Abstract
Although low serum bicarbonate level is known to be associated with adverse outcomes in patients with chronic kidney injury, it is unclear whether low serum bicarbonate level is associated with the development of acute kidney injury (AKI). The purpose of our study was to determine whether serum bicarbonate levels at admission could be a risk factor for AKI development and mortality in hospitalised patients. We retrospectively enrolled 17,320 adult patients who were admitted to the academic teaching hospital from January 2013 to December 2013. Patients were divided into 2 groups based on the first measurement of serum bicarbonate level at admission. The incidence of AKI was higher in patients with low serum bicarbonate level than in those with normal serum bicarbonate level (8.0% vs. 4.1%). Low serum bicarbonate levels at admission were significantly associated with the development of AKI. In addition, low serum bicarbonate levels also independently predicted the 90-day mortality. Pre-existing low bicarbonate levels and subsequent development of AKI increased in-hospital mortality by 15 times compared with that in patients with normal bicarbonate levels and no AKI. Low serum bicarbonate levels may be associated with the development of AKI and high mortality in hospitalised patients.
Similar content being viewed by others
Introduction
Metabolic acidosis (MA), indicated by low serum bicarbonate levels, is a disorder that frequently develops in hospitalised patients. The presence of MA is strongly related to increased mortality, as it is implicated in multiple complications including cardiac dysfunction, hypotension, and increased risk of infection1,2,3.
Acute kidney injury (AKI) is also a common complication in hospitalised patients. Similar to MA, the presence of AKI is strongly related to mortality4. Once AKI develops, the chances of MA are increased. Decline in renal function causes an inability to excrete metabolic wastes and maintain proper acid-base balance, which results in MA5. Thus, clinical practice guidelines recommend the initiation of alkali therapy when the serum bicarbonate level is <22 mmol/L, although a recent Cochrane review has demonstrated that the benefit of sodium bicarbonate in AKI management is equivocal6,7.
Several observational studies have shown a crosstalk between MA and decline in renal function in patients with chronic kidney disease (CKD)8. A significant association of acidosis with all-cause mortality in patients with CKD has also been reported9,10,11. However, the impact of acidosis on the development of AKI has not yet been fully elucidated. In this study, we investigated whether lower serum bicarbonate level at the time of admission could predict the development of AKI, and whether AKI and low serum bicarbonate level have a combined effect on patient mortality.
Results
A total of 17,320 patients were enrolled and divided into 2 groups according to the serum bicarbonate level. In the enrolled cohort, 25.91% (n = 4,488) were acidotic initially. During a median (interquartile range) hospital stay of 6.0 (3.0–10.0) days, AKI of all stages was detected in 882 (5.1%) patients, of whom 662 (3.8%) were in stage I and 220 (1.3%) were in stage II and stage III (Supplementary Table S1). Of the patients, 3.1% died of all causes within 90 days after admission. No patient died before the development of AKI.
Baseline characteristics according to serum bicarbonate level
The patient demographics and clinical parameters at the time of admission are summarised in Table 1. Patients with low serum bicarbonate level were older than those with normal serum bicarbonate level, and more likely to have pre-existing comorbidities such as diabetes, hypertension, cardiovascular disease, and heart failure, except cancer. However, there was no significant difference in the Charlson comorbidity index score between the 2 groups.
The median serum bicarbonate levels in the low serum bicarbonate group and normal serum bicarbonate group were 20.0 (7–21) and 24.0 (22–29) mmol/L, respectively. Additionally, patients with low serum bicarbonate level at admission were more likely to have higher serum creatinine and to develop AKI. Compared with patients with normal serum bicarbonate level, those with low serum bicarbonate level had lower serum sodium, albumin, and estimated glomerular filtration rate (eGFR) at admission.
