253–260 (Elsevier, 2006).
Hammond, C., Bergman, H. & Brown, P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 30, 357–364 (2007).
Rouder, J. N., Speckman, P. L., Sun, D., Morey, R. D. & Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 16, 225–237 (2009).
Gûnel, E. & Dickey, J. Bayes factors for independence in contingency tables. Biometrika 61, 545–557 (1974).
Acknowledgements
The experiment was carried out at NatMEG (http://natmeg.se; Karolinska Institutet, Stockholm, Sweden), supported by the Knut & Alice Wallenberg Foundation. The authors thank Robert Oostenveld for discussions and suggestions on the statistical analysis.
Author information
Authors and Affiliations
Contributions
M.C.V., P.T., H.P., V.J., P.S. and D.L. designed the study and protocol. M.I., P.S. and D.L. provided financial support. M.C.V., P.T., J.W. and P.S. was responsible for recruiting patients and participants. M.C.V., P.T. and J.W. collected data. M.C.V. analysed the data, with suggestions from P.T., H.P., V.J. and D.L. M.C.V. and D.L. wrote the first draft of the manuscript. All authors comments on the manuscripts and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vinding, M.C., Tsitsi, P., Piitulainen, H. et al. Attenuated beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci Rep 9, 2604 (2019). https://doi.org/10.1038/s41598-019-39204-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39204-3
This article is cited by
-
Oscillatory and non-oscillatory features of the magnetoencephalic sensorimotor rhythm in Parkinson’s disease
npj Parkinson's Disease (2024)
-
The Swedish National Facility for Magnetoencephalography Parkinson’s disease dataset
Scientific Data (2024)
-
Biomarkers in amyotrophic lateral sclerosis: current status and future prospects
Nature Reviews Neurology (2023)
-
Simulated proximity enhances perceptual and physiological responses to emotional facial expressions
Scientific Reports (2022)
-
Effects of the Mirror Box Illusion on EEG Sensorimotor Rhythms in Voluntary and Involuntary Finger Movements
Neuroscience and Behavioral Physiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.