Low serum bicarbonate, AKI, and mortality
A significantly higher rate of AKI development was observed in the low bicarbonate group than in the normal bicarbonate group (Fig. 1). Patients with AKI had lower serum bicarbonate levels than those without AKI. To evaluate the independent risk factors predicting the development of AKI, Cox regression analysis was performed (Supplementary Table S2, Table 2). Variables that were significant in univariate analysis, such as albumin, history of intensive care unit stay, hypertension, and diabetes mellitus, were included as adjusting covariates in multivariate analysis. As a result, lower serum bicarbonate level was independently associated with an increased risk of AKI development, with a hazard ratio (HR) of 1.574 (95% confidence interval [CI], 1.273–1.949; P < 0.001) in multivariate Cox proportional hazard regression analysis.
The low bicarbonate group also showed a statistically higher risk of severe AKI, end-stage renal disease at 1 year, and mortality of all causes within 90 days or 1 year after admission than the normal bicarbonate group (Fig. 1, Fig. 2a). Patients with AKI also had considerably higher risk of 90-day than those who did not develop AKI (Fig. 2b). As shown in Table 2, both low serum bicarbonate level and AKI were significant predictors of 90-day mortality in multivariate Cox proportional regression analysis (adjusted HR, 1.302; 95% CI, 1.008–1.682; P = 0.043 and adjusted HR, 2.472; 95% CI, 1.900–3.217; P < 0.001, respectively; Table 2). As low serum bicarbonate level was associated with the development of AKI, we assessed the interaction between low serum bicarbonate levels and AKI for mortality by using the relative excess risk due to interaction (Table 3). Compared with patients with normal serum bicarbonate level and without AKI, patients with low serum bicarbonate level and those with AKI had worse mortality (odds ratio [OR], 2.723; P < 0.001 and OR, 15.200; P < 0.001, respectively). In addition, patients in the low bicarbonate group with AKI had an increased risk of 90-day mortality by 18.863 times compared with patients in the normal bicarbonate group (P < 0.001). We also observed that the hazards of AKI and mortality decreased as the serum bicarbonate level increased in the restricted cubic regression models, and that higher serum bicarbonate level within the normal range seemed beneficial with respect to avoiding the development of AKI and mortality (Fig. 3).
Cumulative survival rate according to serum bicarbonate level and acute kidney injury (AKI). (a–c) Show the survival curves of serum bicarbonate, AKI, and combined serum bicarbonate and AKI groups for the 90-day mortality, respectively. *And †indicate P < 0.001 when compared with normonatraemic patients without AKI and hyponatraemic patients without AKI, respectively; ‡indicates P < 0.05 when compared with normonatraemic patients with AKI in the log-rank test.
Discussion
In our large cohort study, we investigated the effect of low serum bicarbonate level at admission on the subsequent development of AKI in hospitalised patients. Patients with low serum bicarbonate level had an approximately 1.57-fold higher risk of AKI development than those with normal serum bicarbonate level. Low serum bicarbonate level also negatively affected survival and increased the risk of mortality in the presence of AKI. In addition, both AKI development and mortality proportionally increased with the severity of acidosis.
MA has been implicated in the pathogenesis of renal injury. The underlying exact mechanism for the association between MA and AKI is not clear; however, several possibilities can be considered on the basis of the results of previous animal and human studies. In MA, increased endothelin mediates urinary acidification and kidney injury12,13. Additionally, along with increased endothelin, excessive acid loading results in renin-angiotensin-aldosterone system activation14. In another study, MA was related to increased ammonia level, which, in turn, activated the alternative complement pathway and aggravated tubulointerstitial injury in an animal model of AKI15,16.
Furthermore, several observational studies have shown a clear relationship between MA and rapid decline in renal function in patients with CKD8. Although the mechanism of CKD progression due to MA cannot be directly applied to patients with AKI, reduced renal blood flow and inflammation may also be related to renal injury in patients with AKI. Several small trials suggested that the treatment of acidosis with oral alkali can slow the progression of kidney disease in patients with CKD17. In our study, patients treated with bicarbonate had higher rates of AKI development and mortality (data not shown). However, as patients treated with sodium bicarbonate had more severe disease and lower initial bicarbonate levels than patients who did not receive sodium bicarbonate, further well-designed trials are needed.
The higher number of AKI risk factors in patients with low serum bicarbonate level could affect the association between low serum bicarbonate level and AKI development. Therefore, we performed multivariate regression analysis, in which low serum bicarbonate level remained a significant risk factor of AKI development even after adjusting for baseline eGFR and other clinical and demographic factors. Consistent with these results, MA in kidney transplant recipients was a significant risk factor for graft failure and patient mortality, even after adjustment for eGFR18. Jung et al. also demonstrated that patients with low serum bicarbonate levels before cardiac surgery had a higher incidence of postoperative AKI independent of other risk factors for AKI development19. Similarly, recent observational studies found that lower serum bicarbonate levels predicted the development of AKI5,20, thereby supporting a close association between serum bicarbonate level and renal dysfunction.
There were some interactions between AKI and low serum bicarbonate level; thus, we analysed whether hospital-acquired AKI modified the effect of low serum bicarbonate level on short-term patient mortality. AKI can be further exacerbated by MA because of the reduction of renal blood flow and increase of inflammatory mediator release. Moreover, MA exacerbates renal injury, a phenomenon that was associated with a high nuclear factor-κB expression in an experimental AKI model21. These results could be a potential explanation for the synergistic interaction between MA and AKI. MA also affected long-term survival and increased mortality when AKI was concomitantly present.
Our study has several limitations. First, confounding factors originating from the retrospective design may have existed. Particularly, the different severities of illness on admission between the low and normal serum bicarbonate groups would be a major confounding variable. However, we excluded patients with community-acquired AKI. By excluding patients who had AKI before admission, we could clearly identify that MA preceded the development of AKI, reinforcing the relationship between MA and AKI. Moreover, the Charlson comorbidity index score was comparable between the 2 groups, and low serum bicarbonate level was a significant predictor of both AKI and 90-day mortality after adjusting for the severity of illness and other confounding variables. Second, our study was conducted in a single country and a single centre, limiting the generalisation of our findings. However, we collected data from a very large administrative cohort, and every variable was well measured; thus, there were few lost cases and laboratory data during the study period. Third, as our electronic medical records system does not keep records of the patients’ hourly urine output, we defined AKI only according to the serum creatinine criteria. Finally, measurements of arterial blood gas were not taken in all patients. Arterial pCO2 and pH were not determined, which leaves the possibility that the serum carbon dioxide levels may not represent the true serum bicarbonate levels in conditions such as respiratory alkalosis or sepsis. However, the prevalence of chronic obstructive pulmonary disease and heart failure was comparable between groups.
Low serum bicarbonate level in admitted patients is independently associated with the development of hospital-acquired AKI and long-term survival, and synergizes mortality in the presence of AKI. Therefore, patients with low serum bicarbonate level, which is easily detected using serum bicarbonate levels might need to be periodically monitored for serum creatinine level or urine output to check for AKI development.
Methods
Study population
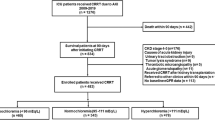
We performed a single-centre, retrospective cohort study of patients aged 18 years or older who were admitted to Seoul National University Bundang Hospital from January 2013 to December 2013. This study initially included a total of 19,534 patients. We excluded patients who met the following exclusion criteria: (1) with community-acquired AKI; (2) with pre-existing end-stage renal disease that required renal replacement therapy before hospitalisation; and (3) with no available data or with high serum bicarbonate level (>31 mmol/L) within 2 days after admission. The remaining 17,320 patients were selected for the final analysis. The study received full approval from the Seoul National University Hospital institutional review board (IRB no. B-1408/264-102). The requirement for written informed consent was waived by the IRB, and this manuscript adheres to the applicable STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
Definitions and measurements
All data were collected from an electronic medical records database. The data included demographic data, comorbidities, and physiological data at admission. The time-weighted average mean arterial pressure (MAP) was calculated as the area under the curve of the MAP measurements divided by the total measurement time within 2 days after admission22. Total serum carbon dioxide levels, which are generally used as indirect measures of serum bicarbonate levels18, were determined using an electrode-based method (UniCel DxC 800; Beckman Coulter Inc., Brea, CA, USA). According to the first serum bicarbonate measurement within 2 days after admission, the patients were divided into 2 groups: the low bicarbonate group, which included patients whose serum bicarbonate level was <22 mmol/L, and the normal bicarbonate group, which included patients whose serum bicarbonate level was ≥22 mmol/L6.
Serum creatinine level was measured using the rate-blanked compensated kinetic alkaline picrate Jaffe method with an automatic analyser (TBA-200FR; Toshiba, Tokyo, Japan). The eGFR was calculated using the variable Modification of Diet in Renal Disease Study equation23. Baseline creatinine was defined as the lowest value within 6 months before the index admission or the calculated value from the Modification of Diet in Renal Disease study equation, assuming that the baseline glomerular filtration rate is 75 mL·min−1·1.73 m−2 if creatinine was not available24. AKI was defined as an increase in serum creatinine level of 26.5 mmol/L (0.3 mg/dL) greater than the baseline value or a 1.5-fold higher value than the baseline level determined during the hospital stay25. We defined AKI solely according to changes in measured serum creatinine values because urine output data were not consistently available for all inpatients. We defined severe AKI as stage 2 and 3 AKI based on the Kidney Disease: Improving Global Outcomes classification25. If the first creatinine level at the index admission met the criteria for AKI, we defined it as a community-acquired AKI. The development of end-stage renal disease was determined from the registry database of the Korean Society of Nephrology. Patient mortality was determined from the death certificates, and 1-year mortality was determined from the database of the Ministry of Interior.
Statistical analysis
Values were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and as percentage for categorical variables. The difference was analysed using Student’s t-test for continuous variables and the chi-square test for categorical variables. For the estimated survival, the Kaplan-Meier method was employed, and the statistical significance was calculated using the log-rank test. A Cox proportional hazards regression analysis was performed to determine the association between low serum bicarbonate level and AKI or mortality. We fitted a multivariate Cox regression model with significant variables (P < 0.05) from the univariate Cox regression analysis. Further, the probability of AKI and mortality in relation to serum bicarbonate level at admission was presented using restricted cubic splines. For the estimated survival, the Kaplan-Meier method was employed, and the statistical significance was calculated using the log-rank test. The additive interaction was assessed using the relative excess risk due to interaction, attributable proportion due to interaction, and synergistic index26, with R statistics (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria). Unless specified, all analyses were performed using SPSS Statistics (version 20; IBM, Armonk, NY, USA).
References
Gunnerson, K. J., Saul, M., He, S. & Kellum, J. A. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Critical care (London, England) 10, R22, https://doi.org/10.1186/cc3987 (2006).
Kraut, J. A. & Madias, N. E. Metabolic acidosis: pathophysiology, diagnosis and management. Nature reviews. Nephrology 6, 274–285, https://doi.org/10.1038/nrneph.2010.33 (2010).
Masevicius, F. D. et al. Relationship of at Admission Lactate, Unmeasured Anions, and Chloride to the Outcome of Critically Ill Patients. Critical care medicine 45, e1233–e1239, https://doi.org/10.1097/ccm.0000000000002730 (2017).
Nash, K., Hafeez, A. & Hou, S. Hospital-acquired renal insufficiency. American journal of kidney diseases: the official journal of the National Kidney Foundation 39, 930–936, https://doi.org/10.1053/ajkd.2002.32766 (2002).
Gujadhur, A. et al. Serum bicarbonate may independently predict acute kidney injury in critically ill patients: An observational study. World journal of critical care medicine 4, 71–76, https://doi.org/10.5492/wjccm.v4.i1.71 (2015).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clinical practice 120, c179–184, https://doi.org/10.1159/000339789 (2012).
Hewitt, J., Uniacke, M., Hansi, N. K., Venkat-Raman, G. & McCarthy, K. Sodium bicarbonate supplements for treating acute kidney injury. The Cochrane database of systematic reviews, Cd009204, https://doi.org/10.1002/14651858.CD009204.pub2 (2012).
Dobre, M. et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. American journal of kidney diseases: the official journal of the National Kidney Foundation 62, 670–678, https://doi.org/10.1053/j.ajkd.2013.01.017 (2013).
Kovesdy, C. P. Metabolic acidosis and kidney disease: does bicarbonate therapy slow the progression of CKD? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 27, 3056–3062, https://doi.org/10.1093/ndt/gfs291 (2012).
Menon, V. et al. Serum bicarbonate and long-term outcomes in CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation 56, 907–914, https://doi.org/10.1053/j.ajkd.2010.03.023 (2010).
Navaneethan, S. D. et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN 6, 2395–2402, https://doi.org/10.2215/cjn.03730411 (2011).
Wesson, D. E. & Simoni, J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney international 78, 1128–1135, https://doi.org/10.1038/ki.2010.348 (2010).
Wesson, D. E., Nathan, T., Rose, T., Simoni, J. & Tran, R. M. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney international 71, 210–217, https://doi.org/10.1038/sj.ki.5002036 (2007).
Ng, H. Y., Chen, H. C., Tsai, Y. C., Yang, Y. K. & Lee, C. T. Activation of intrarenal renin-angiotensin system during metabolic acidosis. American journal of nephrology 34, 55–63, https://doi.org/10.1159/000328742 (2011).
Nath, K. A., Hostetter, M. K. & Hostetter, T. H. Increased ammoniagenesis as a determinant of progressive renal injury. American journal of kidney diseases: the official journal of the National Kidney Foundation 17, 654–657 (1991).
Pangburn, M. K., Schreiber, R. D. & Muller-Eberhard, H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. The Journal of experimental medicine 154, 856–867 (1981).
Chen, W. & Abramowitz, M. K. Metabolic acidosis and the progression of chronic kidney disease. BMC nephrology 15, 55, https://doi.org/10.1186/1471-2369-15-55 (2014).
Park, S. et al. Metabolic Acidosis and Long-Term Clinical Outcomes in Kidney Transplant Recipients. Journal of the American Society of Nephrology: JASN 28, 1886–1897, https://doi.org/10.1681/asn.2016070793 (2017).
Jung, S. Y. et al. Preoperative Low Serum Bicarbonate Levels Predict Acute Kidney Injury After Cardiac Surgery. Medicine 95, e3216, https://doi.org/10.1097/md.0000000000003216 (2016).
Hu, J. et al. Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. Experimental and therapeutic medicine 13, 2362–2374, https://doi.org/10.3892/etm.2017.4292 (2017).
Magalhaes, P. A. et al. Metabolic acidosis aggravates experimental acute kidney injury. Life sciences 146, 58–65, https://doi.org/10.1016/j.lfs.2016.01.007 (2016).
Octavio, J. A. et al. Time-weighted vs. conventional quantification of 24-h average systolic and diastolic ambulatory blood pressures. Journal of hypertension 28, 459–464, https://doi.org/10.1097/HJH.0b013e328334f220 (2010).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 130, 461–470 (1999).
Manjunath, G., Sarnak, M. J. & Levey, A. S. Prediction equations to estimate glomerular filtration rate: an update. Current opinion in nephrology and hypertension 10, 785–792 (2001).
Section 2: AKI Definition. Kidney international supplements 2, 19–36, https://doi.org/10.1038/kisup.2011.32 (2012).
Rothman, K. J. Synergy and antagonism in cause-effect relationships. American journal of epidemiology 99, 385–388 (1974).
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI16C2221).
Author information
Authors and Affiliations
Contributions
S.Y.L. designed the study and drafted the manuscript; Y.M.P. analysed the data; H.J.C., K.Y.N. and D.W.C. contributed to the acquisition of data; S.J.K. contributed to the interpretation of data and provided critical revisions of the manuscript. All authors have given final approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, S.Y., Park, Y., Chin, H.J. et al. Short-term and long-term effects of low serum bicarbonate level at admission in hospitalised patients. Sci Rep 9, 2798 (2019). https://doi.org/10.1038/s41598-019-38892-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38892-1
This article is cited by
-
Serum bicarbonate levels and kidney outcomes in critically ill patients: a prospective cohort study
International Urology and Nephrology (2024)
-
Accuracy of SCORTEN in predicting mortality in toxic epidermal necrolysis
BMC Medical Informatics and Decision Making (2022)
-
Lower serum bicarbonate is associated with an increased risk of acute kidney injury
Journal of Nephrology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.